Abstract
Objectives. The aim of the study was to assess the long-term influence of catheter ablation (CA) of different arrhythmias on cardiovascular implantable electronic devices (CIED) endocardial leads durability. Design. This was a retrospective case-control study. Ablation protocols and in- or outpatient medical records were reviewed to identify and extract data on adult patients with CIED undergoing a CA. A cohort of patients with hypertrophic cardiomyopathy and implantable cardioverter-defibrillators (ICD) served as a historical control group. The primary endpoint was the diagnosis of lead damage defined as permanent loss of proper function demanding replacement or removal. Results. Among 145 patients n = 177 catheter ablations were performed. Patients’ mean age was 66.4 ± 10.5, 66.1% had an ICD or ICD with cardiac resynchronization function (CRT-D), 18.1% had >1 CA. During median 812 days [IQR 381-1588] of follow-up, there were 11 (6.2%) cases of lead damage in the examined and 13 cases (13%) in the control group, p = 0.054. None of the technical aspects of the CA (indication, type of catheter, transseptal sheath) influenced the primary outcome. Both the number of leads and observation time after CA were significantly related to the risk of endocardial lead damage. Conclusion. This study did not find any significant influence of CA on the long-term durability of CIED endocardial leads. Reported risk factors were consistent with general population of CIED patients.
Introduction
Indications for catheter ablation (CA) in patients with cardiovascular implantable electronic devices (CIED) span from standard situations common with general population to device specific issues: adequate and inadequate interventions or low percentage of biventricular pacing (BiV) [Citation1–5]. CA in a CIED is generally considered safe for the patient and the device, but a general concept is to avoid the direct contact between ablation catheter and endocardial leads during applications [Citation6]. However, there have been reports of an acute lead damage during the procedure and periprocedural disturbances in a device program [Citation7–9]. The effect of radiofrequency or cryoablation applied in the direct contact with endocardial leads was tested in vitro in a recent study, which found no short-term deleterious effect on the integrity of leads [Citation10]. Much less is known about a long-term performance of endocardial leads after CA procedures. During 18 ± 12 months of follow-up there were no reports of a lead damage in the recipients of a cardiac resynchronization therapy (CRT) undergoing atrio-ventricular node (AVN) ablation [Citation11].
The aim of this study was to assess the long-term influence of catheter ablation of heart arrhythmias on cardiovascular implantable electronic devices endocardial leads.
Material and methods
This was a retrospective case-control study. Ablation protocols and in- or outpatient medical records were reviewed to identify adult patients with CIED undergoing a CA procedure. General patient information together with indications, technical aspects of the procedure, acute CIED complications and long-term performance of the CIED leads were extracted.
The analysis was approved by the local Bioethics Committee.
General information consisted of: age, gender, type of CIED, type and number of endocardial leads, indication for CA.
CA technical aspects comprised: type of ablation catheter, utilization of a transseptal sheath (both transseptal puncture or catheter stabilization in right heart, left or right heart procedure, left heart access).
Acute CIED complications were defined as lead dislodgement, a significant rise of pacing threshold or impedance, inappropriate ICD therapy.
The primary endpoint of the study was the diagnosis of a lead failure defined as permanent loss of a proper function demanding replacement or removal. Transient fluctuations of lead impedance, sensing or pacing thresholds were deemed clinically non-significant and therefore not analyzed. We calculated and analyzed the following time periods: the total follow-up from the first implantation to the last CIED control visit, the time from CA to lead damage diagnosis, the time from CA to the last CIED control visit.
Study group
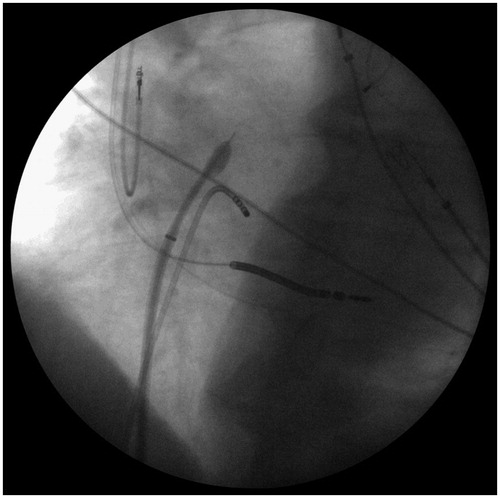
All patients in the study group underwent CA at our center. All equipment used during CA was left to discretion of the operator, but generally the ablation procedure in a CIED patient was similar to a standard one. CIED was interrogated in the EP room directly before CA and immediately after the removal of vascular sheets. The standard catheters used were non-irrigated 4 mm tip RF MarinrMC (Medtronic, Inc, Minneapolis, MN) or 8 mm Dual Sensor (Biosense Webster, Inc, Diamond Bar, CA). The irrigated catheter group consisted of the following: Allcath Flux eXtra (Biotronik GmbH & Co KG, Germany), Flexability or Cool Flex (Abbott Medical/Jude Medical, Inc, St. Paul, MN), Navistar Thermocool or Thermocool Smart Touch (Biosense Webster, Inc, Diamond Bar, CA). For cryoablation Arctic Front 2AF281 or Arctic Front Advanced 2AF283 balloons together with 15 F Flexcath Advance 4FC12 (all Medtronic, Inc, Minneapolis, MN) sheath were used. For catheter stabilization in the right heart and procedures in the left atrium or ventricle standard 7-10 F non-steerable transseptal sheaths (St. Jude Medical, Inc, St. Paul, MN) were used. All radiofrequency (RF) applications were delivered in a power controlled mode using Stockert (Stockert, Freiburg, Germany) or Atakr (Medtronic, Inc, Minneapolis, MN) generators with the following standard settings: 4 mm tip – 50 W/50 °C/60s, 8 mm tip – 70 W/50 °C/60s, irrigated tip 20-45 W/43 °C/30-60s depending on the site of ablation. We used oblique views extensively to manipulate beneath and around endocardial leads, avoided placing a diagnostic catheter into coronary sinus in CRT patients (where available) and opted for a retrograde access to the left ventricle in the cases of particularly difficult transseptal puncture in ventricular tachycardia ablations ().
Figure 1. Transseptal puncture in a patient with cardiac resynchronization therapy (CRT) device. In absence of a diagnostic catheter in coronary sinus the left ventricle lead was used as a landmark for the puncture (LAO 30° projection).
In the majority of cases the long-term follow-up was conducted in our outpatient clinic with personal visits up to three months after CA and then annually for pacemakers and twice a year for other CIEDs. For patients followed-up elsewhere we contacted those centers to obtain information on the most recent CIED interrogation and on any important clinical outcomes. Since we are a reference center for CIED complications and transvenous lead extractions (TLE), it was unlikely to miss any significant lead complications in the study group. In patients lost to follow-up, information from the most recent CIED interrogation was used for the analysis.
Historical control group
The outcomes in the CA group were compared to the previously published historical control group of patients with hypertrophic cardiomyopathy (HCM) implanted and followed-up at our center [Citation12]. Originally, the group comprised 104 patients: mean age 35.6 ± 16.2 years old, 45.2% men, 75% primary prophylaxis of sudden cardiac death, mean ejection fraction 63.5 ± 10.4%, history of atrial fibrillation 26%. The mean follow-up was 4.6 ± 2.6 years, range 2.0-12.7 years. During this time five patients were treated with CA and therefore included in the study and not the control group. Lead dysfunction was observed in 13 (12.5%) patients. Immediate intervention was necessary due to the replacement of one atrial and eight ventricular leads. The remaining cases – one atrial and three ventricular leads were replaced together with a pulse generator during elective procedure [12]. Contrary to the study group, patients comprising the control group were younger and all had single or dual chamber implantable cardioverter-defibrillators (ICD). On the other hand, all their invasive procedures and follow-ups were conducted at our Center, therefore they were similar to the study group it terms of risk factors related to implantation, pocket revisions, generator replacements etc., all of them established risk factors of endocardial lead failure [Citation13–16]. Therefore, despite differences in clinical profiles, we decided to include them in the analysis as a control group understanding the limitation of such an approach.
Statistical analysis
Continuous variables were tested for normal distribution by Shapiro-Wilk test, and are presented as a mean ± SD or median (interquartile range), as appropriate . Categorical variables are reported as frequencies and percentages. Differences between groups were assessed using the chi-2 Pearson’s test or Fisher’s Exact test for categorical variables and Mann-Whitney test or Student’s t-test for continuous data.
Binominal logistic regression models were constructed. The stepwise selection method was applied on the basis of likelihood ratio statistics. The improvement in predictive accuracy was evaluated by C-statistic (area under Receive Operating Curve). Odds ratios (OR) with corresponding 95% confidence interval (CI) were estimated.
A p < 0.05 was considered to indicate statistical significance. SAS software (version 9.4, SAS Institute Inc, Cary, USA) was used for all analyses.
Results
In years 2005-2015 among 145 patients n = 177 catheter ablations were performed. The baseline characteristics of the study group were summarized in . There were 6 (3.4%) cases of lead damage and 3 (1.7%) cases of transvenous lead extraction (TLE) occurring before the CA, which therefore were not included in the analysis. The median time from the implantation to CA was 895 [IQR 266-2251] days. Excluding patients with CRT, an atrial lead was present in 58 cases, including 11 dual chamber ICDs.
Table 1. Baseline characteristics of patients in the study group and historical control group.
Indications and technical aspects of the CA were summarized in . There were no periprocedural cases of lead damage, although in one case the AFL ablation was terminated prematurely due to an operator’s concern over the proximity of the ICD lead. On the follow-up, there was no indication of any significant lead damage. The median follow-up after the CA in the study group was 812 days [IQR 381-1588] and the median time from the CIED implantation to CA was 895 days [IQR 266-2251].
Table 2. Indications and technical aspects of the catheter ablation.
Comparisons between the study and control groups have been presented in . The median time from the CA to the lead damage diagnosis was 1211 days [IQR 215-1590]. Five out of 11 (45%) cases of lead failures were observed in Riata (two) or Sprint Fidelis (three) lead recipients. TLE was performed in seven (64%) cases while an additional lead was implanted in the remaining four patients. . depicts a failure-free survival in all study patients and ICD/CRT-D recipients against the control group. The univariate analysis of the lead failure risk factors in the study group has been presented in . In multivariate analysis, both the number of leads and the observation time after CA were significantly related to the risk of endocardial lead damage while patients’ age was showed to be a mildly protective factor ().
Figure 2. Lead failure-free survival (days) in all patients (A) and ICD/CRT-D recipients (B) in comparison to the control group (study group: continuous line; control group: dashed line).
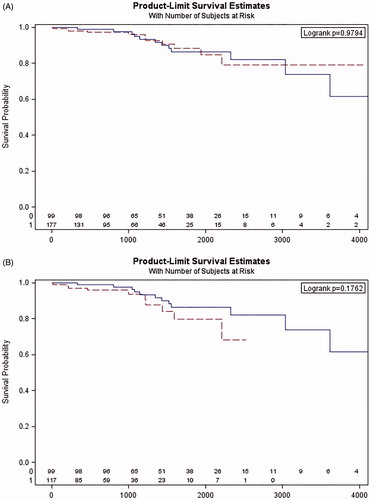
Table 3. The comparison of the catheter ablation and control groups.
TABLE 4. The univariate analysis of risk factors of the lead damage after catheter ablation.
Table 5. The binominal multivariable logistic regression analysis of risk factors of lead damage after catheter ablation.
The median time of observation after CA was significantly longer in patients with lead damage than in the rest of the study group – 1691 [1092-2274] days vs. 791 [342-1493] days, p = 0.029, respectively.
Discussion
Our study has suggested that catheter ablation had no significant impact on the long-term performance of endocardial leads in patients with cardiovascular implantable electronic devices. We analyzed 177 procedures performed over a six years’ time span and observed 11 (6.2%) cases of lead damage. The median time from the index ablation to the lead damage diagnosis was 1211 days [IQR 215-1590] and it exceeded the median follow-up of the group as a whole (median 812 days, IQR 381-1588).
In comparison to the control group, there was a trend towards lower risk of lead damage in the CA group but it did not reach statistical significance (). When high energy devices in the CA group were analyzed, neither percentage nor time to lead damage were different from the control group ( and ). What is worth mentioning, while the total observation time was longer in the control group, the time from implantation to lead damage was similar in both groups ().
In this cohort, neither the indication for the CA nor technical aspects of the procedure (type of catheter, transseptal sheath utilization) played any significant role. This was somehow counterintuitive, as we had hypothesized that the cavo-tricuspid isthmus ablation or utilization of the transseptal sheath might have posed an elevated risk to the CIED leads. It was less surprising in the light of a recent experimental study where intensive radiofrequency and cryoablation in close vicinity did not show any deleterious effects on endocardial leads in the in vitro setting [Citation10]. Our study confirms those observations in the clinical setting.
Both the number of endocardial leads and a longer follow-up after CA appeared to be significant risk factors of the endocardial lead damage ( and ). Those findings were in concordance with the literature published, where the more complex CIED and the longer time of follow-up the higher risk of the lead damage [Citation13–18]. What is interesting, in our cohort almost all lead dysfunctions happened in high energy devices with a single case of CRT-P and no classical pacemaker leads failure. Not surprisingly, five out of 11 (45%) cases happened in recipients of Riata or Sprint Fidelis leads.
Previous studies concerning only AF ablations have suggested rare occurrences of endocardial lead malfunction, either dislodgement or insulation defect, during or shortly after the procedure [Citation3,Citation19]. All patients required lead replacement. There were no cases of lead damage or dislodgement during the procedure in our series where 27% of procedures were conducted using the transseptal sheath (). In our opinion, careful manipulation around the right atrium with the extensive use of oblique projections for optimal positioning of guidewires and transseptal sheaths is crucial for avoiding damage to CIED leads. What is more, almost 24% of our patients had ablation of cavo-tricuspid isthmus dependent atrial flutter without short-term complications. The recent publication estimated the long-term risk of CIED lead malfunction in this population to be about 7% during 55.4 ± 38.1 months of observation [Citation19]. This figure is comparable to our results – 8.5% (), which was observed in a diverse population of patients undergoing a wide variety of catheter ablations although the observation period was clearly shorter ( and ). It should be noted that, in our series percentage of ICD or CRT devices accounted for about 68% of all patients (), contrary to 27% in the cited publication, and those CIEDs are known to be at a higher risk of lead malfunction than pacemakers [Citation13–19].
Another finding of the study was that older patients were at a lower risk of lead damage than younger CIED recipients. This phenomenon had been described among recipients of Sprint Fidelis leads but less had been known about other lead types [Citation20,Citation21]. In our study high energy devices accounted for about 66% of all cases. At this point, this finding has not been thoroughly studied in our cohort.
Limitations
The study has all the standard limitations of a retrospective study including potential selection bias and missing data. To limit these issues we thoroughly searched all available medical records and included all patients regardless of the indication, length of follow-up, history of the CIED, etc.
We included the previously published, historical control group of patients implanted and followed-up at our Center for reference, when a control group comprising all CIED patients from the corresponding time period would be more appropriate. While remaining the second biggest limitation to the study, an additional analysis of high energy devices ( and ) showing no significant differences between groups lends additional credibility to our decisions.
The procedures analyzed were performed by experienced operators or EP fellows under a strict supervision, hence the outcomes might not be representative for less experienced centers.
As it was a single center study, the number of patients analyzed was relatively low, which further decreases the generalizability of our results.
Conclusions
This study suggests there may be no significant influence of catheter ablation on the long-term performance of endocardial leads in patients with cardiovascular implantable electronic devices. Larger, multicenter studies would be advisable to confirm these results.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Di Biase L, Mohanty P, Mohanty S, et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644.
- Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017;19:465–511.
- Lakkireddy D, Di Biase L, Ryschon K, et al. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol. 2012;60:1531–1539.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200.
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867.
- Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm. 2011;8:1114–1154.
- Lakkireddy D, Patel D, Ryschon K, et al. Safety and efficacy of radiofrequency energy catheter ablation of atrial fibrillation in patients with pacemakers and implantable cardiac defibrillators. Heart Rhythm. 2005;2:1309–1316.
- Newby KH, Zimerman L, Wharton JM, et al. Radiofrequency ablation of atrial flutter and atrial tachycardias in patients with permanent indwelling catheters. Pacing Clin Electro. 1996;19:1612–1617.
- Sadoul N, Blankoff I, de Chillou C, et al. Effects of radiofrequency catheter ablation on patients with permanent pacemakers. J Interv Card Electrophysiol. 1997;1:227–233.
- Darrat YH, Agarwal A, Morales GX, et al. Radiofrequency and Cryo-Ablation Effect on Transvenous Pacing and Defibrillatory Lead Integrity: An In Vitro Study. J Cardiovasc Electrophysiol. 2016;27:976–980.
- Proclemer A, Facchin D, Pagnutti C, et al. Safety of pacemaker implantation prior to radiofrequency ablation of atrioventricular junction in a single session procedure. Pacing Clin Electro. 2000;23:998–1002.
- Syska P, Przybylski A, Chojnowska L, et al. Implantable cardioverter-defibrillator in patients with hypertrophic cardiomyopathy: efficacy and complications of the therapy in long-term follow-up. J Cardiovasc Electrophysiol. 2010;21:883–889.
- Borleffs CJ, van Erven L, van Bommel RJ, et al. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411–416.
- Kirkfeldt RE, Johansen JB, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–1194.
- Kirkfeldt RE, Johansen JB, Nohr EA, et al. Risk factors for lead complications in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Heart Rhythm. 2011;8:1622–1628.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551.
- Ellenbogen KA, Hellkamp AS, Wilkoff BL, et al. Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol. 2003;92:740–741.
- Hauser RG, Hayes DL, Kallinen LM, et al. Clinical experience with pacemaker pulse generators and transvenous leads: an 8-year prospective multicenter study. Heart Rhythm. 2007;4:154–160.
- Dinshaw L, Schäffer B, Akbulak Ö, et al. Long-term efficacy and safety of radiofrequency catheter ablation of atrial fibrillation in patients with cardiac implantable electronic devices and transvenous leads. J Cardiovasc Electrophysiol. 2019;30:679.
- Girerd N, Nonin E, Pinot J, et al. Risk of Sprint Fidelis defibrillator lead failure is highly dependent on age. Arch Cardiovasc Dis. 2011;104:388–395.
- Hauser RG, Maisel WH, Friedman PA, et al. Longevity of Sprint Fidelis implantable cardioverter-defibrillator leads and risk factors for failure: implications for patient management. Circulation. 2011;123:358–363.
