Abstract
Objectives. The normal aortic diameter (AD) varies with gender, age and body surface area (BSA). The aortic size index (ASI) is defined as the AD divided by BSA. The primary aim of this study was to investigate if ASI is a predictor of development AAA, and to compare the predictive impact of ASI to that of the absolute AD. Design. Population-based prospective study including 4161 individuals (53.2% women) from the Tromsø study with two valid ultrasound measurements of the AD and no AAA at baseline (Tromsø 4, 1994). The primary outcome was AAA (AD ≥30 mm) in Tromsø 5 (2001). A secondary outcome was aortic growth of >5 mm over 7 years. Estimates of relative risk were calculated in logistic regression models. The main exposure variable was ASI. Adjustments were made for age, gender, smoking, body mass index, total and high-density lipoprotein (HDL) cholesterol, and hypertension. Results. In total, 124 incident AAAs (20% among women) were detected. In adjusted analyses, both ASI and AD were strong predictors of AAA, with similar results for men and women. Both ASI and AD were also significant predictors of aortic growth >5 mm. In comparison, AD was superior to ASI as a predictor of both endpoints. Conclusions. ASI was a significant predictor of both AAA development and aortic growth of >5 mm for both men and women, but not a better predictor of either outcomes compared to the AD. The role of ASI compared to the AD as a predictor of AAA development seems to be limited.
Introduction
There is no universally accepted definition of an abdominal aortic aneurysm (AAA). Several definitions have been proposed, resulting in significantly different prevalence of the disease [Citation1–4]. In clinical practice, AAA is usually defined as a localized aortic diameter (AD) of 30 mm or more, independent of gender [Citation1,Citation5].
The normal AD varies both with gender and age [Citation6,Citation7], and correlates positively to the body surface area (BSA) [Citation8]. Normal infrarenal AD values, as measured by ultrasound, range from 11.9 to 18.7 mm for women and 14.1 to 20.5 mm for men [Citation9]. A standardized way to define an AAA is a localized dilatation of the abdominal aorta exceeding the normal diameter by more than 50% [Citation9].
A large, but non-aneurysmal absolute abdominal AD has previously been reported as a strong risk factor for development of AAA [Citation10]. The BSA in general is smaller in women than in men. Hence, the relative increase in diameter might be larger in women than in men with an AAA of the same size, when an AAA is defined as ≥30 mm. Women with AAA might may therefore have a more advanced stage of AAA at the time of diagnosis [Citation11]. This might be connected to the higher relative mortality and a rupture risk that is three to four times higher in women compared to men [Citation12–14].
The aortic size index (ASI) is defined as the AD divided by the BSA. ASI has been associated with an increased risk of AAA rupture, particularly among women [Citation11,Citation15]. A recent study reported that ASI used as a diagnostic criterion of AAA significantly altered the AAA prevalence and evened the gender gap [Citation16]. The association between the absolute AD versus ASI and AAA development may be affected by how an AAA is defined [Citation1].
The aim of this study was to investigate if ASI is a significant predictor of development of AAA or aortic growth >5 mm. The predictive ability of ASI was compared to that of the AD, when AAA was defined as ≥30 mm.
Materials and methods
The Tromsø study
The Tromsø study is a multi-phase population-based prospective study in Norway. In Tromsø 4 (1994–1995), 6892 men and women aged 25–82 underwent an ultrasound examination of the infrarenal aorta to measure the AD and to investigate the occurrence of AAA. In Tromsø 5 in 2001, 5087 (85%) of the same individuals attended a follow-up ultrasound examination. The cohort eligible for the ultrasound examinations were all individuals aged 55–74 years (in Tromsø 4), a random sample of 5% in the age groups from 25 to 54 years and a random sample of 0.5% aged 75–82 years [Citation17]. Throughout this text, Tromsø 4 and Tromsø 5 are denoted T4 and T5, respectively.
Study cohort
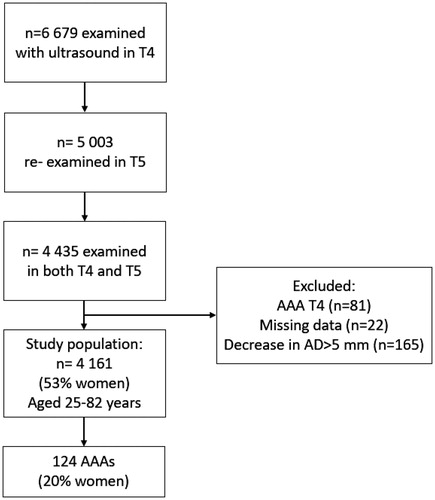
presents the study cohort, which included all individuals with ultrasound measurements of the infrarenal aorta both in T4 and T5 and no prevalent AAA at baseline in T4. The final study population consisted of 4161 individuals aged 25–82 years, after exclusion of individuals with a present AAA in T4 (n = 76) or previous surgery on the abdominal aorta (n = 5), a noted decrease in AD of >5 mm at follow-up in T5 (n = 165) or without complete data (n = 22). Among the 76 individuals excluded due to an AD ≥30 mm in T4 (men, n = 71, women, n = 10), the mean AD in T4 was 35.3 mm (range 30–69 mm). The largest AD was 59 mm for women (mean 36.1 mm) and 69 mm for men (mean 35.3 mm).
Definition of AAA and outcome measures
The primary outcome was an incident AAA, defined as an AD of ≥30 mm in T5.
A secondary outcome was defined as growth of the aorta between T4 and T5 of >5 mm. The purpose of including this outcome was to define a marker of dilating aortic disease with a potential to develop into an AAA, and thereby identify individuals with a significant growth of their aorta, but where the AD in T5 did not necessarily reach the 30 mm threshold. The main exposure was ASI. The impact of ASI was compared to that of the AD as predictors of both outcomes in separate multivariable models.
Assessment of the ultrasound measurements
An ultrasound machine (Acuson, 128 XP B-mode Doppler, Acuson Corporation, Mountain View, CA, USA) with a 3.5 Hz probe was used during both study evaluations. The methods have been described previously [Citation18]. The number of individuals in the study cohort without any growth of the aorta (AD in T4 = AD in T5) was 553 (13.2%). A minimum of 1 mm increase in diameter from T4 to T5 was observed for 1 893 individuals (45%). An observed decrease in the measured AD from T4 to T5 > 5 mm was interpreted as an inaccuracy in the ultrasound measurement. Accordingly, individuals with >5 mm decrease in the AD from T4 to T5 were excluded.
Assessment of risk factors
Baseline data for age, systolic and diastolic blood pressure, height and weight, total cholesterol and high-density lipoprotein (HDL) cholesterol were obtained from T4 measurements and included as continuous variables. Information about risk factors for AAA was obtained from self-reported questionnaires (antihypertensive medication, smoking status). Smoking was categorized into three groups (never, past, current). Hypertension was defined as a dichotomized categorical variable (yes, no), and based on systolic blood pressure above 140 mmHg, diastolic pressure above 90 mmHg or reported use of antihypertensive medication. Because of high correlation between hypertension and systolic blood pressure (R = 0.75), only hypertension was included in the adjusted analyses. BSA was calculated according to the Dubois and Dubois formula [Citation18], whereas the ASI was calculated as AD (cm)/BSA (m2) [Citation11]. Data on self-report diabetes (yes, no) were included in T4. Valid answers were available for only 2 626 out of 4161 persons (63%). This included 77/124 individuals with AAA and 149/235 with aortic growth >5 mm. These numbers were not sufficient for diabetes to be included in the main regression analyses.
Statistical analyses
Baseline characteristics are presented as mean with standard deviation (SD), median (min–max) or number/frequencies. Continuous variables were compared using the independent-sample t-tests or the Mann–Whitney U-test. The chi-square test was used to assess differences in categorical variables. Due to the high correlation between ASI and AD (Pearson correlation coefficient (R = 0.7, p < .001), these variables were included and evaluated as independent variables in separate models. Odds ratios (OR) with 95% confidence intervals (CI) for incident AAA were estimated in univariable and multivariable logistic regression models including individuals with complete data for all variables considered. Adjustments were made for age, gender, smoking, BMI, total cholesterol, HDL cholesterol and hypertension. As a measure of goodness-of-fit, receiver-operating characteristic (ROC) curves were generated and areas under the curve for adjusted models were compared. For each model, values for the pseudo r2 (the proportion of the variation explained by the model) and the information measures AIC (Akaike’s information criterion) and BIC (Bayesian information criterion) were evaluated. The AIC and BIC measured are estimators of the relative quality of a statistical model for a given set of data. The preferred models were the ones with the lowest AIC or BIC. The statistical analyses were performed using Stata (version IC.15.1, Stata Corp, College Station, TX, USA). p-values <.05 were considered statistically significant (two-sided tests).
Subgroup analyses, sensitivity analyses and missing values
To test for heterogeneity in the association with ASI across subgroups, interaction terms between ASI and each of the adjustment factors were included in the model one at a time. The same analyses were performed for AD. The amount of missing values was low (BSA and ASI, n = 4, smoking, n = 2, cholesterol, n = 22), and complete case analyses are therefore presented. The number of individuals under the age of 50 at baseline who developed incident AAAs (n = 4) or had significant aortic growth (n = 20) during follow-up was very low. Sensitivity analyses for both outcome measures (incident AAA and aortic growth >5 mm) where age at baseline was restricted to ≥50 years (n = 3 606) were therefore performed. In an additional sensitivity analysis AAA was defined as an AD ≥27 mm among women in the study population T5 (n = 2 214).
Ethics
This study was approved by the Tromsø Study board of directors and a Regional Ethics Committee (project number 7820.00002) and was conducted in accordance with the Helsinki Declaration. Every participant included in the Tromsø Study has provided written informed consent.
Results
Characteristics of the study population
shows the characteristics of the study population. The gender distribution was fairly even (53.2% women). Mean age at baseline was 59.3 years, with women being slightly older than men (59.8 vs. 58.7 years, p < .001). The mean ASI was 1.1 cm/m2 for both men and women (0.6–1.9 cm/m2) (p = .14). Men had significantly larger BSA than women (1.95 vs. 1.71 m2, p < .001), whereas BMI was similar (women 25.8 kg/m2 vs. men 26.0 kg/m2, p = .19). Mean AD at baseline was 19.9 mm (11.0–29.0 mm), and higher among men than women (21.3 vs. 18.8 mm, p < .001). In T5 the mean AD was 20.3 mm (10.0–56.0 mm), significantly larger among men than women (21.9 vs. 18.9, p < .001). Among those with at least 1 mm increase in AD during follow-up (n = 1 893), the mean increase in AD was 3.1 mm (range 1–24 mm).
Table 1. Characteristics of the study population, in total and by AAA.
Incident AAA
The number of incident AAAs was 124 (2.9% over 7 years, annual incidence 0.4%), and 79.8% were detected among men. Individuals with AAAs were older than those without AAA (p < .002). Moreover, this group was in general taller, heavier, and had larger BMI and BSA, higher blood pressure, total cholesterol levels, and more reported hypertension and smoking. Levels of HDL cholesterol were significantly lower among those with than without AAA (p < .001) (). The data for diabetes was insufficient (37% missing values). In the subgroup with valid data (n = 2 626) 60 persons had diabetes (2.3%) while 2 566 did not have diabetes (97.7%). Among individuals with diabetes, only 2 persons developed AAA (3.3%) while 4 had aortic growth >5 mm (6.7%). The corresponding proportions with AAA and aortic growth >5 among those without diabetes were 2.9% and 5.6%, respectively.
Table 2. Odds ratios (OR) with 95% confidence intervals (95% CI) for the association between ASI, other risk factors and incident AAA in T5a.
ASI at baseline was significantly higher in the group that developed AAA compared to the rest of the population (p < .001). Among those with AAAs, more than 60% were in the highest ASI quartile. ASI was higher among women with AAA than men with AAAs (1.4 cm/m2 vs. 1.2 cm/m2, p = .002), whereas men had larger BSA (2.0 m2 vs. 1.74 m2, p < .001). There was no significant difference in AD at baseline for men and women in the AAA group (men 24.9 mm vs. women 24.0 mm p = .14).
The AAA diameters in T5 ranged from 30 to 39 mm among women (mean 33.5 mm) and 30 to 45 mm among men (mean 33.1 mm) (p = .49). The increase in AD from T4 was substantially higher among those with incident AAA compared to those without (8.4 vs. 0.1 mm, p < .001). In the AAA group the mean growth in AD corresponded to a 40% mean increase for women (9.6 mm) compared to 33% for men (8.1 mm), but the difference was not significant (p = .09). The mean AD at baseline for women and men with AAAs were 18.8 mm and 21.3 mm, respectively. To be diagnosed with AAA in our study, this would require a 60% increase in AD for women and a 41% increase for men. Only 34 individuals (16 women, 18 men) had more than 50% increase in AD from T4 to T5.
ASI and risk of AAA development
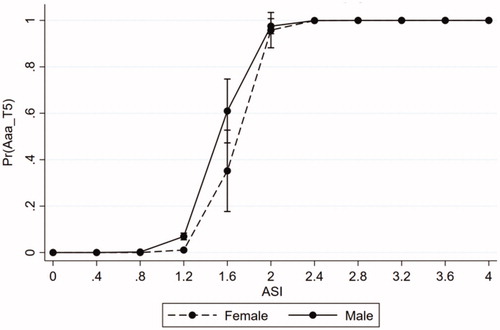
displays the risk of AAA by ASI for women compared to men. The risk of AAA started to increase for men at an ASI of 0.8 cm/m2, whereas the corresponding increase for women occurred at 1.2 cm/m2. In comparison, when the AD was used to predict AAA, the risk of AAA development started to increase at approximately 20 mm in both genders.
Figure 2. Prediction of AAA using ASI, by gender. AAA: abdominal aortic aneurysm; ASI: aortic size index.
In univariable models, increasing ASI, BMI, BSA, total cholesterol and HDL, age, male gender, previous and current smoking, were all significantly associated with AAA development (). Adjusted for these factors, increasing ASI remained a highly significant predictor of AAA (OR 2.58, 95% CI 2.2–3.0). Significant associations were also observed for male gender, smoking (current and past), increasing total cholesterol and BMI. Increasing HDL cholesterol was associated with a reduced risk (OR 0.50, 95% CI 0.28–0.88), whereas no significant associations were found for age or hypertension.
The association between ASI and incident AAA appeared slightly stronger for women (OR 2.9, 95% CI, 2.2–3.8) than men (OR 2.5, 95% CI, 2.1–2.9), but the difference was not significant (p = .3 for interaction). Moreover, the association between ASI and AAA did not differ significantly in subgroups defined by age, BMI, hypertension, total or HDL cholesterol, or smoking status.
ASI versus AD as predictors of AAA
The impact of the two predictors was evaluated by a comparison of the two separate models due to significant correlation between ASI and AD. AD was significantly associated with increased risk of AAA when replacing ASI in an identically adjusted model (OR, 1.83, 95% CI, 1.7–2.0). No change in the main results occurred when BSA was also added as a covariate in the AD model (p = .98).
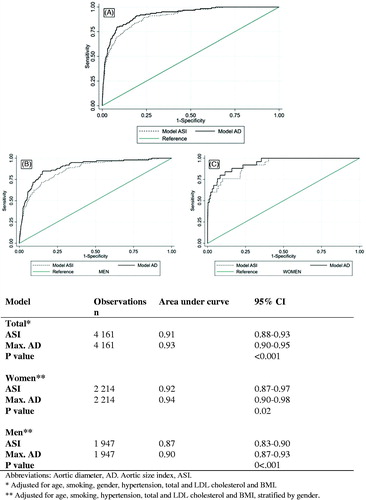
Based on low AIC and BIC values, the model including AD (AIC, 719, BIC, 783) had substantially better fit than the model with ASI (AIC, 767, BIC, 830). The proportion of the variation explained by the regression model was lower for the model with ASI than the model with AD (pseudo r2: 0.33 vs. 0.38). ROC curves based on the final models for both ASI and AD showed excellent discrimination (ASI: AUC, 0.91, AD: AUC, 0.93, p < .001) (), but the AUC for AD was significantly larger than for ASI. Stratified by gender, the AUC was significantly larger for AD than ASI for both women and men. The difference between ASI and AD was less pronounced for women (ASI: AUC 0.92 vs. AD: 0.94, p = .02) than men (AUC ASI, 0.87 vs. AD, 0.90, p < .001).
ASI versus AD as predictors of >5 mm aortic growth
A total of 235 individuals (35% women) had >5 mm increase in AD between T4 and T5, including 104 persons with incident AAAs. Only 20 individuals (3 women) with an AAA (AD ≥30 mm) in T5 had less or equal to 5 mm growth of the aorta (16.1%). Using the same multivariable models for the secondary outcome aortic growth, both ASI (OR 1.13, 95% CI 1.02–1.24, p = .016) and AD (OR 1.09, 95% CI 1.03–1.15, p = .002) were independent predictors of aortic growth. The association with growth for both ASI and AD, respectively, did not differ significantly for men and women (ASI: p = .7 for interaction, AD: p = .6 for interaction).
The AIC and BIC for ASI (AIC 1 687, BIC 1 751) and the AD (AIC, 1 684, BIC, 1 747) values were similar, and the pseudo r2 values were low for both models (ASI, 0.06 vs. AD, 0.07). Each model showed similar and acceptable levels of discrimination in ROC analyses based on the adjusted analyses (ASI: AUC 0.70, AD: AUC 0.70, p = .79). The AUC was significantly larger for AD than ASI for women (ASI, 0.60 vs. AD, 0.68, p < .001), but not for men (p = .99).
Sensitivity analyses
The associations between ASI and incident AAA (OR 2.58, 95% CI 2.21–3.00) and AD and incident AAA (OR 1.82, 95% CI 1.67–1.99) were not affected in the analyses including only individuals aged ≥50 years a baseline in T4 (n = 3 606). In the same subpopulation, the associations between aortic growth >5 mm and ASI (OR 1.16, 95% CI 1.05–1.30) versus AD (1.10, 95% CI 1.04–1.16) remained practically unchanged. The sensitivity analysis of women where incident AAA was defined as ≥27 mm, showed essentially the same results as the main models. The change in the definition doubled the number of AAAs among women, from 25 in the main analyses (proportion with AAA in women, 1.12%) to 51 (2.29 %). Both ASI (OR = 2.16, 95% CI 1.75–2.66) and the absolute AD (OR = 1.69, 95% CI 1.49–1.93) were significantly associated with development of the disease. When the two models were compared, the AD (pseudo r2 0.37, AIC 321, BIC 372) was a significantly better predictor of AAA than ASI (pseudo r2 0.34, AIC 339, BIC390). The AUC was significantly larger for the AD compared to ASI (0.92 vs. 0.90, p = 0.009).
Discussion
This prospective population-based study showed that ASI was a highly significant and independent predictor of incident AAA for both men and women. In addition, ASI was a significant predictor of aortic growth >5 mm over 7 years. However, compared to the AD, ASI was inferior as a predictor of both incident AAA and aortic growth.
Predictors of AAA development
Strong positive associations with the major risk factors smoking and male gender were confirmed [Citation17–20]. In addition, a positive association was found for BMI, were previous studies have been inconsistent [Citation20,Citation21]. Increasing total cholesterol was associated with increased risk of AAA, whereas high HDL cholesterol had a protective effect, in line with previous results from the Tromsø study [Citation17].
With the inclusion of ASI in the adjusted analyses, the expected association with age became non-significant. This could relate to the short time between T4 and T5 or the age distribution of the study population but might also illustrate the impact of ASI as a predictor of AAA. In comparison, age was significantly associated with AAA in the model including the AD (p < .001). A large but non-aneurysmal AD has previously been reported as a risk factor for AAA in the Tromsø study [Citation10], which was confirmed in this study. It is not surprising that an increasingly large non-aneurysmal diameter predicts a disease also defined by an AD.
The association between ASI and AAA was not significantly different between women and men. However, the AD was a significantly better predictor of AAA than ASI in both genders. This finding is somewhat in contrast to a previous study, where ASI was a better predictor of rupture than the AD in women in a population of both men and women surgically treated for AAAs [Citation19]. However, different factors are associated with rupture and development of AAA, and a direct comparison of these two end-points is not straightforward.
ASI vs. AD and definitions of AAA
Different definitions of AAA substantially affect the prevalence of AAA in a population [Citation1]. Previous studies have developed nomograms to predict normal ADs and thereby relative increase in diameter in individuals with AAAs [Citation6,Citation8], but this method has not been generally implemented. Hence, the widely used ≥30 mm definition was applied in this study. However, defining AAA as an AD ≥30 mm may be open to discussion. Notably, a recent study from New Zealand applied an ASI ≥1.5 as a diagnostic criterion of an AAA, which led to a more gender equal prevalence [Citation16]. In our study, individuals with incident AAAs had higher BMI, larger BSA as well as larger baseline AD than those with normal aortas in T5. This could indicate that the definition of AAA as ≥30 mm tends to diagnose large persons to a greater extent than small persons, and by extension also men over women. The risk of AAA started to increase at a higher ASI in women than in men. In comparison, the risk increased at an AD of 20 mm in both genders. BSA was larger among men, which could explain the difference. Alternatively, the results could indicate that the AD in women who develop AAAs are relatively larger. However, in our study lowering the threshold of the AAA definition to 27 mm among women lead to the same results as the main analyses, the AD was a better predictor of AAA than ASI.
A standardized definition of AAA is a 50% increase compared to the normal diameter [Citation9]. Applying this definition resulted in only 34 incident AAAs in this study, but notably, among them 47% were women. To be diagnosed with AAA in our study, an increase of 60% in the AD was necessary for women, with a corresponding 41% increase for men. The overall increase in AD from T4 to T5 was slightly higher for women than men with AAAs (40% vs. 33%), but not significantly different. This supports previous reports that the relative increase in diameter is larger in women with AAA than in men with an AAA of the same size, and that female patients might potentially have a more advanced stage of AAA at the time of diagnosis [Citation8,Citation22]. The newly revised guidelines for AAA recommend defining AAA as ≥30 mm among men, but also state that a lower threshold may be appropriate for women [Citation5]. Our results support the later argument, but the definition of “lower” is somewhat unclear. On the other hand, it is debatable whether men with a 30 mm infrarenal aorta corresponding to a 40% increase in diameter should be diagnosed with an AAA.
It is possible that ASI may be a better predictor than the AD with another definition of AAA. The number of included AAA in this study, particularly for women, and not knowing the normal AD for each included individual, restricted the ability to compare the impact of ASI versus AD as predictors on other suggested definitions of AAA. However, an increase in AD >5 mm from T4 to T5 was considered a marker of vessel wall pathology for both men and women in this study. Seven years of follow up might be considered short to evaluate the development of aortic changes. On the other hand, previous studies have shown that nearly 50% of sub-aneurysms (25–29 mm) can progress to an aneurysm already within five years [Citation23]. The aim of investigating a significant growth of the aorta was to include vessel wall changes that might mirror a pathology in the aortic wall, without necessary reaching the threshold of 30 mm in diameter. A total of 124 persons developed AAA and 235 individuals had aortic growth during follow-up. We therefore consider the time-window for follow-up to be sufficient to cover the aims of our study. Among the 235 individuals with an aortic growth >5 mm, approximately 84% had an incident AAA in T5. Both ASI and the AD were significantly associated with growth >5 mm for both genders. Stratified for gender, however, the AD was a significantly better predictor for growth >5 mm than ASI in women, whereas the results were similar among men. This further supports the results from this study, which suggest that ASI has less value as a predictor of AAA development than the AD.
Strengths and limitations
The strengths of this study include a population-based and prospective design. The proportion of missing values was very low for all variables considered. Adjustments were made for known major risk factors for the disease. The data in our study did not allow for adjustment for diabetes in the regression analyses. This may potentially have affected the results, as current evidence seems to point towards an inverse association between diabetes and both development of AAA as well as aortic growth [Citation24–27]. The detection of AAA was ultrasound-based, and the attendance in both T4 and T5 was high. There are also limitations that should be addressed. The number of AAAs was relatively small, particularly for women. This made it impossible to evaluate the impact of other common definitions of AAA in this population, and probably influenced the results from the interaction analyses. Inaccuracies in the ultrasound measurements may have led to misclassification. Those with a negative aortic difference between T4 and T4 > 5mm were excluded. Some of the covariate responses were based on self-reported questionnaires, potentially leading to information bias [Citation28,Citation29].
Conclusion
The ASI is a strong predictor of incident AAA for both men and women, when AAA is defined as ≥30 mm. However, our results suggest that the role of ASI as a predictor of AAA development in comparison to the AD is limited but supports further evaluation of ASI as predictor of AAA using other definitions of the disease.
Acknowledgements
This study has used data from the Norwegian Tromsø Study, and we thank Professor Bjarne K. Jacobsen for the collaboration. The Statistiska Konsultgruppen by Aldina Pivodic in Gøteborg, Sweden, contributed to the statistical analyses.
Disclosure statement
None of the listed authors have any conflict of interest.
Data availability statement
The results presented are based on analyses of the much larger Tromsø Study database, and each project has to be authorized and data cannot be shared. Requests to access the dataset from qualified researchers may be directed to the Tromsø study ([email protected]).
Additional information
Funding
References
- Wanhainen A. How to define an abdominal aortic aneurysm––influence on epidemiology and clinical practice. Scand J Surg. 2008;97:105–109. discussion 9.
- Sterpetti AV, Schultz RD, Feldhaus RJ, et al. Factors influencing enlargement rate of small abdominal aortic aneurysms. J Surg Res. 1987;43:211–219.
- McGregor JC, Pollock JG, Anton HC. Ultrasonography and possible ruptured abdominal aortic aneurysms. Br Med J. 1975;3:78–79.
- Collin J, Araujo L, Walton J, et al. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet. 1988;2:613–615.
- Wanhainen A, Verzini F, Van Herzeele I, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg. 2019;57:8–93.
- Sonesson B, Lanne T, Hansen F, et al. Infrarenal aortic diameter in the healthy person. Eur J Vasc Surg. 1994;8:89–95.
- Lederle FA, Johnson GR, Wilson SE, et al. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg. 1997;26:595–601.
- Lanne T, Sandgren T, Sonesson B. A dynamic view on the diameter of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1998;15:308–312.
- Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on reporting standards for arterial aneurysms, ad hoc committee on reporting standards, society for vascular surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452–458.
- Solberg S, Forsdahl SH, Singh K, et al. Diameter of the infrarenal aorta as a risk factor for abdominal aortic aneurysm: the Tromso Study, 1994-2001. Eur J Vasc Endovasc Surg. 2010;39:280–284.
- Lo RC, Lu B, Fokkema MT, et al. Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. J Vasc Surg. 2014;59:1209–1216.
- Michel JB, Martin-Ventura JL, Egido J, et al.; For the FAD EU consortium. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27.
- Scott RA, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–285.
- Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Annals Surg. 1999;230:289–296. discussion 96–7.
- Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Annals Thor Surg. 2006;81:169–177.
- Jones GT, Sandiford P, Hill GB, et al. Correcting for body surface area identifies the true prevalence of abdominal aortic aneurysm in screened women. Eur J Vasc Endovasc Surg. 2019;57:221–228.
- Forsdahl SH, Singh K, Solberg S, et al. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromso Study, 1994-2001. Circulation. 2009;119:2202–2208.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition (Burbank, Los Angeles County, Calif). 1989;5:303–311. discussion 12–3.
- Tang W, Yao L, Roetker NS, et al. Lifetime risk and risk factors for abdominal aortic aneurysm in a 24-year prospective study: the ARIC study (atherosclerosis risk in communities). Arterioscler Thromb Vasc Biol. 2016;36:2468–2477.
- Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548.
- Stackelberg O, Bjorck M, Sadr-Azodi O, et al. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100:360–366.
- Forbes TL, Lawlor DK, DeRose G, et al. Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg. 2006;20:564–568.
- Söderberg P, Wanhainen A, Svensjö S. Five year natural history of screening detected sub-aneurysms and abdominal aortic aneurysms in 70 year old women and systematic review of repair rate in women. Eur J Vasc Endovasc Surg. 2017;53:802–809.
- Raffort J, Lareyre F, Clement M, et al. Diabetes and aortic aneurysm: current state of the art. Cardiovasc Res. 2018;114:1702–1713.
- Tanaka A, Ishii H, Oshima H, et al. Inverse association between diabetes and aortic dilatation in patients with advanced coronary artery disease. Atherosclerosis. 2015;242:123–127.
- De Rango P, Farchioni L, Fiorucci B, et al. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47:243–261.
- Le MT, Jamrozik K, Davis TM, et al. Negative association between infra-renal aortic diameter and glycaemia: the Health in Men Study. Eur J Vasc Endovasc Surg. 2007;33:599–604.
- Drieling RL, LaCroix AZ, Beresford SA, et al. Validity of self-reported medication use compared with pharmacy records in a cohort of older women: Findings from the women's health initiative. Am J Epidemiol. 2016;184:233–238.
- Myrtveit SM, Ariansen AM, Wilhelmsen I, et al. A population based validation study of self-reported pensions and benefits: the Nord-Trondelag health study (HUNT). BMC Res Notes. 2013;6:27.