Abstract
Objectives. Infective endocarditis has potential for severe complications and high mortality. The number of patients with prosthetic valves has risen, and an increase in incidence of infective endocarditis has been suggested. We aimed to examine the epidemiology, etiology, treatment and outcome of patients admitted to Division of Cardiovascular and Pulmonary Diseases at Oslo University Hospital, and explore changes in incidence over the last four years. Design. We conducted a retrospective study including all patients admitted to a tertiary hospital in Oslo, Norway, and diagnosed with infective endocarditis according to ICD-10 between 2014 and 2017. Results. Two hundred and ninety-one patients ≥18 years were included (61.3 ± 13.8 years, 75.6% men). 36.4% had previous valve surgery and this proportion decreased during the period. The aortic valve was most commonly affected (51.9%). Streptococci were the most frequent microorganisms (35.1%), while staphylococci accounted for 26.8%. 81.8% were treated surgically, at a median of 6.5 (0–120) days after admission. Hemodynamic changes or instability was the primary surgical indication (51.5%). One-year mortality was 20.6%. Surgery within a week after admission resulted in poorer 1-year prognosis than surgery after one week. Also, surgically treated patients who died were significantly older than those who survived. Conclusions. In this cohort, streptococci were the most common causative microorganism. Approximately, one-third of the patients had prosthetic valves. Mortality remains high, underscoring the need for continuous medical awareness. A high number of streptococcus infections in this cohort suggest dental origin.
Introduction
Infective endocarditis is defined as an infection of native or prosthetic heart valves, the endocardium or a cardiac device [Citation1–4]. It is most often presenting as an acute or subacute disease, and has potentially severe complications and high mortality. Treatment consists of antibiotics and possibly surgery, with surgery increasingly being used [Citation5]. Prophylactic antibiotics during dental or other surgical procedures in high risk patients is reasonable, but has disadvantages and lacks sufficient evidence [Citation6]. As the number of patients with prosthetic valves and cardiac devices has risen, an increase in the number of patients with infective endocarditis has been suggested. However, previous reports have shown stable or only a slight increase in incidence [Citation7–9].
Our hypothesis was that the incidence of patients with infective endocarditis referred to our hospital is on the rise, and that prosthetic valve endocarditis contributes to an increasing share of these cases. To investigate this, we explored patients with infective endocarditis admitted to the Department of Cardiology and the Department of Cardiothoracic Surgery at Division of Cardiopulmonary and Pulmonary Diseases, Oslo University Hospital (OUH). This division serves as a tertiary referral center for surgical treatment of endocarditis in southern and eastern Norway, covering approximately 50% of the Norwegian population of 5.3 million (2018).
We wanted to characterize the patients referred to the division over a four-year period, and to describe and discover possible changes in epidemiology, etiology and treatment in this population.
Materials and methods
Patient selection
All records of patients admitted to the Department of Cardiology or the Department of Cardiothoracic Surgery at Division of Cardiovascular and Pulmonary Diseases at OUH with new infective endocarditis according to ICD-10 codes, I33-Acute and subacute endocarditis and I38-Endocarditis, valve unspecified [Citation10], from January 2014 through December 2017, were reviewed. Cases of infective endocarditis to the same patient more than 12 months apart, or any new infection with a different causative microorganism, were counted as new cases of infective endocarditis.
A total of 322 cases in 319 patients were retrieved. Eleven patients were excluded as they were diagnosed before the study’s timeframe, and four patients were only admitted for echocardiographic control after treatment. Infective endocarditis was ruled out in one patient on the basis of negative clinical features and negative cultures of excised valve tissue, and one patient had a coding error in the medical records. Two patients were excluded due to lacking medical records, and nine patients were omitted because they were <18 years old. All patients were diagnosed according to the Dukes criteria of endocarditis [Citation11] by the doctor writing the discharge report. A total of 294 cases of infective endocarditis in 291 patients were finally included. In three patients with relapsing infective endocarditis, only the first episode was registered. These three patients had significant comorbidity (drug abuse, severe cancer or immunosuppressive treatment).
Data collection
Epidemiological data, microbial agent, infection site, use of echocardiography, choice of treatment and mortality were registered. Epidemiological data included age, sex and status of the infected valve. Valve status was divided into native, unoperated valves or previous valve surgery. The latter was further divided into biological valve replacements, mechanical valve replacements and valvuloplasties.
We separated between conservative treatment and surgical treatment. Surgical indications were divided into four groups: (1) hemodynamic indication including acute heart failure and major valve insufficiency, (2) uncontrolled infection or sanitizing the focus of infection, (3) septic embolization or high risk of septic embolization, and (4) multiple indications. Time to surgery was calculated from admission date to the date of operation in our hospital. One-year mortality and total mortality was registered. Total all-cause mortality was calculated in the complete population, with a mean observational time of 36 months (spread 12–61 months).
Statistical analysis
Data were systematized and analyzed in Microsoft Excel 2017. Upon comparing two groups, chi-square test was used for categorical variables and two-tailed t-tests for continuous variables. Categorical data were presented with frequencies and percentages and continuous data with average and standard deviation, unless otherwise specified. Relative risk (RR) and hazard ratio (HR) were presented with 95% confidence intervals. Two-tailed p value <.05 was considered statistically significant.
This retrospective study was approved by the data protection officer at OUH. All patient data were anonymized.
Results
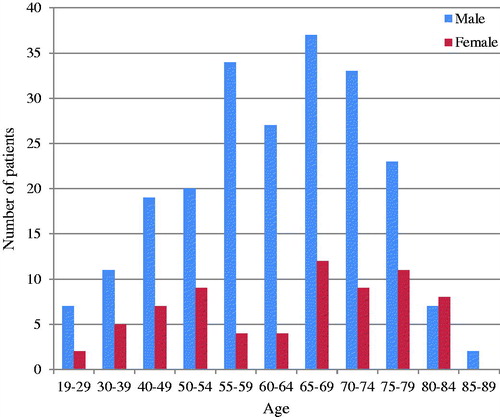
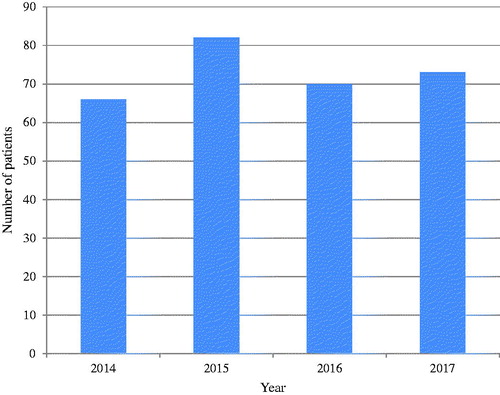
Two hundred and ninety-one patients were included, with a male to female ratio of 3:1. The annual number of patients with new infective endocarditis was fairly consistent as illustrated in . Average age was 61.3 ± 13.8 years and median was 64 years (IQR 53–72), with showing that patients aged 65.0–69.9 were most frequently admitted. There was no significant difference (p=.44) in average age between sexes (women (62.4 ± 15.4 years) vs. men (61.0 ± 13.3 years)). Furthermore, there were no significant differences in gender distribution between the microbial agents. Regarding age, patients with Staphylococcus aureus endocarditis were significantly younger compared to the other microbial agents (57.5 ± 14.1 vs. 62.2 ± 13.7; p=.02).
Figure 1. Yearly incidence of infective endocarditis: Number of patients admitted to Division of Cardiovascular and Pulmonary Diseases at Rikshospitalet, Oslo University Hospital, between 2014 and 2017.
Valve involved
Approximately, 2/3 of the patients had native valves when diagnosed with infective endocarditis, while the remaining 1/3 had undergone previous valve surgery (). The aortic valve was most commonly affected, being the sole infection site in half of the cases. Second most affected was the mitral valve, contributing to a quarter of all cases. 14.8% had infection in multiple valves, most often the combination of the aortic and mitral valve ().
Figure 3. Percentage of infected valves that were native, unoperated or previously operated. Previously operated valves were further divided into biological valve replacements, mechanical valve replacements and valvuloplasties.
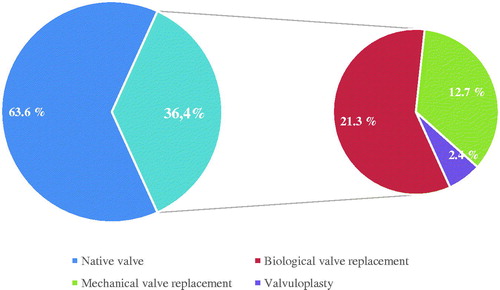
Table 1. Characteristics of the 291 patients with infective endocarditis.
Biological valve replacements accounted for more than half of all previous valve surgeries and mechanical valve replacements for roughly 1/3. The proportion of patients with previous valve surgery decreased significantly (p=.03) when comparing the two first years (2014–2015, 42.6%) with the two latter (2016–2017, 30.1%). Also, “other staphylococci” (not including Staphylococcus aureus) infected patients who had undergone previous valve surgery significantly more often than Staphylococcus aureus, enterococci and streptococci (RR = 2.44; 95% CI 1.72–3.46; p<.001). This difference was a result of other staphylococci infecting biological valves more frequently than the other main agents (RR = 3.48; 95% CI 2.23–5.43; p<.001).
Microbial agent
Streptococci were the most frequent microbial agent, followed by staphylococci and enterococci (). Summarized, they were the causative organism in 3/4 patients. Alpha-hemolytic streptococci accounted for roughly 2/3 of all streptococcus infections, consisting mainly of Streptococci viridans (n = 61). 12.4% had either unspecified, unknown or negative blood culture yields (). In the whole group, streptococci accounted for significantly more cases (p<.001) in native valve infective endocarditis (n = 79, 42.7%) than in prosthetic valve- or valvuloplasty-endocarditis (n = 23, 21.7%).
Table 2. Distribution of microbial agents.
Echocardiography
89.0% (n = 259) were examined by transthoracic echocardiography (TTE) at the referring hospital while 77.0% (n = 224) received a transesophageal echocardiography (TEE). Including examinations done at local hospitals, 99.3% (n = 289) received a TTE and 88.3% (n = 257) a TEE in total.
Treatment
The vast majority, 81.8% (n = 238), were treated surgically, whereas 18.2% (n = 53) only received conservative treatment with antibiotics (). Upon arrival at our hospital, the median time to operation was 6.5 days (IQR 3–14, spread 0–120). Surgically treated patients were significantly younger than conservatively treated patients.
Table 3. Characteristics of patients with infective endocarditis treated surgically (n = 238) and conservatively (n = 53).
Hemodynamic indication was the main surgical indication in half of the operations. One in four patients had surgery performed due to multiple indications, whilst septic embolization was listed as primary indication in 15.5% ().
Patients with native valve infective endocarditis received surgery at significantly higher rates than those with previous valve surgery, while patients with biological valve replacements received surgery significantly less often than other patients. Furthermore, patients with mechanical valve replacements (83.8%) were operated non-significantly (p=.15) more often than those with biological valve replacements (71.0%).
Rate of surgery was not significantly affected by gender. However, alpha-hemolytic streptococci-endocarditis was treated with surgery significantly more often than other microbial agents (RR = 1.15; 95% CI 1.05–1.27; p=.02). Also, strictly left sided infective endocarditis was operated significantly more often than other cases of infective endocarditis (84.2% vs. 56.0%, p<.001). No differences where seen regarding surgical indication between the microbial agents.
Mortality
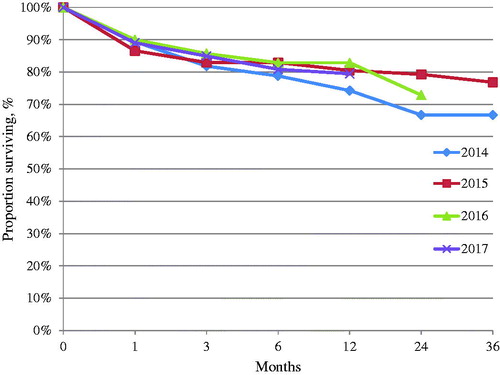
One-year mortality was 20.6% (n = 60), with yearly variation as illustrated in . Total all-cause mortality in the cohort was 27.1% (n = 79). One-year mortality was higher in women than in men (RR = 1.67; 95% CI 1.06–2.64; p=.03). Additionally, patients who died within a year were significantly older than the survivors (). Mortality in patients with previous valve surgery did not differ significantly from patients with native valve endocarditis (23.6% vs. 18.9%, p=.34).
Table 4. Characteristics of patients with infective endocarditis who were alive (n = 231) and dead (n = 60) after 1 year.
Endocarditis with alpha-hemolytic streptococci had significantly lower 1-year mortality (RR = 0.35; 95% CI 0.16–0.78; p=.02) rates than other agents (). Staphylococcus infections were only non-significantly (p=.056) more lethal than streptococci. Staphylococcus aureus, other staphylococci, beta-hemolytic streptococci and enterococci had non-significantly higher 1-year mortality rates than other agents (25.5% vs. 21.9%, p=.13). However, for total mortality, they were associated with significantly higher mortality rates (32.4% vs. 21.9%; RR = 1.48; 95% CI 1.01–2.18; p=.04). Infection site had no significant impact on mortality, yet a trend indicated worse outcome in left sided infective endocarditis (21.4% mortality) vs. other infection sites (12% mortality).
Close to half of all conservatively treated patients deceased within a year, the portion being significantly higher (HR = 4.16; 95% CI 2.18–7.95; p<.001) than in those who were surgically treated. Patients who received surgery within a week after admission had poorer 1-year prognosis than patients surgically treated after one week (RR = 2.03; 95% CI 1.03–3.99; p=.03). Moreover, surgically treated patients who died (69.1 ± 10.2 years) were significantly (p<.001) older than those who survived (58.9 ± 13.7 years).
Discussion
Our study is in accordance with other reports at some topics, but also highlights points that may be specific for surgical referral centers and therefore differ from previous investigations.
First of all, our results regarding patient characteristics are in consistency with what is previously reported regarding age and the majority of men [Citation8,Citation12–16]. Since our hospital is a tertiary care referral center for cardiothoracic surgery, cases in elderly, comorbid patients and eventual other patients deemed clearly inoperable by cardiologists at the community hospitals were most likely not referred. Therefore, a population-based study in our region would probably result in even higher patient age. On the other hand, younger intravenous drug-abusers are perhaps also underrepresented in our population, as indicated by a relatively low number of right sided valve endocarditis in our study. Still, our data support the fact that infective endocarditis is a disease mostly affecting the elderly. The increased life expectancy in the general population results in more frequent degenerative valve disease and an increasing amount of people living with structural heart disease, thereby predisposing for infective endocarditis [Citation1,Citation4,Citation7,Citation17,Citation18].
The overall proportion of patients who had undergone previous valve surgery (36.4%) is higher in our study than in other studies, where it mostly ranges from 15% to 31% [Citation8,Citation12,Citation15,Citation16,Citation19–21]. With an increasing number of valve replacements performed and increasing survival among these patients [Citation22], the at-risk population for infective endocarditis is numerically growing. However, so is also the general population, and we have not found that there is an increase in the portion of patients with prosthetic valve endocarditis relative to native valves. The high proportion of patients who had undergone previous valve surgery in our study is most likely because of guidelines, which define endocarditis in previously valve-operated patients as complex and suggest referral of these patients to tertiary centers.
The valve involvement varies in previous studies. Aortic valve involvement was most common in our population, as also seen in other studies [Citation14,Citation15,Citation19]. On the other hand, newer investigations present the mitral valve as the primary point of infection [Citation8,Citation13]. The frequent involvement of the aortic valve as seen in our material may be because the aortic valve is a common site of degenerative valve disease, thus making these valves vulnerable for infection. It might also reflect a tendency to refer and operate aortic valves more often than other sites of infective endocarditis.
Interestingly, streptococci prevail as the most common microorganism in this study, which is similar to some population-based studies [Citation9,Citation15]. However, the findings contradicts most studies, both Norwegian [Citation14,Citation20] and international [Citation8,Citation12], which have documented S. aureus as the most causative microbial agent. Considering this, our discovery probably does not represent the true distribution of microorganisms in patients with infective endocarditis in Norway. It does, however, confirm that many cases still arise from dental foci, despite broad international consensus that invasive procedures, health care contact, prosthetic valves and other S. aureus-related risk factors are now the most important predisposing factors [Citation2,Citation3,Citation7,Citation18].
In relation to this, questions regarding antibiotic prophylaxis still need to be focused on. Prophylaxis has been restricted to only the highest-risk patients in guidelines [Citation23], explained by the low risk of infective endocarditis in relation to dental procedures, and focusing more on the cumulative risk from repetitive bacteremia through daily activities like toothbrushing [Citation24]. No conclusion regarding prophylaxis can be drawn from our study, as it would need a different study design, and data on how the patients have been treated according to guidelines was not available. However, the high number of oral streptococci suggests the need for maintained focus on prophylaxis.
Furthermore, in the last couple of decades, tendency to treat infective endocarditis with early valve surgery has become clearer [Citation5]. Still, surgery was performed (81.8%) considerably more often at our division than in other reports, ranging from 32% to 63% [Citation1,Citation8,Citation12,Citation14,Citation15,Citation19]. It mainly reflects our position as a referral center for complex endocarditis and surgery, but may also to some extent reflect a lower threshold to perform surgery in patients with infective endocarditis. Surgery has been shown to be essential for survival in some previous investigations [Citation12,Citation13,Citation19], while other studies have suggested that there is no survival benefit from surgery in infective endocarditis [Citation25,Citation26]. Finally, one study shows that such benefit only exists when there are clear surgical indications [Citation27]. Therefore, the effect of surgery in infective endocarditis needs further evaluation.
We observed that native valve infective endocarditis was surgically treated significantly more often than prosthetic valve endocarditis. This probably reflects that patients with prosthetic valve endocarditis are generally more comorbid, making them more likely to have contraindications for surgery, but this was not investigated further in the study. Nevertheless, this is an interesting finding.
Different end-points are used in different studies, but the 1-year mortality rate among our patients (20.6%) is still in the lower region of what is seen in other reports at 6 months [Citation1,Citation13]. The microbial distribution in our study might explain this finding, with streptococci being less virulent than staphylococci. Nonetheless, mortality remains high, in spite of improved diagnostic tools, clear guidelines and better surgical treatment than in previous decades. High age, female gender and conservative treatment were associated with high 1-year mortality in our study, whereas infection with alpha-hemolytic streptococci and surgery seemed prognostically beneficial for the patients. Patients in the conservatively treated group included those who had contraindications for surgery, thereby contributing to this difference. The reason for female gender being associated with worse outcome is unclear, but may be due to comorbidity such as diabetes mellitus and immunosuppression as discussed in other reports [Citation21].
Limitations
Our study was conducted at a single tertiary center with a cardiac surgery program. Therefore, patients included here are subject to referral bias, and the data are unlikely to reflect the true epidemiology in the Norwegian population. However, many studies on infective endocarditis are from tertiary centers, making the data comparable to previous investigations [Citation12,Citation13,Citation19]. Second, the diagnosis of infective endocarditis is based on the ICD-10 coding by the clinical cardiologist or surgeon, and not by a stringent, systematical use of the Dukes criteria. On the other hand, these criteria form the basis of the clinical work at our division. Given the high rate of surgical treatment and verification of the diagnosis, probability of false positive diagnosis seems low. Third, being a retrospective study, the study relies on adequate journal documentation. Finally, variation over time or between centers can also be explained by a change in virulence in the bacterial flora, of which data were not available in this study.
Conclusions
To conclude, the three major findings in our study are: (1) oral streptococci still cause a significant number of infective endocarditis cases, (2) patients who have undergone previous valve surgery are contributing to a high proportion of all infective endocarditis cases, and (3) mortality rates remain high. This underscores the seriousness of infective endocarditis, and the need for continuous medical awareness to reduce mortality.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387:882–893.
- Werdan K, Dietz S, Loffler B, et al. Mechanisms of infective endocarditis: pathogen–host interaction and risk states. Nat Rev Cardiol. 2014;11:35–50.
- Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8:322–336.
- Beynon RP, Bahl VK, Prendergast BD. Infective endocarditis. BMJ. 2006;333:334–339.
- Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132:1025–1035.
- Kaya CT, Erol C. How to achieve infective endocarditis prophylaxis. ESC. 2018 [updated 2018 Dec 12; cited 2019 Feb 4]. Available from: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-16/vol16no33
- Ambrosioni J, Hernandez-Meneses M, Tellez A, et al. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep. 2017;19:21.
- Selton-Suty C, Celard M, Moing L, et al. Pre-eminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infec Dis. 2012;54:1230–1239.
- Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–3028.
- WHO. ICD-10. International Statistical Classification of Diseases and Related Health Problems 10th Revision; 2016. Available from: https://icd.who.int/browse10/2016/en
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638.
- Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169:463–473.
- Hill EE, Herijgers P, Claus P, et al. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J. 2006;28:196–203.
- Gulati G, Hole T, Eide E. Infectious endocarditis in a Norwegian hospital 1997–2006. Tidsskriftet. 2011;131:115–117.
- Hoen B, Alla F, Selton-Suty C, et al. Changing profile of infective endocarditis: results of a 1-year survey in France. JAMA. 2002;288:75–81.
- Curlier E, Hoen B, Alla F, et al. Relationships between sex, early valve surgery and mortality in patients with left-sided infective endocarditis analysed in a population-based cohort study. Heart. 2014;100:1173–1178.
- Yew HS, Murdoch DR. Global trends in infective endocarditis epidemiology. Curr Infect Dis Rep. 2012;14:367–372.
- Prendergast BD. The changing face of infective endocarditis. Heart. 2006;92:879–885.
- Loupa C, Mavroidi N, Boutsikakis I, et al. Infective endocarditis in Greece: a changing profile. Epidemiological, microbiological and therapeutic data. Clin Microbiol Infect. 2004;10:556–561.
- Husebye T, Smith G, von der Lippe E, et al. Infectious endocarditis at Ulleval hospital 1988–94. Echocardiographic investigation. Tidsskr nor Laegeforen. 1998;118:222–225.
- Aksoy O, Meyer LT, Cabell CH, et al. Gender differences in infective endocarditis: pre- and co-morbid conditions lead to different management and outcomes in female patients. Scand J Infect Dis. 2007;39:101–107.
- Reinöhl J, Kaier K, Reinecke H, et al. Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med. 2015;373:2438–2447.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–3128.
- Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125.
- Tleyjeh IM, Ghomrawi HM, Steckelberg JM, et al. The impact of valve surgery on 6-month mortality in left-sided infective endocarditis. Circulation. 2007;115:1721–1728.
- Sy RW, Bannon PG, Bayfield MS, et al. Survivor treatment selection bias and outcomes research: a case study of surgery in infective endocarditis. Circ Cardiovasc Qual Outcomes. 2009;2:469–474.
- Chu VH, Park LP, Athan E, et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation. 2015;131:131–140.