Abstract
Objectives: Protamine reduces platelet aggregation after cardiopulmonary bypass (CPB). We studied the inhibitory effect of a reduced protamine dose, the duration of impaired platelet function and the possible correlation to postoperative bleeding. Design: Platelet function was assessed by impedance aggregometry in 30 patients undergoing cardiac surgery with CPB at baseline, before protamine administration, after 70% and 100% of the calculated protamine dose, after 20 minutes and at arrival to the intensive care unit. Adenosine diphosphate (ADP), thrombin receptor activating peptide-6 (TRAP), arachidonic acid (AA) and collagen (COL) were used as activators. Blood loss was measured during operation and three hours after surgery. Results are presented as median (25th–75th percentile). Results: Platelet aggregation decreased markedly after the initial dose of protamine (70%) with all activators; ADP 89 (71–110) to 54 (35–78), TRAP 143 (116–167) to 109 (77–136), both p < .01; AA 25 (16–49) to 17 (12–24) and COL 92 (47–103) to 60 (38–81) U, both p < .05. No further decrease was seen after 100% protamine. The effect was transient and after twenty minutes platelet aggregation had started to recover; ADP 76 (54–106), TRAP 138 (95–158), AA 20 (10–35), COL 70 (51–93) U. Blood loss during operation correlated to aggregometry measured at baseline and after protaminization. Conclusions: Protamine after CPB induces a marked decrease in platelet aggregation already at a protamine-heparin ratio of 0.7:1. The impairment seems to be transient and recovery had started after 20 minutes.
Introduction
Cardiac surgery with cardio pulmonary bypass (CPB) is associated with postoperative bleeding [Citation1] that often requires blood transfusion. It has been shown that platelet dysfunction increases bleeding [Citation2]. CPB impairs platelet function [Citation3–6] although the cause of the dysfunction is incompletely understood [Citation7]. Many factors are known to contribute to platelet dysfunction, for instance the foreign material in the CPB-circuit, hypothermia, hemodilution and pharmaceuticals given during and after surgery [Citation4]. Platelet function has been described to be affected by excessive protamine administration [Citation8] and by the heparin-protamine complex formed during protamine reversal of heparin coagulation [Citation9–11]. Protamine in commonly used doses has also been shown to have a negative effect on platelet aggregation in whole blood both in vivo and in vitro [Citation12,Citation13].
There are different ways to calculate how much protamine is needed to reverse the heparin anticoagulation. One way is to use a fixed protamine-heparin ratio, usually 1 mg protamine for every 100 U of heparin, based on the initial dose of heparin given pre bypass. Another way is to use a statistic model or a point–of–care (POC) device to calculate how much protamine is needed to reverse the remaining heparin in the blood [Citation14,Citation15]. Extended knowledge of the inhibitory effect of protamine on platelet aggregation and hemostasis, and optimization of protamine dosing could be of importance for the management of bleeding patients during cardiac surgery.
The purpose of this study was to examine the duration and possible dose dependence of the inhibitory effect of protamine on platelet aggregation. A secondary aim was to evaluate a possible correlation between the effect on platelet aggregation and blood loss during and after surgery.
Method
Patients
The study was performed at the cardio-thoracic clinic in Karlskrona, Sweden. Thirty patients who underwent elective coronary artery bypass grafting (CABG) or aortic valve replacement (AVR) with CPB were included. The exclusion criteria were known bleeding diathesis, treatment with clopidogrel or ticagrelor within 5 days prior to surgery, re-heparinization after administration of protamine and reinstitution of CPB. All patients were treated with acetylsalicylic acid until the day of surgery.
The study was approved by the ethical review board, Linköping University, Sweden, Dnr 2013/455-31, and was performed in agreement with the declaration of Helsinki. All patents gave their written consent to participate in the study.
Clinical management
Treatment with clopidogrel or ticagrelor was discontinued five days before surgery according to clinical routine. Anesthesia was induced with 300–500 µg fentanyl and 1–3 mg/kg propofol and rocuronium 0.6 mg/kg was used to achieve muscle relaxation (all B.Braun, Melsungen, Germany). Anesthesia was maintained with sevoflurane (Baxter Medical AB, Kista, Sweden) during the operation. Two grams of tranexamic acid (Pfizer AB, Sollentuna, Sweden) was given to all patients before and after CPB. The residual blood in the heart-lung machine was retransfused with the Ringer’s chase-technique [Citation16].
Perfusion was performed with a S5 heart-lung machine (Sorin Group, Deutschland GmbH, München) and the CPB-circuit was primed with 1500 ml Ringers Acetate and 100 ml Mannitol (both Fresenius Kabi AB, Uppsala, Sweden). Heparin 10 000 U (LEO Pharma AB, Malmö, Sweden) was added in the prime. Cardiac arrest was induced with cold blood cardioplegia in the aortic root with a ratio of 4:1.
Heparin and protamine treatment
The initial dose of heparin was 350 U/kg and the goal was to achieve an activated clotting time (ACT) of > 480 s before initiation of CPB. A supplementary dose of heparin was given if the ACT target was not achieved. An additional dose of heparin was given, if needed, during CPB to maintain an ACT above 480 s. The heparin anticoagulation was reversed with protamine after weaning from CPB. The protamine dose was calculated based on the initial dose of heparin given pre bypass, with 1 mg of protamine for every 100 U of heparin. The 10,000 U heparin in the prime was not included in the calculation. Protamine was administered in two steps. Initially, 70% of the calculated dose was given. Three minutes after the administration the remaining 30% of the calculated protamine dose was administered. An additional dose of protamine was given if the ACT target of 130 or lower not was reached or if deemed needed by the attending surgeon.
Blood samples
Platelet count and aggregation was analyzed at six time points:
at baseline (before induction of anesthesia)
immediately before protamine administration
three minutes after 70% of the calculated protamine dose
three minutes after full protamine dose (100% of the calculated dose)
20 minutes after the completed protamine dose and finally
on arrival in the intensive care unit (ICU).
Hemoglobin and leukocyte concentrations were analyzed at three time points; at baseline, three minutes after full protamine administration and on arrival in the ICU. All blood samples were drawn from an indwelling arterial catheter. Hemoglobin, leukocyte and platelet concentration was analyzed by the Department of Clinical chemistry, Blekinge Hospital, Karlskrona, Sweden. ACT was measured in the operating theatre before, during and after CPB with a Hemochron Jr, Signature, ICT, Edison, USA.
Platelet function
Platelet aggregation was measured by impedance aggregometry according to the manufacturers specification using the Multiplate® (Dynabyte, München, Germany now ROCHE, Basel, Switzerland) device. The test is considered to be reliable when the platelet count is above 100 × 109 L. The technique is based on the increase in impedance between two electrodes when platelets aggregate on the electrode surfaces. After the blood had been stabilized for 30 minutes, 300 µL of whole blood was mixed with 300 µL NaCl and incubated 3 minutes at 37 degrees Celsius. The platelets were then activated with adenosine diphosphate (ADP-test), thrombin activating peptide-6 (TRAP-test), arachidonic acid (ASPI-test) or collagen (COL-test). The electrical impedance, reflecting the platelet aggregation on the surface of the electrodes, was registered for 6 minutes and the result was reported as units of aggregation (U).
Blood loss
Blood loss during surgery was measured in two intervals, from start of surgery until end of CPB and from end of CPB until end of surgery. During surgery blood loss was registered as volume in suction bottles and swabs. Cell saver was not used. Coronary suction was connected to the CPB circuit during bypass. Postoperative blood loss through chest tubes was measured at three hours after surgery.
Statistics
Normality was tested with the Kolmogorov–Smirnov test. Normally distributed data are presented as mean ± SD and were evaluated with repeated measures ANOVA followed by Bonferroni correction. Platelet aggregometry was not normally distributed and is presented as median values with 25th–75th percentile and assessed with Friedman test followed by Wilcoxon signed rank-test with Bonferroni correction. Spearman non-parametric correlation (rs) was used to evaluate correlation between platelet aggregation and blood loss. Simple linear regression was used to calculate the relation between platelet aggregation and platelet count. Platelet levels after CPB corrected for hemodilution were calculated using hemoglobin as a marker, excluding patients who received blood transfusion during surgery.
Statistical significance was defined as p < .05. Before the start of the study a sample size calculation was made based on results from our previous study [Citation12], where ADP-test was reduced by more than 50% after protamine administration. The analysis indicated that a 2α = 0.05 in a sample of n = 30 would detect a 50% difference with a power higher than 85%. Statistical calculations were made with IBM SPSS statistics v23 (New York, USA) and Statistica 12 (Tulsa, OK, USA).
Results
Clinical characteristics of the patients and intraoperative data are shown in and .
Table 1. Clinical characteristics.
Table 2. Operative data.
The aggregometry results are presented in and . All baseline levels were within or above the reference range in the ADP-test and TRAP-test. Most patients had baseline values below normal range with the ASPI-test. With the collagen test half of the patients had levels below the normal reference range. The samples taken before protamine did not differ significantly from baseline with any of the activators. When 70% of the calculated protamine dose was given, platelet aggregation decreased markedly compared to before protamine with all activators, ADP and TRAP both p = .001, ASPI p = .001 and collagen p = .01.
Figure 1. Platelet aggregation before and after protamine. Boxplots (25th–75th percentiles) with the line as median and the whiskers total range for platelet aggregation at baseline, before protamin, after 70% protamine, after 100% protamine, 20 minutes after protamine and at arrival at the intensive care unit (ICU). U: Units, Dotted lines indicate normal range, ADP: adenosine phosphate, TRAP: thrombin receptor activating peptide-6, ASPI: arachidonic acid, COL: collagen, * denotes p<.05 compared to before protamine, ** denotes p<.01 compared to before protamine.
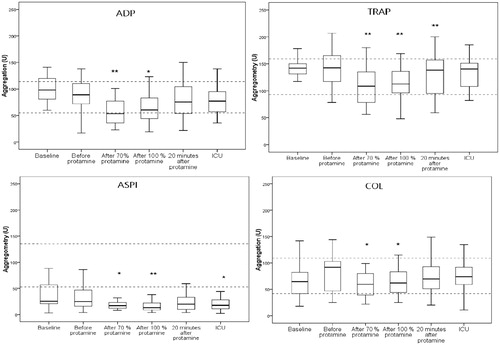
Table 3. Hemoglobin, platelet count and platelet aggregation.
When the remaining 30% of protamine was given no further decrease in aggregation was seen with activators TRAP, ASPI or COL. On the contrary a slight increase in aggregation with the ADP-test compared to the sample taken at 70% was observed (p=.03). There was no correlation between the doses of protamine (70 or 100%) and the decrease in platelet aggregation.
Twenty minutes after full protamine administration, platelet aggregation had started to recover compared to nadir after the initial dose (70%) of protamine, with ADP (p=.001) and TRAP (p=.01).). ASPI and collagen stimulated aggregation also increased, but the rise did not reach significance. On arrival to the ICU TRAP and collagen tests had reached baseline levels, while ADP and ASPI tests were still lower than baseline (both p=.01).
The hemoglobin concentration decreased from 136 ± 18 g/L at baseline to 107 ± 10 g/L after CPB and was 109 ± 12 g/L at arrival to the ICU (both p = .001 compared to baseline, ).
The leukocytes increased during CPB compared to baseline (8.5 ± 1.9 × 109 L to 15.0 ± 5 × 109 L, p = .03) and were still higher than baseline at arrival to the ICU, 13.8 ± 4.7 × 109 L, p = .01. The platelet count was reduced from 258 ± 83 × 109 L preoperatively to 203 ± 53 before protamine, p = .001. When 70% of the protamine was administered, the platelet count was 159 ± 37 and after full dose of protamine 162 ± 39 × 109 L (both p = .001 compared to before protamine). On arrival to the ICU the platelet level had increased to 193 ± 62 x× 109 L but was still significantly lower than baseline ().
Hemodilution during CPB resulted in a lower platelet count. To evaluate if the aggregometry results could be related to platelet concentration a linear regression analysis was done on baseline values. Platelet aggregation with ADP-test was related to platelet count with a regression coefficient of 0.314 U/109 platelets (ADP-test = 20 + 0.314* platelet count, r2 = 0.38; p < .001). From this result an expected theoretical reduction of ADP aggregation after protamine in relation to the reduction in platelet count was calculated. The expected median decrease of the ADP-test due to lower platelet concentration after 70% of protamine would be 17 U (27–5). However, the actual median decrease in platelet aggregation with the ADP-test was 37 U (47–20) after 70% of protamine. The difference between calculated and actual reduction of aggregometry values was significant (p = .02).
When correcting the platelet level for hemodilution the platelet count (×109 L) was 276 ± 76 before protamine, 211 ± 60 after 70% protamine and 216 ± 59 after 100% protamine (both p < .001 compared to before protamine).
Heparin and protamine
The total dose of heparin given before CPB was 31,433 ± 6643 U, and 3500 ± 5153 U was given as additional doses during bypass. The protamine dose given at 70% of the calculated dose was 219 ± 47 mg, with a protamine – heparin ratio of 0.7:1 ± 0.02. The total dose of protamine given was 312 ± 64 mg with a ratio of 1:1 ± 0.03 (1 mg protamine/100 U heparin). No additional dose of protamine was given.
ACT before start of CPB was 559 ± 107 s, the last ACT measured during bypass before weaning was 488 ± 63 s. ACT after 70% of the calculated protamine dose was 118 ± 10 s and subsequently, after the complete 100% dose, the ACT was 118 ± 9 s.
Blood loss and blood transfusion
The median total blood loss during surgery and postoperatively is shown in .
Total blood loss during surgery correlated to platelet aggregation before surgery with ADP and collagen tests, both rs = –0.40, p = 0.03. Total blood loss during surgery also correlated to aggregometry after 70% of protamine (ADP: rs = –0.43, p=.03; ASPI: rs = –0.43, p=.02), after 100% protamine (ADP rs = –0.46, p=.01) and 20 minutes after full protamine dose (ADP: rs = –0.37, p=.05). Blood loss from start of surgery until end of CPB correlated to platelet aggregometry at baseline with TRAP (rs = –0.47, p=.01), ASPI (rs = –0.37, p=.05), and collagen test (rs = –0.53, p=.001). There was no correlation between postoperative bleeding and aggregometry results at baseline, before or after protamine with any activator.
Seven patients were transfused with erythrocytes during surgery and were excluded from the hemodilution calculations. Two patients received erythrocytes within three hours in the ICU (). No patient was treated with fibrinogen or aprotinin during or after surgery.
Discussion
We found a marked decrease in platelet aggregation measured by impedance aggregometry after the initial dose (70% of the total calculated dose) of protamine with all four activators; ADP, TRAP, ASPI and collagen. The remaining 30% of protamine (up to 100% of the calculated dose) did not affect the platelet aggregation further with the TRAP-, ASPI- and COL-tests. ADP-test was still below baseline values, but significantly higher compared to samples after 70% protamine.
It has previously been demonstrated that a standard dose of protamine can impair platelet aggregation [Citation17,Citation18] and a dose-dependent effect has been seen [Citation19]. We found that protamine has an immediate inhibitory effect on stimulated platelet aggregation. This reduction was not more pronounced after the additional 30% dose of protamine and ADP-stimulated aggregation had even started to recover. The negative effect on the platelets therefore seems to be transient, but it is not clear whether a new exposure to protamine after a longer time period would again result in reduced platelet aggregation.
The mechanism of the platelet inhibition caused by protamine is not fully understood and probably multifactorial [Citation7]. Reduced thrombin generation through inhibition of factor V activation [Citation20] and inhibition of interaction between glycoprotein Ib and platelet-bound von Willebrand factor [Citation21] could be of importance. The effect has been shown to be more pronounced in vivo after CPB [Citation12] and the phenomenon could probably be modulated by many factors related to both surgery and CPB.
When the platelet count was corrected for hemodilution, the consumption of platelets seemed to be insignificant during CPB, when comparing the samples taken before protamine with baseline. However, the platelet count after the initial dose of protamine was significantly lower than before protamine. This indicates a consumption of platelets in connection to reversal of the heparin effect, since no further hemodilution could be expected to occur during that short time frame.
It could be argued that the reduced aggregation seen after protamine could be a result of the lower platelet concentration and not an effect on the function of the remaining platelets. When calculating an expected corrected ADP aggregation at the measured platelet concentration, the expected median reduction in aggregation U (25th–75th) after protamine administration would be 17 (27–5), whereas the actual measured median reduction in ADP stimulated aggregation was 37 (47–20). This would indicate that the function of the remaining platelets was lower and not a measurement artifact due to hemodilution. The measured lower platelet aggregability could be the result of protamine inhibiting all platelets, or it could indicate that the platelets remaining in the circulation are a less reactive subpopulation of platelets, and that the more reactive platelets were consumed after protamine administration.
We found a negative correlation between total blood loss during surgery and platelet aggregation with ADP-test at all sample times during surgery. A negative correlation between ADP-test at baseline and total blood loss during operation has previous been demonstrated in patients treated with thienopyiridine [Citation22].
The decrease in platelet aggregation, caused by protamine should theoretically be of greater importance for blood loss after CPB, since protamine administration occurs in connection to decannulation after end of CPB. Blood loss from start of surgery correlated to TRAP, ASPI and collagen tests but blood loss after CPB until end of surgery could not be related to the platelet aggregation in this study.
The dosing of protamine is delicate since platelet function could be affected by excessive protamine administration. In a review from 2003 [Citation8] it was concluded that high ratios of protamine-heparin (>2.6:1) could impair platelet function. It has recently been suggested that postoperative blood loss could increase already at protamine-heparin ratios of >1:1 due to protamine-induced coagulopathy [Citation7], but platelet dysfunction could also contribute in this setting.
When the protamine-heparin dose is empirically calculated, the consumption of heparin during CPB is often not considered. The protamine dose is usually based on the initial dose of heparin given pre-bypass with a ratio of 1 mg protamine for every 100 U of heparin. Recently point–of–care (POC) devices has become available which makes it possible to titrate the heparin dose pre-bypass and the protamine dose needed to reverse the heparin effect after CPB. There are pro [Citation14] and cons [Citation23] with this titration and it is not clear if the heparin and protamine titration improves the hemostasis after cardiac surgery. Statistic models for protamine titration has also been introduced [Citation15,Citation24] as an alternative to the POC devices. Still, many cardio thoracic centers calculate the protamine-heparin ratio empirically when reversing the heparin effect and use an ACT value to assess the treatment. ACT measures whole-blood coagulation and is known to be affected by hemodilution and does not seem to correlate to actual plasma heparin concentration. Falsely elevated ACT values are often caused by depletion of coagulation factors during CPB [Citation14]. A prolonged ACT does not necessary indicate residual heparin after CPB [Citation25], and excessive protamine administration can also prolong the ACT [Citation11].
Based on the ACT values taken during this study, the protamine-heparin ratio of 0.7:1 seemed to be sufficient to reverse the anticoagulative effect of heparin. All ACT values were below 130 s after the initial dose of protamine (70%), which was the desired ACT target after CPB. The remaining 30% of protamine did not influence the ACT value further. Previous studies [Citation8,Citation26] indicate a more pronounced impairment of platelet function after higher doses of protamine. Thus, there seems to be multiple reasons for optimizing protamine dosage and avoiding over dosage when reversing heparin anticoagulation after CPB.
Limitations
This is a small study and findings need to be confirmed in a larger patient cohort. The patients also underwent different types of surgery and were operated by different surgeons, which might have an impact on the blood loss.
Conclusion
Protamine induces a marked but transient decrease in platelet aggregation already at a protamine-heparin ratio of 0.7:1. No further decrease is observed when additional protamine is given within three minutes. In this study, a protamine-heparin ratio of 0.7:1 was sufficient to reverse the heparin anticoagulation as measured by ACT. Together this underlines the importance of optimizing protamine dosage. Twenty minutes after the initial dose of protamine platelet aggregation has started to recover and is almost back to baseline values at arrival in ICU, an hour after the full protamine dose. The mechanism behind the protamine effect needs to be further investigated together with the possible clinical impact on blood loss during surgery.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med. 2004;30:1873–1881.
- Samson S, Shore-Lesserson L. Platelet function and cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth. 2001; 5:273–281.
- Harker LA. Bleeding after cardiopulmonary bypass. N Engl J Med. 1986; 314:1446–1448.
- Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76:1680–1697.
- Kestin AS, Valeri CR, Khuri SF, et al. The platelet function defect of cardiopulmonary bypass. Blood. 1993;82:107–117.
- Harker LA, Malpass TW, Branson HE, et al. Mechanism of abnormal bleeding in patients undergoing cardiopulmonary bypass: acquired transient platelet dysfunction associated with selective alpha-granule release. Blood. 1980;56:824–834.
- Boer C, Meesters MI, Veerhoek D, et al. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth. 2018;120:914–927.
- Mclaughlin KE, Dunning J. In patients post cardiac surgery do high doses of protamine cause increased bleeding?. Interact Cardiovasc Thorac Surg. 2003;2:424–426.
- Carr ME, Jr, Carr SL. At high heparin concentrations, protamine concentrations which reverse heparin anticoagulant effects are insufficient to reverse heparin anti-platelet effects. Thromb Res. 1994;75:617–630.
- Ellison N, Edmunds LH, Jr, Colman RW. Platelet aggregation following heparin and protamine administration. Anesthesiology. 1978;48:65–68.
- Mochizuki T, Olson PJ, Szlam F, et al. Protamine reversal of heparin affects platelet aggregation and activated clotting time after cardiopulmonary bypass. Anesth Analg. 1998;87:781–785.
- Olsson A, Alfredsson J, Håkansson E, et al. Protamine reduces whole blood platelet aggregation after cardiopulmonary bypass. Scand Cardiovasc J. 2016;50:58–63.
- Ammar T, Fisher CF. The effects of heparinase 1 and protamine on platelet reactivity. Anesthesiology. 1997;86:1382–1386.
- Ural K, Owen C. Pro: The Hepcon HMS should be used instead of traditional activated clotting time (ACT) to dose heparin and protamine for cardiac surgery requiring cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2016;30:1727–1729.
- Hällgren O, Svenmarker S, Appelblad M. Implementing a statistical model for protamine titration: effects on coagulation in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2017;31:516–521.
- Olsson A, Alfredsson J, Ramström S, et al. Better platelet function, less fibrinolysis and less hemolysis in re-transfused residual pump blood with the Ringer’s chase technique – a randomized pilot study. Perfusion. 2018;33:185–193.
- Gertler R, Wiesner G, Tassani-Prell P, et al. Are the point-of-care diagnostics MULTIPLATE and ROTEM valid in the setting of high concentrations of heparin and its reversal with protamine?. J Cardiothorac Vasc Anesth. 2011;25:981–986.
- Ortmann E, Klein AA, Sharples LD, et al. Point-of-care assessment of hypothermia and protamine-induced platelet dysfunction with multiple electrode aggregometry (Multiplate(R)) in patients undergoing cardiopulmonary bypass. Anesth Analg. 2013;116:533–540.
- Khan NU, Wayne CK, Barker J, et al. The effects of protamine overdose on coagulation parameters as measured by the thrombelastograph. Eur J Anaesthesiol. 2010;27:624–627.
- Ni Ainle F, Preston RJS, Jenkins PV, et al. Protamine sulfate down-regulates thrombin generation by inhibiting factor V activation. Blood. 2009;114:1658–1665.
- Barstad RM, Stephens RW, Hamers MJ, et al. Protamine sulphate inhibits platelet membrane glycoprotein Ib-von Willebrand factor activity. Thromb Haemost. 2000;83:334–337.
- Ranucci M, Baryshnikova E, Soro G, et al. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg. 2011;91:123–129.
- Gilly G, Trusheim J. Con: The Hepcon HMS should not be used instead of traditional activated clotting time to dose heparin and protamine for cardiac surgery requiring cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2016;30:1730–1732.
- Kjellberg G, Sartipy U, van der Linden J, et al. An adjusted calculation model allows for reduced protamine doses without increasing blood loss in cardiac surgery. Thorac Cardiovasc Surg. 2016;64:487–493.
- Yamamoto T, Wolf H-G, Sinzobahamvya N, et al. Prolonged activated clotting time after protamine administration does not indicate residual heparinization after cardiopulmonary bypass in pediatric open heart surgery. Thorac Cardiovasc Surg. 2015;63:397–403.
- Griffin MJ, Rinder HM, Smith BR, et al. The effects of heparin, protamine, and heparin/protamine reversal on platelet function under conditions of arterial shear stress. Anesth Analg. 2001;93:20–27.
