Abstract
Objectives: To investigate cardiac implantable electrical device (CIED) first implants in patients with hypertrophic cardiomyopathy (HCM) in a Swedish tertiary university hospital. Design: Clinical and technical data on pacemaker, implantable cardioverter defibrillator (ICD), and cardiac resynchronization therapy (CRT) first implants performed in HCM patients at the Karolinska University Hospital from 2005 to 2016 were extracted from the Swedish Pacemaker and ICD Registry. Echocardiographic data were obtained by review of hospital recordings. Results: The number of first pacemaker implants in HCM patients was 70 (1.5% of total pacemaker implants). The mean age of HCM pacemaker patients was 71 ± 10 years. Pacemaker implants were almost uniformly distributed between genders. Dual-chamber pacemakers with or without CRT properties were prevalent (6 and 93%, respectively). The number of first ICD implants in HCM patients was 99 (5.1% of total ICD implants). HCM patients receiving an ICD were 53 ± 15 years and prevalently men (70%). Sixty-five (66%) patients were implanted for primary prevention. Dual-chamber ICDs with or without CRT were 21 and 65%, respectively. Obstructive HCM was present in 47% pacemaker patients and 25% ICD patients with available pre-implant echo. Conclusions: This retrospective registry-based study provides a picture of CIED first implants in HCM patients in a Swedish tertiary university hospital. ICDs were the most commonly implanted devices, covering 59% of CIED implants. HCM patients receiving a pacemaker or an ICD had different epidemiological and clinical profiles.
Introduction
The Swedish Pacemaker and ICD Registry provides a real-time picture of the use of pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices in Sweden [Citation1,Citation2]. The Registry is highly representative, covering almost 100% of the implanting activity across the country.
The aim of this study was to investigate the use of pacemaker and ICD first implants in patients previously diagnosed with hypertrophic cardiomyopathy (HCM) at the Karolinska University Hospital from 2005 to 2016, based on the Swedish Pacemaker and ICD Registry data. Expanded data concerning patient echocardiographic characteristics and device features were analysed.
Methods
Implant and patient data retrieval
Retrospective data on pacemaker and ICD (including CRT) first implants in patients diagnosed with HCM, performed at the Karolinska University Hospital between January 2005 and September 2016, were retrieved from the Swedish Pacemaker and ICD Registry with the support of the Registry administrator. Since 2002 the Swedish Pacemaker Registry has become accessible online (http://www.pacemakerregistret.se), and pacemaker data have been reported by the participating centres using direct data entry on the website. Data on ICD implants have been entered online since 2004. Informed consent for data entry is required by the ethics committee of each participating hospital. Collected data are regularly checked for internal consistency by the Registry administrator, and online statistics are updated on a daily basis. The following variables were retrieved from the Registry: number of pacemaker and ICD implants related to the selected centre (i.e. Karolinska University Hospital) in patients with a diagnosis of HCM, patient demographics (i.e. age and gender), clinical and electrocardiographic indication (brady- or tachyarrhythmias), technical information on devices, and perioperative complications. Complications leading to a reintervention or other medical intervention include infections, lead electrical dysfunction, perforation/tamponade, pneumothorax, electrode displacement, subclavian or other related thrombosis. Primary prevention indication identifies an ICD implant based on risk marker identification according to ESC guidelines [Citation3]. Secondary prevention refers to an ICD implant in patients with documented ventricular tachyarrhythmias. According to ESC guidelines [Citation4], sudden cardiac death (SCD) was defined as a non-traumatic, unexpected fatal event occurring within 1 h of the onset of symptoms, most likely caused by an arrhythmic event, in the absence of obvious extra-cardiac causes identified by postmortem examination. Patient survival data were obtained by matching the Pacemaker and ICD Registry to the Swedish population register database (SPAR database).
Echocardiographic evaluation
We limited our analysis to echocardiographic pre-implant examinations performed at the Karolinska University Hospital, excluding evaluations conducted at other referring centres. Transthoracic Doppler echocardiography was performed using a Vivid 7 system (Vingmed-General Electric, Horten, Norway) equipped with a phased array 3.5 MHz transducer (Doppler frequency 5.0–3.5 MHz). Images were digitally stored on a dedicated server and data analysis was performed off-line on the EchoPAC workstation (GE EchoPAC sw only, version 2.0.2, Horten, Norway) by one investigator. The mean value of 3 cardiac cycles was calculated for each variable. In the event of atrial fibrillation, measurements were averaged over 5 cardiac cycles.
Left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volumes were measured from the apical four-chamber view according to Simpson’s equation, and ejection fraction (LVEF) was derived. End-stage HCM was defined by LVEF <50% [Citation5]. Septal and posterior wall thicknesses were measured by two-dimensional imaging in parasternal long-axis view at end-diastole. The transmitral inflow velocity pattern was used to assess left ventricular (LV) diastolic function, and E/E′ ratio was calculated by measuring the early diastolic velocity (E′) at the septal border of the mitral annulus by pulsed-wave tissue Doppler imaging [Citation6]. Left atrial (LA) volume was measured at ventricular end-systole from the apical four-chamber view and indexed to body surface area [Citation5]. The maximal gradient in the LV outflow tract (LVOT), reflecting the severity of dynamic obstruction, was determined by measuring the peak LVOT velocity by continuous-wave Doppler from the apical view. LVOT gradient was assessed at rest and following Valsalva maneuver. LV outflow obstruction was defined as a LVOT gradient ≥ 30 mmHg [Citation7]. Mitral regurgitation (MR) was assessed using color Doppler and described as central or eccentric. MR degree was expressed on a scale of 0 to 4 score with steps of 0.5, based on the percentage jet area relative to the LA size in the apical four-chamber view [Citation8].
Statistics
Data were analysed using a commercially available statistical package (IBM SPSS Statistics 25). Continuous variables were presented as mean ± standard deviation or median ± interquartile range according to their distribution, and categorical variables were reported as percentages. Independent samples t test was used for comparisons between continuous data of two unrelated groups. Chi-squared test was used for comparisons between ordinal data of two independent groups. Patient survival was evaluated according to the Kaplan–Meier estimate. Factors affecting survival probability were analysed by univariable models. Risk of death from cardiac failure, SCD or other causes was analysed using hazard ratio statistics. p<.05 were considered statistically significant.
Results
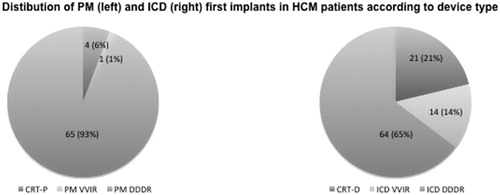
From 2005 to 2016, the total number of first pacemaker implants at the Karolinska University Hospital, independently of cardiac disease etiology, was 4789. In the same time frame, the number of first ICD implants was 1914. By limiting the analysis to patients diagnosed with HCM, 70 first pacemaker implants were found to be performed from 2005 to 2016 (1.5% of total pacemaker implants). In 4 cases (6%) a CRT-P device was implanted. In the same period, 99 patients were implanted with an ICD (5.1% of total ICD implants), 21 of whom (21%) receiving a CRT-D device. Pacemaker and ICD implant numbers in HCM patients over time are displayed in . A progressive decrease in pacemaker first implants has occurred over the last years, while the number of ICD implants has remained substantially stable in 2015–2016. Clinical and echocardiographic data of HCM patients included in this study, receiving a pacemaker or ICD, are presented in and discussed below.
Figure 1. Pacemaker and ICD implant numbers per year in HCM patients at the Karolinska University Hospital from 2005 to 2016.
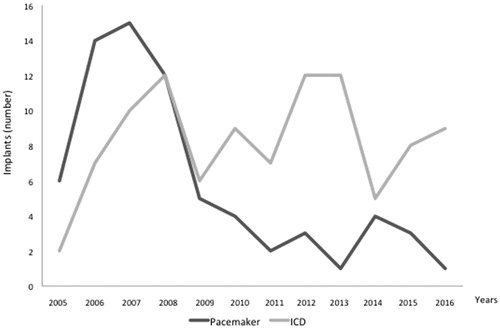
Table 1. Baseline clinical and echocardiographic variables of HCM study population.
Pacemaker implants
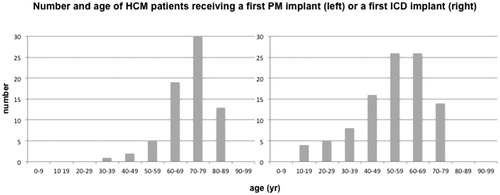
The mean age of HCM patients requiring a first pacemaker implant was 71 ± 10 years. shows the distribution of first pacemaker implants by age. Most HCM patients receiving a first pacemaker implant were aged 70–79 years (n = 30). Pacemaker implants were almost uniformly distributed between men (47%) and women (53%). Clinical indications were mainly breathlessness/tiredness (67%), dizziness (11%), and syncope (7%). Electrocardiographic indications were related to atrioventricular conduction disorders in 12 (17%) patients, sick sinus syndrome in 6 (9%) patients, and paroxysmal/chronic atrial fibrillation with low heart rate in 4 (6%) patients. Pacing implantation was performed in the attempt to reduce LVOT gradient in 46 (66%) patients. This indication was seen mainly in years between 2006 and 2008. As shown in , dual-chamber pacemakers (DDDR) with or without CRT properties were implanted in 6% and 93% of the patients, respectively. A single chamber pacemaker (VVIR) was implanted only in one (1%) patient with chronic atrial fibrillation. Perioperative complications were observed in two (3%) cases and were represented by electrode displacement and acute arrhythmia requiring drug administration, respectively. Alcohol septal ablation was performed in 14 (20%) pacemaker patients distributed over years 2003–2010 with start in March 2003. In 9 (64%) cases, pacemaker implant occurred after alcohol septal ablation.
ICD implants
The mean age of HCM patients implanted with an ICD was 53 ± 15 years. As shown in , most ICD implants were performed in patients aged 50–59 (n = 26) and 60–69 years (n = 26). Most patients were males (70%). Sixty-five (66%) patients were implanted for primary prevention of SCD. Among the patients receiving ICD therapy for secondary prevention (34%), sustained ventricular tachycardia was the most frequent arrhythmia (41%), followed by non-sustained ventricular tachycardia (26%), ventricular fibrillation (21%), and ventricular tachycardia plus ventricular fibrillation (12%). Most frequent clinical manifestations were syncope (24%), breathlessness/tiredness (19%), palpitations (10%), and heart failure (6%). Aborted SCD had been reported in 10% of patients undergoing ICD implant. Twenty-three (23%) ICD patients had paroxysmal/chronic atrial fibrillation. Atrioventricular conduction disorders and sick sinus syndrome had been diagnosed in 11 and 4% of ICD recipients, respectively. As shown in , dual-chamber ICDs with or without CRT were implanted in 21 and 65% of the patients, respectively, whereas single-chamber ICDs accounted for 14% of the implants. One patient received first an ICD and was then upgraded to CRT-D. No perioperative complications were observed. Alcohol septal ablation was performed in 16 (16%) ICD patients distributed over years 2003–2016 with start in April 2003. In 15 (94%) cases, ICD implant occurred after alcohol septal ablation.
Echocardiographic data
Echocardiographic preimplant data were available in 49 HCM patients receiving a pacemaker and 79 HCM patients implanted with an ICD. As presented in , ICD recipients had higher LV volumes and slightly lower LVEF than pacemaker recipients (p<.05). Septal and posterior wall thicknesses were similar in both groups. Regarding LV diastolic function, higher E/A ratio and E slope, together with lower deceleration time, were found in ICD patients (p≤.01). LA size was higher in ICD than pacemaker patients (p=.004). MR was detectable in 39 (80%) HCM patients receiving a pacemaker and 64 (81%) HCM patients receiving an ICD. MR degree was similar in both groups. MR jet was central in 21 (54%) pacemaker patients and 38 (59%) ICD patients, being eccentric in the remaining cases.
Among HCM patients receiving a pacemaker, LV outflow tract obstruction was found in 19 (39%) cases at rest (median peak gradient 55 mmHg, range 37–61 mmHg) and in 4 (8%) cases during Valsalva maneuver (range 37–62 mmHg). Clinical and echocardiographic parameters of obstructive and non-obstructive HCM patients in the pacemaker population with available echo data are presented in . As shown, no significant differences in systolic or diastolic functional echo parameters were observed between the two groups.
Table 2. Clinical and echocardiographic variables of obstructive and non-obstructive HCM patients in the pacemaker population with available echo data (n = 49).
Among HCM patients receiving an ICD, similar clinical and echocardiographic characteristics were found between primary and secondary preventive ICD recipients (). In the entire ICD population, LVOT obstruction was observed in 12 (15%) cases at rest (median peak gradient 70 mmHg, range 51–92 mmHg) and in 8 (10%) cases during Valsalva maneuver (range 40–69 mmHg). The percentage of obstructive HCM was not significantly different between primary and secondary preventive ICD recipients (23 vs. 31%, respectively, p=.43).
Table 3. Clinical and echocardiographic variables of HCM patients with primary and secondary preventive ICD.
End-stage HCM was identified in 4 (8%) patients implanted with a pacemaker. In this group, median LVEF was 42% (range 34–49%). Two of the patients received a CRT-P. Eighteen (23%) patients receiving an ICD had end-stage HCM with median LVEF of 35% (range 29–49%). Twelve of the patients received a CRT-D.
Survival
shows the Kaplan–Meier estimates of survival from implant date to last follow-up date. Numbers and confidence intervals of long-term survival estimates for pacemaker and ICD patients are presented in .
Figure 4. Kaplan–Meier survival estimates (upper part) in pacemaker (left) and ICD (right) patients, respectively. In the lower part, probability of death at different years from pacemaker (left) and ICD (right) implant according to clinical etiology. HF: heart failure; SCD: sudden cardiac death.
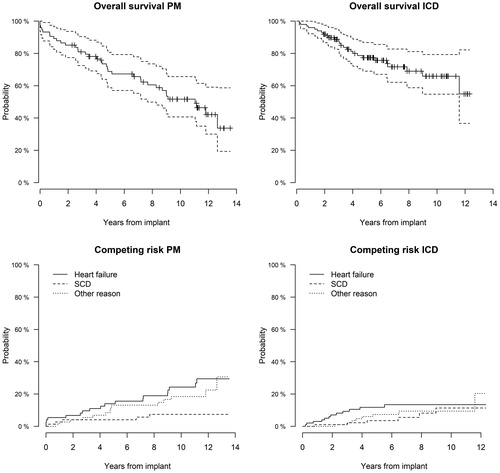
Table 4. Long-term survival estimates for pacemaker and ICD patients with HCM.
As presented in , by using univariable models to investigate how each variable affects survival in pacemaker and ICD patients, respectively, the only variable affecting the outcome (time to death) was the age at implant, and this applied to both groups. The risk of death was 1.07 and 1.11 for the pacemaker and ICD populations, respectively (p<.001 for both). Gender, rhythm (sinus vs. atrial fibrillation), and previous alcohol septal ablation did not significantly affect the survival of pacemaker and ICD patients. The age grouping was based on the total distribution of pacemaker and ICD patients (quartiles). Reference group for age grouping was the age class (64–72) years, which was most representative of pacemaker and ICD patient distribution. Concerning pacemaker patients, none of the age groups was significantly different from the reference group (). Among ICD patients, the age class [10, 53] showed a 0.11 times lower risk of death than the reference group (p=.001). Notably, only one ICD patient was <16 years old.
Table 5. Univariable models investigating how each individual variable affects survival for the PM group and the ICD group, separately.
When analysing the risk of death divided into various causes of death (SCD, heart failure, and other), the ICD group showed a significantly higher risk of death related to heart failure, while the pacemaker group showed a comparable risk of death due to heart failure and other causes (). In the ICD group, the estimated risk of death at 5 years was 11.8% for heart failure, 3.5% for SCD, and 7.3% for other reasons. Overall, in the ICD group 6 (6%) patients died of SCD. Two of them had sudden arrhythmic death caused by ICD shock failure. In the pacemaker group, the estimated risk of death at 5-year follow-up by different etiology was 14.0% for heart failure, 4.1% for SCD, and 13.1% for other reasons.
Discussion
In recent years, the use of cardiac implantable electrical devices (CIED) has increased in Europe as a consequence of large clinical trials, development of scientific guidelines, and implementation of evidence in clinical practice [Citation9–12]. While CIED implant rates in the entire population of different geographic contexts are now being published [Citation9,Citation12], registry-data on the utilization of CIED in specific heart diseases, like HCM, are still under-reported.
This retrospective registry-based study provides a real-world picture of pacemaker and ICD first implants in HCM patients over the last decade in a Swedish tertiary university hospital. Data were extracted from the Swedish Pacemaker and ICD Registry, which has a 97% coverage of procedures when validated with the Patient Care Registry kept by the National Board of Welfare and gathers demographic, clinical, and technical data [Citation1,Citation2]. Overall, ICDs were the most commonly implanted devices, covering 59% of the total amount of implants in HCM patients. A progressive decrease in pacemaker first implants has occurred from 2005 to 2016, while the number of ICD implants has remained substantially stable in the last years. This may reflect the effect of wider diffusion of risk stratification models for SCD in HCM [Citation13,Citation14], and progressive implementation of ESC guidelines for cardiac pacing and SCD prevention in HCM patients (3).
HCM patients implanted with a pacemaker or an ICD in our study showed different epidemiological and clinical profiles. HCM patients receiving a pacemaker were comparatively older than those receiving an ICD. While pacemaker implants were almost uniformly distributed between men and women, ICD implants were higher in men. This is in line with previous findings, showing similar gender differences in the use of ICD therapy for primary and secondary prevention [Citation1,Citation15]. The reasons for lower ICD implant rates in women are still debated, and seem not to be entirely justified by epidemiological and clinical factors [Citation15]. Prevalent indication for pacing therapy in our population was LVOT gradient reduction [Citation16,Citation17], and it was combined with alcohol septal ablation in less than one-quarter of the patients. As regards ICDs, primary prevention indication, according to contemporary ESC guidelines, accounted for 66% of first implants. CRT-P and CRT-D systems were 6 and 21% of the total amount of implanted pacemakers and ICDs, respectively, and were performed in case of systolic dysfunction and heart failure symptoms. Complication rates were low and observed only in the pacemaker population. As previously commented, so far few registry-based data have been published on CIED implants in HCM patients. In the Portuguese Registry of Hypertrophic Cardiomyopathy, over the years 2013–2015 the main indication (88%) for ICD was primary prevention; a pacemaker was implanted for conduction disorders in 70% of the patients and for gradient reduction in 21% [Citation18].
Pacing therapy with atrioventricular sequential pacing can reduce LVOT obstruction and improve symptoms in obstructive HCM (HOCM) patients [Citation19]. The underlying mechanisms are not completely known, but seem to be related to reduced hypercontractility, asynchronous septal activation with delayed septal thickening, limitation of abnormal mitral valve motion, improved LV filling, and reverse ventricular remodelling [Citation19]. Despite the documented benefits of atrioventricular pacing on LVOT obstruction and quality of life [Citation17,Citation19], still the evidence for reduction in morbidity and mortality is insufficient [Citation20]. Therefore, 2014 ESC guidelines (3) suggest that atrioventricular pacing with optimal atrioventricular delay may be used to reduce LVOT gradient or to facilitate medical treatment only in selected symptomatic HOCM patients who are unsuitable or unwilling to undergo invasive septal reduction therapies. We think that clinical studies on pacing in HCM patients and implementation of ESC guidelines in clinical practice have influenced pacing activity over the past decade, and this may explain the progressive decrease in pacemaker first implants in HCM patients in the selected centre, as shown by our registry-based data. In our opinion, registries have indeed the potential to reflect the clinical adherence to contemporary guidelines in selected geographic contexts.
Echocardiographic assessment of HCM patients receiving a CIED showed a more compromised LV systolic and diastolic function in ICD compared to pacemaker group. HOCM was more represented in the pacemaker than in the ICD group, while the percentage of end-stage HCM was higher among ICD patients. Of note, despite a similar degree of MR in pacemaker and ICD groups, LA size was significantly higher in the latter, suggesting other factors involved, most probably more severe diastolic dysfunction in end-stage HCM. It is known that LA enlargement in HCM is determined by multiple factors, including diastolic dysfunction, atrial fibrillation, MR severity, and atrial myopathy [Citation5]. The assessment of LA size provides important prognostic information [Citation21], and has been integrated in a risk prediction model for SCD in HCM [Citation13].
In line with the previous findings [Citation22], alcohol septal ablation did not significantly affect the survival of HCM patients. Survival of HCM patients receiving a pacemaker or an ICD was mostly affected in both groups by the age at implant. The earlier the patient was implanted with a pacemaker or ICD, the better the outcome was. In our opinion, these highlights the relevance of proper implant timing of CIED in HCM patients. The ICD group showed a higher risk of death related to heart failure, while the pacemaker group showed comparable risk of death from heart failure and other causes. It is known that the implantation of an ICD in a HCM patient influences and reduces the risk for SCD. Since younger age at diagnosis is an independent predictor of poor outcomes in HCM [Citation23], appropriately timed CIED treatment in selected patients may improve the clinical course and prognosis.
Study limitations
The study was based on observational data concerning a single tertiary university hospital. However, the results may be representative of real-life implanting activity in similar contexts. Echo results concerned only patients who had undergone pre-implant examinations at the Karolinska University Hospital, since partial echo data were available from evaluations conducted at other referring centres. Since the Registry does not report all the clinical features associated with an increased risk of SCD in adults, it was not possible to better identify the risk profile of patients receiving a primary prophylactic ICD. Primary prevention indication was based on risk marker assessment [Citation3] and physician judgment. ICD intervention rates were not reported in the Registry.
Conclusions
In this registry-based study on CIED first implants in HCM patients at the Karolinska University Hospital over the years 2005–2016, ICDs were the most commonly implanted devices, whereas a progressive decrease in pacemaker implants was observed. Within the pacemaker population, obstructive and non-obstructive HCM patients showed similar systolic and diastolic functional parameters, as assessed by echocardiography. Within the ICD population, similar clinical and echocardiographic characteristics were found between primary and secondary preventive ICD recipients. Primary prevention was the main indication for ICD implant. Less than one-quarter of the patients received a CRT-D device. Complication rates were low and observed only in the pacemaker population. Survival of HCM patients receiving a CIED was mostly affected by the age at implant.
Acknowledgments
We acknowledge Eva Hagel for statistical support. The authors are grateful to Anita Fredenson and Zsolt Palfi for expert and thorough administration of the Registry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace. 2015;17(1):69–77.
- Magnusson P, Gadler F, Liv P, et al. Hypertrophic cardiomyopathy and implantable defibrillators in Sweden: inappropriate shocks and complications requiring surgery. J Cardiovasc Electrophysiol. 2015;26(10):1088–1094.
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–2779.
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17(11):1601–1687.
- Nagueh SF, Bierig SM, Budoff MJ, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2011;24(5):473–498.
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133.
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet. 2017;389(10075):1253–1267.
- Valzania C, Gadler F, Eriksson MJ, et al. Electromechanical effects of cardiac resynchronization therapy during rest and stress in patients with heart failure. Eur J Heart Fail. 2007;9(6–7):644–650.
- Raatikainen MJP, Arnar DO, Merkely B, et al. A decade of information on the use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2017 Report from the European Heart Rhythm Association. Europace. 2017;19(suppl_2):ii1–ii90.
- Sponder M, Khazen C, Dichtl W, et al. Specific indications and clinical outcome in patients with subcutaneous implantable cardioverter–defibrillator (ICD) – a nationwide multicentre registry. Eur J Intern Med. 2018;48:64–68.
- Albouaini K, Egred M, Rao A, et al. Cardiac resynchronisation therapy: evidence based benefits and patient selection. Eur J Intern Med. 2008;19(3):165–172.
- Valzania C, Torbica A, Tarricone R, et al. Implant rates of cardiac implantable electrical devices in Europe: a systematic literature review. Health Policy. 2016;120(1):1–15.
- O’Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014;35:2010–2020.
- Maron BJ, Maron MS. Contemporary strategies for risk stratification and prevention of sudden death with the implantable defibrillator in hypertrophic cardiomyopathy. Heart Rhythm. 2016;13(5):1155–1165.
- Curtis LH, Al-Khatib SM, Shea AM, et al. Sex differences in the use of implantable cardioverter–defibrillators for primary and secondary prevention of sudden cardiac death. JAMA. 2007;298(13):1517–1524.
- Gadler F, Linde C, Juhlin-Dannfelt A, et al. Long-term effects of dual chamber pacing in patients with hypertrophic cardiomyopathy without outflow tract obstruction at rest. Eur Heart J. 1997;18(4):636–642.
- Gadler F, Linde C, Daubert C, et al. Significant improvement of quality of life following atrioventricular synchronous pacing in patients with hypertrophic obstructive cardiomyopathy. Data from 1 year of follow-up. PIC study group. Pacing in cardiomyopathy. Eur Heart J. 1999;20(14):1044–1050.
- Cardim N, Brito D, Rocha Lopes L, et al. The Portuguese Registry of Hypertrophic Cardiomyopathy: overall results. Rev Port Cardiol. 2018;37(1):1–10.
- Daubert C, Gadler F, Mabo P, et al. Pacing for hypertrophic obstructive cardiomyopathy: an update and future directions. Europace. 2018;20(6):908–920.
- Qintar M, Morad A, Alhawasli H, et al. Pacing for drug-refractory or drug-intolerant hypertrophic cardiomyopathy. Cochrane Database Syst Rev. 2012;(5):CD008523.
- Nistri S, Olivotto I, Betocchi S, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy). Am J Cardiol. 2006;98(7):960–965.
- Jensen MK, Havndrup O, Hassager C, et al. Survival and sudden cardiac death after septal ablation for hypertrophic obstructive cardiomyopathy. Scand Cardiovasc J. 2011;45(3):153–160.
- Maron BJ, Rowin EJ, Casey SA, Link MS, et al. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J Am Coll Cardiol. 2015;65(18):1915–1928.