Abstract
Objective. (i) To compare daily physical activity (PA) levels evaluated by the International Physical Activity Questionnaire (IPAQ) and by triaxial accelerometry in heart failure with preserved ejection fraction (HFpEF) patients; (ii) to describe daily PA patterns based in objective measurements; and (iii) to observe the association between prognostic indicators and PA measurements. Design. This is a cross-sectional study with 24 stable HFpEF patients. PA was assessed through the IPAQ short version and triaxial accelerometer. Time spent in moderate-to-vigorous PA (MVPA) from IPAQ was computed as self-reported walking and MVPA. Prognostic indicators were: distance on the 6-minute-walking test (6MWT), oxygen consumption (VO2) during the test, quality of life (QoL), BNP plasma level, and E/e′ ratio. Results. Compared to accelerometry, IPAQ underestimated sedentary time (253 ± 156 vs. 392 ± 104 min/day, p = .001) and overestimated MVPA (44 ± 56 vs. 19.3 ± 26 min/day, p < .001). Accelerometer-derived data showed that HFpEF patients spent 50% of their waking time in sedentary behaviours and 2.5% in MVPA. Of measured surrogate prognostic markers, functional capacity (6MWT, r = 0.652, p = .04; VO2, r = 0.512, p = .02) and QoL (r=−0.490, p = .04) were correlated with MVPA. Conclusions. The IPAQ underestimated sedentary time and over-estimated MVPA in HFpEF patients. Using accelerometer-derived data, HFpEF patients spent only a minority of their time involved in MVPA, which was the only PA pattern positively associated with prognostic indicators.
Introduction
Epidemiologic evidence indicates that heart failure (HF) prevalence has been increasing in the last decade, with heart failure with preserved ejection fraction (HFpEF) accounting up to half of HF population [Citation1]. It is expected that HFpEF will become the most common cause of hospitalization in older adults over the coming years [Citation2]. Since HFpEF continues to be resistant to current therapies, primary prevention strategies might be useful to control its growing burden at the population level [Citation3]. Therefore, in addition to understanding the natural history and pathophysiology of HFpEF, it will be important to identify modifiable risk factors that can be targeted.
Lifestyle risk factors such as low physical activity (PA) and high sedentary time are recognized as primary factors for most chronic diseases, including HF [Citation4]. While it remains to be clarified which HF phenotypes is most affected, recent observations have more strongly implicated low PA in the development of HFpEF [Citation5]. It was recently demonstrated that lower levels of PA were associated with higher risk of HFpEF, but not HF with reduced ejection fraction [Citation5]. In addition, evidence shows that a sedentary lifestyle is associated with many underlying cardiac and skeletal muscle abnormalities often present in HFpEF [Citation6,Citation7]. For those with established HFpEF, lower PA levels have been associated with poor quality of life (QoL) and worse clinical outcomes such as functional class [Citation8], hospitalisation rate, and mortality [Citation9]. Overall, these observational studies suggest that PA and/or sedentary time are important treatment targets to improve clinical outcomes in HFpEF.
Regardless the population, daily PA assessment is a challenging task. There are different methods (e.g. questionnaires and accelerometry), and each one has its own limitations and strengths. Accelerometry seems to be superior than questionnaires in terms of accuracy, reliability, representatively of daily activities [Citation10]. Additionally, in clinical settings accelerometry might represent a challenge compared to questionnaires, which are better in terms of costs and practicality. However, correlation between questionnaires and accelerometer measurements in the large majority of studies is poor [Citation11]. Additionally, there are no data comparing questionnaire and accelerometer-derived data in HFpEF patients. Therefore, this study aimed: (i) to determine the validity of the International Physical Activity Questionnaire (IPAQ) against accelerometer-measured PA in HFpEF patients; (ii) to describe daily PA patterns and sedentary time based in objective measurements; (iii) to observe the association between prognostic indicators and PA and sedentary time measurements.
Materials and methods
Study design
This is a cross-sectional study conducted in a Portuguese public hospital. Inclusion criteria were diagnosis of HFpEF according to ESC guidelines [Citation12]. Patients were excluded if they presented medical or orthopaedic conditions that precluded independent ambulation and exercise testing.
Twenty-four stable and well-medicated HFpEF patients (17 female and seven male) were evaluated, but only 22 had valid accelerometer data. The study was approved by the Ethics Committee of CHP-Hospital de Santo Antonio (N/S: 2015.125). All procedures were conducted according to the Declaration of Helsinki, and participants signed informed consent to participate.
Data collection
Blood pressure: A trained researcher performed blood pressure measurements after 10 min resting in seated position. Blood pressure was assessed (Colin, BP 8800; Critikon, Inc., Tampa, FL) in both arms, and the arm with the highest BP was used. SBP and DBP were computed as the average of three readings, with 2 min apart between. Additional readings were performed when differences between readings exceeded 5 mmHg [Citation13].
Blood collection and biochemical determinations: Blood samples were collected early in the morning by venepuncture of antecubital vein after 10 min resting in seated position. Samples were collected into EDTA-coated tubes and immediately placed on ice before centrifugation (15 min at 1000×g). The plasma was stored at −80 °C. Brain natriuretic peptide (BNP) was quantified using an Architect i2000 automated analyser (Abbott, Lisbon, Portugal).
Anthropometric measures: Body height was measured standing upright against a stadiometer (Holtain Ltd., Crymych, UK) [Citation14]. Weight, body mass index, fat mass and free fat mass were measured using an electronic segmental body composition analyser (Tanita, BC-418, Tokyo, Japan). Waist circumference was measured as previews described [Citation15]. Obesity was determined as BMI ≥ 30 kg/m2 [Citation16].
Self-reported physical activity: Self-reported PA was assessed with the short form of IPAQ (IPAQ-SF) [Citation17], through personal interview. The IPAQ-SF estimates PA frequency and duration during the previous seven days. It focusses on moderate, vigorous and walking physical activities lasting at least 10 min, and time spend sitting. Total weekly PA in METs was estimated using the instrument's scoring protocol (3.3 METs to walking, 4.0 METs to moderate, and 8.0 METs vigorous activity) [Citation18]. Meeting international PA guidelines was defined as ≥150 min/week of moderate-to-vigorous PA (MVPA) data [Citation19]. Thus, to estimate MVPA we merged the reported activities ≥3 METs (walking + moderate + vigorous PA).
Accelerometer-assessed physical activity: Daily PA was measured using a triaxial accelerometer (Actigraph GT3X, Pensacola, FL). Participants were instructed to wear the accelerometer over the right hip for eight consecutive days, except while sleeping, bathing and water-based activities. The accelerometer was programmed to record data at a frequency of 30 Hz and 1 s length epochs. ActiLife software (Actigraph, Pensacola, FL, version 6.9) was used to process the accelerometer data. Data were downloaded and integrated into 60-second epochs. Non-wear time was defined as 90 consecutive minutes of zero counts, with an allowance of two-minutes of nonzero counts provided there were 30-minute consecutive zero count windows up and downstream [Citation20]. Non-wear time was excluded from the analysis. Patients with valid data were those having a minimum of four days with at least 10 h/day of wear-time. The average min/day spent at different categories of PA intensity was defined as: sedentary time (<200 counts/min) [Citation21], light PA (LPA) (200–2751 counts/min) and MVPA (>2752 counts/min) [Citation22]. Meeting international PA guidelines was defined as ≥150 min/week of MVPA. This definition was applied considering: (i) only MVPA that occurred in bouts of ≥10 min’ long (episodes of continuous MVPA lasting for at least 10 min) as specified by the guidelines [Citation19] and (ii) without bout length restriction.
Functional classification: Patients were classified by the physician into subgroups based on their symptoms using the New York Heart Association (NYHA) functional class. Patients’ symptoms are based on how much they are limited during PA (class I to IV) [Citation23].
Echocardiography evaluation: Supine transthoracic echocardiography was performed using a cardiovascular ultrasound Vivid E95® (GE Healthcare, Chicago, IL). All quantitative echocardiographic measurements were performed by a single reader blinded to the results of the other evaluations, using a computerized off-line analysis station. Peak early diastolic tissue velocity was measured at the septal and lateral mitral annulus. Mitral inflow velocity was assessed by pulsed wave Doppler from the apical four-chamber view, positioning the sample volume at the tip of the mitral leaflets. E/e′ ratio was calculated as E wave divided by e′ velocities. LV mass was estimated from LV linear dimensions and indexed to body surface area as recommended by ESC guidelines [Citation24]. LV volumes were estimated by the modified Simpson method using the apical four- and two-chamber views, and LVEF was derived from volumes in the standard manner. LA volume was estimated by the method of disks using apical four- and two-chamber views at an end-systolic frame preceding mitral valve opening and was indexed to body surface area (calculated according to Mosteller's formula) to derive LA volume index.
Cardiorespiratory fitness with pulmonary gas exchange analysis: Cardiorespiratory fitness was assessed by the 6-minute walk test (6MWT) in a 25-m-long unobstructed corridor. The 6MWT was performed wearing a portable gas analyser (K4b2, Cosmed, Rome, Italy). Oxygen uptake (VO2; mL.min−1.kg−1) was measured directly and continuously. Respiratory samplings were collected in a breath-by-breath, and then, data were averaged over 5-s intervals. Data were calculated as the average of measures taken in test total duration.
Health-related quality of life: Health-related QoL was performed by interview through the Minnesota Living With Heart Failure Questionnaire (MLHFQ). The MLHFQ encompasses 21 questions and answers; options ranges from 0 (none) to 5 (very much), where 0 represented no limitation and 105 represented maximal limitation [Citation25]. The MLHFQ total score ranges from 0 to 105 (no impairment to maximum impairment).
Statistical analyses
Statistical analysis was performed using the IBM SPSS 24 software (SPSS, Chicago, IL). Normal data distribution was examined by the Shapiro–Wilk test. Non-normal data were transformed with the square root or its natural logarithm for subsequent analysis. Categorical data are reported as absolute values and percentages. Between gender and age comparisons were performed by Student’s independent t-test and Chi-square test, as appropriated. Cut point to age analyses was defined by median, and it was set as 76 years old. Siting time and walk + moderate + vigorous PA (MVPA) from IPAQ-SF were compared with sedentary time, total MVPA and 10 min-bouts of MVPA derived from GT3X accelerometer, using paired t-test. Partial correlation was used to analyse the relationship between the variables derived from the two methods adjusted by gender and age. The strength and limits of agreement between the two methods were assessed using the Bland–Altman technique [Citation26]. Statistical significance was established for p< .05.
Results
Patients’ characteristics
Demographic and clinical characteristics of the participants are depicted in . On average, participants were 76 ± 4 years old (range 59–85 years), 73% (n = 16) were female and mean ejection fraction was 59.4 ± 6.3%. Hypertension was the most prevalent risk factor (n = 20, 91%), followed by dyslipidaemia (n = 15, 68%) and obesity (n = 12, 55%). The majority of participants (n = 17, 77%) were classified as NYHA class II. The BNP average was 272 ± 191.56 pg/mL. Mean distance walked in the 6MWT was 312 ± 90 m. The average of VO2 during the test was 11.2 ± 2.3 mL.min.kg−1. The total score of MLHFQ was 25.2 ± 24.1 points.
Table 1. Patients characteristics.
Self-reported vs. objective measures of PA
The results of self-reported and objective measures of PA are described in . Accelerometer was used as an average of 6.4 ± 0.9 days, with a mean daily wear time of 790 min (13.2 ± 1 h/day). Mean total activity volume from IPAQ-SF was 152 ± 183 MET/min/day, and 550 ± 239 CPM from accelerometry.
Table 2. Descriptive PA levels from IPAQ and accelerometer variables.
Mean sedentary time was significantly lower in self-reported measurement (253 ± 156 min.day−1) compared to accelerometry (392 ± 104 min.day−1, p = .001). No significant differences were found regarding to gender and age. Considering MVPA, self-reported daily time compared to accelerometer was higher (44 ± 56 vs. 19.3 ± 26 min/day, respectively; p < .001). Applying the ≥10min-bouts at MVPA criterion, the discrepancy between measurements was even higher (IPAQ = 44 ± 56 min/day; accelerometry = 1.1 ± 2.4 min/day, p < .001). From accelerometry data, it was possible to verify that only five patients accomplished with at least one bout of MVPA per week.
Regarding to LPA, comparisons are not possible because it is measured only via accelerometry. Mean time in LPA was 379 ± 128 min/day.
Correlation and agreement between self-reported and objective measures of PA
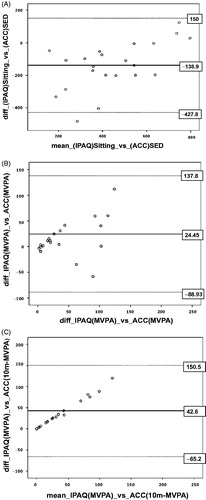
Comparison between self-report and accelerometer measurements are shown in . Validation coefficients (correlation between self-report and accelerometer measured) were not significant in all PA levels. A significant mean difference (systematic error; p < .05) between self-reported and accelerometer-derived PA levels was detected in sedentary time and in 10-min-bouts-MVPA. By analysing the 95% limits of agreement, both PA levels presented a higher variation, ranging from –427.8 to 150 min/day for sedentary time, and from –65.2 to 150.5 min/day in 10-min-bouts-MVPA. The SEE was 96.7 min/day for sedentary time, 26.7 min/day for MVPA and 2.4 min/day for 10-mim-bouts-MVPA.
Table 3. Self-report physical activity and differences to objective.
Separate Bland–Altman’s plots were build-up for sedentary time, total MVPA and 10 min-bouts-MVPA (). Both MVPA plots show a linear tendency, which is not acceptable for the agreement between the two methods. Analysis of 10 min-bouts of MVPA revealed that at higher levels of PA, the difference between self-reported data and accelerometers data becomes greater. In these cases, the self-reported levels were greater than what was observed by accelerometry.
Figure 1. The Bland–Altman plot of the mean bias and 95% limits of agreement for time spent in (A) sedentary time, (B) MVPA and (C) 10-min-bouts-MVPA. Full line indicates mean difference (systematic error); dashed lines indicate the 95% limits of agreement (mean ± 1.96 SD). SD: standard deviation. MVPA: moderate-to-vigorous physical activity.
Associations between objective measures of PA with prognostic indicators
The association between objective measures of PA and prognostic indicators is shown in . Total activity (CPM) was significantly associated with VO2 (r = 0.498, p = .04). Furthermore, MVPA was positively correlated with VO2 (r = 0.512; p = .02) and 6MWT (r = 0.652; p = .008), and inversely correlated with QoL (r=–0.490; p = .04). In addition, 10 min-bouts of MVPA was positively correlated with VO2 (r = 0.559; p = .04) and inversely correlated with QoL (r=–0.465; p = .03). No significant correlations were found between sedentary time or LPA with any prognostic indicators.
Table 4. Partial correlations between accelerometer-derived data and prognosis values.
Discussion
The main findings of this study suggest that: (i) in comparison to accelerometer, self-reported PA from IPAQ-SF underestimates sedentary time and overestimates time spent in MVPA in HFpEF patients, (ii) HFpEF patients spent only a minority of their daily activity in MVPA, and (iii) only MVPA measured from accelerometer was correlated with functional capacity and QoL.
Despite the evidence showing that questionnaires are valid and reliable in measuring PA, their correlation with objective measures (e.g. accelerometers) is far from satisfactory [Citation10,Citation11,Citation27], which limits PA-based decisions. In agreement with these observations, our data highlight the disparity of PA measurements obtained with IPAQ-SF in comparison to accelerometer-derived data in HFpEF patients, with the former overestimating MVPA and underestimating sedentary/sitting time. We also verified the absence of agreement between self-reported and accelerometer-derived sedentary time and MVPA. This means that measuring PA levels in the same person with these instruments leads to important variations in magnitude and agreement of the measured outcome.
According to self-reported PA, 59% of our patients would meet the international recommendations for MPVA (>150 min/week of MVPA). However, when we confronted with accelerometer-assessed data, this percentage dropped to 27% (without the 10 min bout criteria) but decreased to zero when the 10 min-bouts criteria were considered. A similar finding was found in the Women’s Health Study, where 67% of women meet PA recommendations when considering results from self-reported methods, but when considering accelerometry (using 10 min-bouts criteria), this percentage was 19% [Citation28]. The lack of correlation and agreement between methods may be related with different constructs measured by the two instruments. While the accelerometer measures the motion through acceleration of body mass, the questionnaires measure the time spent in specific behaviours [Citation29]. Therefore, the use of objective measures of PA seems to have a particular importance to avoid bias in populations with limited physical function and limited past knowledge and experience on regular PA [Citation30].
Given the growing recognition of the impact of PA levels in the prognosis of HFpEF patients, a rigorous characterization of their patterns is crucial for prescribing tailored life-style changes. In our study, descriptive accelerometer data show that HFpEF patients spent 50% of recorded time in sedentary behaviours, 47.5% at LPA and only 2.5% at MVPA (0.1% with 10 min-bout criteria). Similar patterns were recently observed by Yavari et al., where HFpEF patients reported to spent most of their waking time in sedentary behaviours while their daily activity was mainly comprised of LPA, and just a few minutes in MVPA [Citation31]. Overall, these patterns of PA are similar to those described for adults older than 60 years, where sedentary time accounted for approximately for 60% of their waking time, LPA for 40% and 4% for MVPA [Citation32]. Although the patterns of time spent in each category are similar, our results show that the proportion of time spent in LPA was almost 10% higher, while sedentary time was 10% lower. While the fact that our patients were in optimal medical control may have accounted for that, differences in the type of accelerometers and cut-points also may have influenced the results. In fact, we used the GT3X triaxial accelerometer with the low frequency extension filter, which was shown to be more sensitive to capture slower movements, translating into decreased sedentary time and increase time in all PA intensities [Citation33]. In addition, although specific cut-points for GT3X triaxial accelerometer have not been validated in HFpEF patients, we used cut-points for older adults validated with the GT3X, which we believe are more representative for our population.
With respect to prognosis, it was recently shown in the NEAT-HFpEF trial that accelerometer-derived total daily PA in HFpEF patients was associated with better 6MWT, NYHA functional class, QoL, and NT-proBNP levels [Citation8]. Our study adds the novelty that only time spent in MVPA was significantly associated with important clinical outcomes as VO2, 6MWT and QoL, but not with E/e′ or BNP levels. Thus, it seems that intensity is a requirement for significantly impacting the patient’s prognosis. Corroborating this hypothesis, it was recently shown a dose-response relationship between MVPA and risk of hospitalization or mortality in HFpEF patients [Citation9]. In addition, it should be noted that only a minority of patients achieved the weekly recommendations of MVPA (without the 10 min-bouts criteria). While any increase in the amount of PA may translate into some health benefits [Citation34], our data suggest that patients should be educated about the importance of reducing their sedentary time and engage in more MVPA for greater benefits.
Study limitations
The small sample size limits inferences regarding the agreement between PA measurements from IPAQ-SF questionnaire and PA measures derived from triaxial accelerometer. This report has a cross-sectional design, which limits the establishment of causal inferences, and the sample size preclude the determination of predictive models. In addition, for a more precise estimation of sedentary time, longer period of 7–10 days would be necessary. However, once that mostly of our patients are retired (mean age 76 ± 4 years old), the mean time used of 6.4 ± 0.9 days may be representative of sedentary patterns in our sample.
Conclusions
Our results suggest that IPAQ-SF, compared to objectively measured PA, underestimates sedentary time and over-estimates time spent in MVPA in patients with HFpEF, limiting its use to support accurate recommendations. Accelerometer-derived data show that HFpEF patients spent only a minority of their time involved in MVPA, which was the only PA pattern positively associated with prognostic indicators, highlighting the importance of reducing sedentary time and performing MVPA throughout the day.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410.
- Nanayakkara S, Patel HC, Kaye DM. Hospitalisation in patients with heart failure with preserved ejection fraction. Clin Med Insights Cardiol. 2018;12.
- Pandey A, Allen NB, Ayers C, et al. Fitness in young adulthood and long-term cardiac structure and function: the CARDIA study. JACC Heart Fail. 2017;5:347–355.
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211.
- Pandey A, LaMonte M, Klein L, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142.
- Dorfman TA, Rosen BD, Perhonen MA, et al. Diastolic suction is impaired by bed rest: MRI tagging studies of diastolic untwisting. J Appl Physiol. 2008;104:1037–1044.
- Irimia JM, Guerrero M, Rodriguez-Miguelez P, et al. Metabolic adaptations in skeletal muscle after 84 days of bed rest with and without concurrent flywheel resistance exercise. J Appl Physiol. 2017;122:96–103.
- Snipelisky D, Kelly J, Levine JA, et al. Accelerometer-measured daily activity in heart failure with preserved ejection fraction: clinical correlates and association with standard heart failure severity indices. Circ Heart Fail. 2017;10:e003878.
- Hegde SM, Claggett B, Shah AM, et al. Physical activity and prognosis in the TOPCAT Trial (treatment of preserved cardiac function heart failure with an aldosterone antagonist). Circulation. 2017;136:982–992.
- Skender S, Ose J, Chang-Claude J, et al. Accelerometry and physical activity questionnaires – a systematic review. BMC Public Health. 2016;16;16:515.
- Lee PH, Macfarlane DJ, Lam TH, et al. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200.
- Mancia G, Grassi G, Redon J. Manual of hypertension of the European Society of Hypertension. 2nd ed. London: CRC Press; 2014.
- Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign (IL): Human Kinetics Books; 1988.
- Riley L, Guthold R, Cowan M, et al. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. 2016;106:74–78.
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253.
- Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395.
- IPAQ Research Committee. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)-short and long forms; 2005 [accessed 2018 May 21]. Available from: https://sites.google.com/site/theipaq/scoring-protocol
- Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093.
- Choi L, Liu Z, Matthews CE, et al. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43:357–364.
- Aguilar-Farias N, Brown WJ, Peeters GM. ActiGraph GT3X + cut-points for identifying sedentary behaviour in older adults in free-living environments. J Sci Med Sport. 2014;17:293–299.
- Santos-Lozano A, Santin-Medeiros F, Cardon G, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med. 2013;34:975–982.
- Dolgin M. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. The Criteria Committee of the New York Heart Association. 9th ed. Boston: Little, Brown; 1994.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270.
- Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–1025.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310.
- Koolhaas CM, van Rooij FJ, Cepeda M, et al. Physical activity derived from questionnaires and wrist-worn accelerometers: comparability and the role of demographic, lifestyle, and health factors among a population-based sample of older adults. Clin Epidemiol. 2017;10:1–16.
- Shiroma EJ, Cook NR, Manson JE, et al. Comparison of self-reported and accelerometer-assessed physical activity in older women. PLoS One. 2015;10:e0145950.
- Troiano RP, McClain JJ, Brychta RJ, et al. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48:1019–1023.
- Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
- Yavari M, Haykowsky MJF, Savu A, et al. Volume and patterns of physical activity across the health and heart failure continuum. Can J Cardiol. 2017;33:1465–1471.
- Spittaels H, Van Cauwenberghe E, Verbestel V, et al. Objectively measured sedentary time and physical activity time across the lifespan: a cross-sectional study in four age groups. Int J Behav Nutr Phys Act. 2012;9:149.
- Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47:1821–1845.
- Physical Activity Guidelines Advisory Committee Scientific Report. Physical Activity Guidelines Advisory Committee. Washington (DC): U.S. Department of Health and Human Services; 2018.
