Abstract
Objectives
Atrial fibrillation is the most common arrhythmia occurring after cardiac surgery. Less attention has been focused on preoperative atrial fibrillation and anaemia as risk factors for mortality after cardiac surgery. The aim of this study was to determine preoperative risk factors for 30-d mortality after open-heart surgery. Design. The study population consisted of 2015 patients (73.4% men; mean age 68 years) undergoing coronary artery bypass grafting (CABG) (52.0%), aortic valve replacement (AVR) (18.6%), AVR and CABG (10.0%), mitral valve plasty or replacement (14.0%), and AVR and aortic root reconstruction (ARR) (5.5%) in Kuopio University Hospital from January 2013 to December 2016. Univariate and multivariate Cox proportional hazards models were used for statistical analyses. Kaplan–Meier survival curves were generated. Results. Total 30-d mortality was 1.8%. By Cox regression analysis, predictors of 30-d mortality (hazard ratio [HR] [95% confidence interval [CI]]) included female gender (1.95 [1.00–3.77]), preoperative atrial fibrillation, (2.38 [1.12–5.03]) reduced haemoglobin level (3.40 [1.47–7.90]), and pulmonary congestion (3.16 [1.52–6.55]). The combination of preoperative reduced haemoglobin and preoperative atrial fibrillation was a strong predictor (12.37 [4.40–34.77], p < .001). Estimated glomerular filtration rate (eGFR) predicted 30-d mortality in univariate models but was not an independent predictor in multivariate models. Conclusions. According to the main findings of our study, the combination of preoperative atrial fibrillation and reduced haemoglobin level substantially increase the risk of 30-d mortality after cardiac surgery. Identification of high-risk patients pre-operatively could help to make optimal clinical decisions for timing of operation and perioperative treatment.
Introduction
Worldwide, more than 2 million patients undergo cardiac surgery each year [Citation1]. The reported in-hospital mortality rate in cardiac surgery has decreased over the years and ranges from 2.5% to 3.4% in different studies [Citation2,Citation3], while 30-d mortality varies between 1% and 4% [Citation4–6]. Identification of high-risk patients pre-operatively could help to make optimal clinical decisions for timing of operation, surgery technique, and perioperative treatment.
Several risk models have been proposed for predicting mortality in cardiac surgery. The best-known risk models are European System for Cardiac Operative Risk Evaluation (EuroSCORE II) [Citation7], Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models score (STS) [Citation8–10], and Age, Creatinine, Ejection fraction score (ACEF II) [Citation11]. The simplest of these is ACEF II, which takes into account five risk factors. EuroSCORE II includes 18 and STS 64 determinants. The STS score is not only used for assessing mortality risk but also the risk of other post-operative events such as stroke and renal failure, as well as the length of hospital stay. Age, ejection fraction (EF), renal function, and emergency versus elective surgery are included in all these risk models.
This study aims to define predictors for 30-d mortality in cardiac surgery. Consensus is lacking whether preoperative atrial fibrillation, preoperative reduced haemoglobin level, and decreased estimated glomerular filtration rate (eGFR) are independent predictors for increased 30-d mortality.
Patients and methods
Ethics
The study was approved by the Ethics Committee of Kuopio University Hospital (No. 1694/13.02.00/2019). The study complies with the Declaration of Helsinki.
Study design and patient population
The data were collected retrospectively from Kuopio University Hospital cardiac surgery registry, which comprises 2559 adult patients undergoing cardiac surgery in Kuopio University Hospital from January 2013 to December 2016. The registry does not include emergency or urgent surgery patients. Patients undergoing coronary artery bypass grafting (CABG), aortic valve replacement (AVR), AVR in combination with CABG, mitral valve plasty (MVP) or mitral valve replacement (MVR), and aortic root reconstruction (ARR) were included in the study, without an upper age limit. Patients with other cardiac surgery like ventricular or atrial septal defect surgery (n = 349) and patients with incomplete or conflicting data (n = 195) were excluded.
There were altogether 2015 patients in the study, aged from 20 to 90 years with a mean age of 67.7 years. Detailed preoperative clinical characteristics are described in . The median length of the hospital stay was 4 d with an interquartile range (IQR) of 4 − 6 d. A total of 249 patients (12.9%) needed intensive care unit (ICU) treatment and the median length of the ICU stay was 2 d with an IQR of 1 − 5 d and a maximum ICU stay length of 32 d. The outcome was all-cause mortality during the first 30 d after the index surgical procedure.
Table 1. Preoperative clinical characteristics according to the categories of cardiac surgery. The values are means (standard deviations) or n (%).
Data analysis and statistical methods
The data are presented in terms of means with standard deviations, medians with IQRs, or frequencies with percentages when appropriate. Univariate analyses were used to evaluate possible predictors of 30-d mortality. The analysed predictors were age, sex, previous cardiac operations, diabetes (data available for 80% of patients), endocarditis, preoperative atrial fibrillation, current cigarette smoking, preoperative pulmonary congestion, New York Heart Association (NYHA) class, haemoglobin, body mass index (BMI) < 20 kg/m2, BMI ≥ 35 kg/m2, EF < 40%, the clinical diagnosis of arteriosclerosis obliterans (ASO), and eGFR. eGFR was estimated both by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [Citation12] and modification of diet in renal disease (MDRD) [Citation13] equations. Haemoglobin level and eGFR were dichotomized (in case of haemoglobin, separately for men and women) by receiver characteristics curve (ROC) analysis and Youden’s index. The term “reduced” will be used for haemoglobin values below the cutoff values which were 143.5 g/l for males and 127.5 g/l for females. The cutoff for eGFR was 60 ml/min/1.73 m2. Hazard ratios (HR), 95% confidence intervals (CI), and p values were calculated for each predictor using univariate Cox regression model. p Values <.05 were considered statistically significant. Kaplan–Meier curves were generated, and the log-rank test was applied. Finally, a multivariate Cox regression analysis was performed using the backward Wald method. The cutoff probability for stepwise entry and removal were 0.05 and 0.10, respectively. The analyses were performed with the IBM SPSS statistics version 25.0 software for Windows (SPSS Inc., Chicago, IL).
Results
Altogether 37 patients (1.8%) died within the first 30 d after the surgery, with highest (5.5%) 30-d mortality rate in the group of patients with AVR in combination with CABG, and lowest (1.1%) in patients with CABG. In the other groups, the 30-d mortality rates were 1.6% (AVR), 1.8% (MVP/MVR), and 2.7% (ARR). The 30-d mortality rate was slightly higher in women (2.8%; 15/521) than in men (1.5%; 22/1457), with a p value of .053. The mean age of survivors was 67.7 years (SD 10.5) and that of non-survivors 70.1 years (SD 8.8); p = .138.
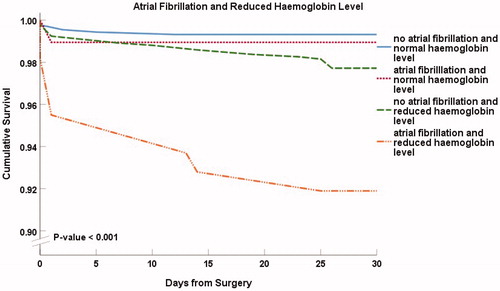
Depicted by Kaplan–Meier curves (), the 30-d survival was lower in patients with pulmonary congestion (93.0% versus 98.6%, p < .001), with NYHA class ≥3 (97.8% versus 98.9%, p = .09), with preoperative atrial fibrillation (95.1% versus 98.5%, p < .001), and with decreased renal function (96.4% versus 98.5%, p < .01) than in those without the condition.
Figure 1. Thirty-day survival curves for selected patient subgroups. Kaplan–Meier presentations with p values from the log-rank tests. Panel A: New York Heart Association (NYHA) class 3 or 4. Panel B: Pulmonary congestion. Panel C: Renal function. eGFR indicates estimated glomerular filtration rate. Panel D: Preoperative atrial fibrillation.
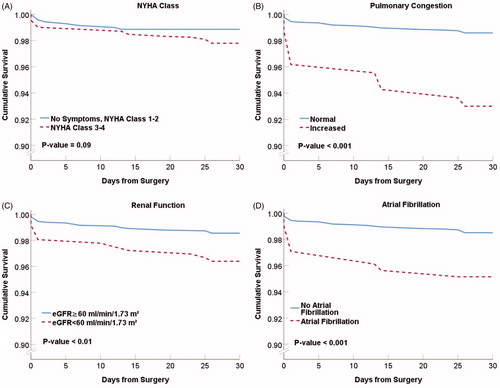
shows Kaplan–Meier 30-d survival curves for males and females with reduced and normal haemoglobin level (p < .001). The survival was lower in females with normal and reduced haemoglobin level compared to males. Thirty-day survival was 99.6% (n = 684) among males and 98.6% (n = 294) among females with normal haemoglobin. Patients with reduced haemoglobin had a lower 30-d survival of 97.6% (n = 790) in males, and 95.5% (n = 242) in females.
Figure 2. Thirty-day survival in male and female patients with normal or reduced haemoglobin levels. Kaplan–Meier presentation with p value from the log-rank test.
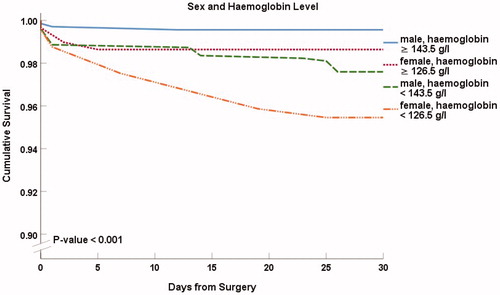
In , we show Kaplan–Meier curves for 30-d survival combining renal function and haemoglobin level (p < .001 between the groups). Among patients with normal haemoglobin level, decreased renal function did not alter survival (99.2%, n = 118) in comparison to patients with normal renal function (99.3%, n = 860). Survival was slightly decreased in patients with reduced haemoglobin level alone (97.7%, n = 791). A marked reduction of survival was observed in patients with both decreased renal function and reduced haemoglobin (95.0%; n = 241).
Figure 3. Thirty-day survival according to renal function and the level of haemoglobin. Hb < 143.5 g/l in males and <127.5 g/l in females was considered reduced. eGFR indicates estimated glomerular filtration rate. Kaplan–Meier presentation with p value from the log-rank test.
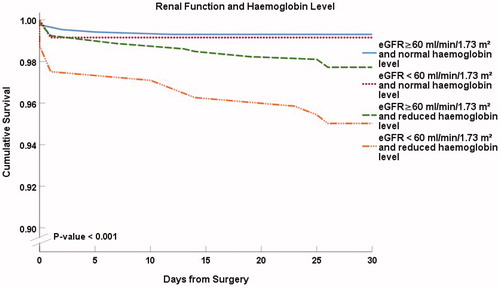
Kaplan–Meier curves in combine preoperative atrial fibrillation and the level of haemoglobin (p < .001 between the groups). Atrial fibrillation alone did not essentially affect 30-d survival, but the patients with reduced haemoglobin level alone had lower survival rate than those with normal haemoglobin level, 97.7% (n = 921) versus 99.3% (n = 883). The patients with the combination of preoperative reduced haemoglobin and preoperative atrial fibrillation had the lowest survival, 91.9% (n = 111) suggesting a more than additive effect. Patients with both a reduced preoperative haemoglobin level and preoperative atrial fibrillation had a high mortality risk with a HR of 12.37 (95% CI 4.40–34.77), p < .001, compared to patients without both atrial fibrillation and reduced haemoglobin.
Figure 4. Thirty-day survival according to the presence of preoperative atrial fibrillation and haemoglobin level. Kaplan–Meier presentation with p value from the log-rank test.
shows HRs (95% CI) derived from univariate Cox regression models for 30-d mortality after cardiac surgery. Age, BMI < 20 kg/m2, BMI ≥ 35 kg/m2, previous cardiac operation, NYHA 3–4 class, diabetes, endocarditis, or current smoking were not statistically significant explanatory factors. Sex was of borderline significance (p = .056). Of surgical procedures, AVR + CABG versus only CABG was associated with 30-d mortality with a close-to five-fold HR. The HR for CKD-EPI eGFR < 60 ml/min/1.73 m2 was 2.52 (95% CI 1.28–4.94) and, for comparison, that for MDRD based eGFR < 60 ml/min/1.73 m2 was 2.35 (1.20–4.62). Higher HRs were observed for lower cutoffs of eGFR: 2.77 (1.08–7.12) for eGFR < 45 ml/min/1.73 m2 and 4.94 (1.19–20.54) for eGFR < 30 ml/min/1.73 m2.
Table 2. Univariate Cox regression model hazard ratios for 30-d mortality.
shows HRs and p values derived from multivariate Cox regression models. The last step of the multivariate Cox regression analysis included ASO (NS), female sex, preoperative atrial fibrillation, preoperative pulmonary congestion, and reduced haemoglobin.
Table 3. Multivariate Cox regression model hazard ratios (HR) for 30-d mortality risk factors.
Discussion
The main result of our study was that preoperative atrial fibrillation, reduced haemoglobin level, pulmonary congestion, and female gender appeared as independent risk factors for 30-d mortality in multivariate regression analysis. eGFR predicted 30-d mortality in univariate models but did not appear as an independent predictor in multivariate models. Most importantly, the combination of preoperative reduced haemoglobin and preoperative atrial fibrillation resulted in a substantial increase of 30-d mortality.
Our study has some limitations. First, the retrospective nature of the study does set inherent limitations for the interpretation of the study results. In addition, our study was a single-centre study, and, therefore, the results cannot be generalized to all cardiac surgery populations. Moreover, data on diabetes were incomplete in our database, including information on 80% of patients. Diabetes is recognized as one of the most important cardiovascular risk factors. However, the role of diabetes on 30-d mortality after cardiac surgery is unclear. In a recent study on diabetes and ethnicity as risk factors for cardiac surgery, diabetes did not increase 30-d mortality although the postoperative hyperglycaemia did increase the risk of acute kidney injury [Citation14]. Another limitation was that data on medication were available only for anticoagulants. Data on cardiovascular medication would have been nice additional information but would have remained mainly descriptive, not allowing meaningful statistical comparisons due to the differences in basic pathology of various heart diseases across patients groups (e.g. ischaemia, pressure versus volume load).
The 30-d mortality observed in our study (1.8%) was on the same level as in previous studies, varying from 1% to 4% [Citation4–6]. In our study, there was a significant difference in 30-d mortality between males and females in multivariate analysis, with a slightly higher mortality rate in women. The finding is in concordance with several previous study results. Vogt et al. [Citation4] described an odds ratio of 1.26 (95% CI 1.07–1.50) for females in a study of 10,525 cardiac surgery patients. In a study of CABG patients, Saxena et al. [Citation15] described an odds ratio of 1.24 (95% CI 0.95–1.61) for females. Higher mortality in females is taken into account in STS and EuroSCORE II but not in ACEF II.
In this study, preoperative pulmonary congestion was the only cardiac failure-associated clinical finding that was shown to be an independent risk factor for 30-d mortality. NYHA class ≥3 was not found to be a risk factor for 30-d mortality in our study. Cardiac failure determined by NYHA class ≥ 3 or EF < 40% has been reported to be a risk factor with a HR of 1.98 (95% CI 1.67–2.34) [Citation4]. In another study [Citation15] NYHA class ≥ 3, EF < 45% and history of congestive heart failure were risk factors with HRs of 1.43 (95% CI 1.10–1.87), 1.94 (95% CI 1.49–2.53), and 1.89 (95% CI 1.43–2.49), respectively. Reduced EF is a determinant in all of the above-mentioned risk models. NYHA class is part of STS and EuroSCORE II, but pulmonary congestion is not taken into account in any of these three risk models.
Acute kidney injury is considered as one of the most important complications after open-heart surgery in adult patients [Citation16]. Among the risk factors associated with acute kidney injury in cardiac surgery, one of the most frequent ones is pre-existing chronic kidney disease [Citation1]. Chronic kidney disease is an independent risk factor for mortality in non-surgical setting [Citation17], and decreased eGFR is an established mortality risk factor, not only after non-cardiac surgery [Citation18], but also after cardiac surgery [Citation19]. In a study on CABG patients, renal failure increased 30-d mortality with a HR of 1.84 (95% CI 1.22–2.77) [Citation15]. In a systematic review and meta-analysis focusing on cardiac surgery, the risk of death within 30 d was three-fold in patients with eGFR less than 60 ml/min/1.73 m2 [Citation20]. The eGFR and renal replacement therapy are included in EuroSCORE II, creatinine level and renal replacement therapy in STS score and creatinine level alone in ACEF II score.
As decreased renal function is often associated with reduced level of haemoglobin, its role as an independent predictor is not settled. Our study suggested a substantial risk of reduced eGFR in association with reduced preoperative haemoglobin in cardiac surgery. However, in multivariate analyses, eGFR was not an independent predictor of 30-d mortality.
Anaemia is common in patients having cardiac surgery. In a large (n = 114,277) meta-analysis the prevalence of anaemia was as high as 20.6%. Anaemic patients undergoing cardiac surgery often have also other risk factors, such as chronic kidney disease, poor left ventricular EF, arrhythmias, advanced age, and female gender [Citation21,Citation22]. Zindrou et al. noted that in-hospital mortality rate after surgery was five-fold among individuals with a preoperative haemoglobin concentration of 100 g/l or less in comparison to those with a higher haemoglobin concentration. Preoperative anaemia was reported as a risk factor for 30-d mortality in a large meta-analysis, including also cardiac surgery patients. Fourteen reports, including close to 900,000 patients, identified, but eight reports (including close to 60,000 patients) did not identify anaemia as an independent risk factor for mortality [Citation23]. A meta-analysis of 22 studies and 114,277 patients showed a HR of 2.74 (95% CI 2.32–3.24) for 30-d or in-hospital mortality after cardiac surgery in anaemic patients [Citation24]. In a study on possible predictors for 30-d mortality after mitral valve replacement, the only independent risk factor was haematocrit level (p = .017) [Citation25]. These findings are in agreement with those of our study, in which reduced haemoglobin was an independent risk factor with a 3.4-fold HR for 30-d mortality after cardiac surgery. Haemoglobin level is not recognized as a risk modifier in EuroSCORE II though haematocrit is recognized in STS and ACEF II.
Several previous studies have described association of preoperative atrial fibrillation with 30-d mortality. However, preoperative atrial fibrillation is considered as a risk factor only in the STS score. Preoperative atrial fibrillation was a risk factor for elderly patients undergoing CABG [Citation26], and an independent risk factor for patients undergoing AVR [Citation27]. Among 21,534 patients who underwent isolated CABG, preoperative atrial fibrillation was associated with increased 30-d mortality with a HR of 1.63 (95% CI 1.17–2.29) [Citation15]. Moreover, 30-d mortality was higher in the preoperative atrial fibrillation group (6.2%) versus sinus rhythm group (4.4%) in 1800 matched patients undergoing any cardiac operation [Citation28]. In contrast to these findings, no association between preoperative atrial fibrillation and 30-d mortality (1.6% versus 1.9%, p = .79) was observed in 257 matched patients undergoing CABG [Citation29]. Overall, the majority of the evidence supports the view that preoperative atrial fibrillation is a risk factor for 30-d mortality, compatible with our own finding.
In conclusion, preoperative atrial fibrillation, reduced haemoglobin level, female gender, and pulmonary congestion were independent risk factors for 30-d mortality after open heart surgery. Most importantly, the combination of preoperative atrial fibrillation and reduced haemoglobin level substantially increased the risk of 30-d mortality after cardiac surgery. The combination of preoperative atrial fibrillation and reduced haemoglobin level should be evaluated for inclusion in future risk models.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711.
- Mazzeffi M, Zivot J, Buchman T, et al. In-hospital mortality after cardiac surgery: patient characteristics, timing, and association with postoperative length of intensive care unit and hospital stay. Ann Thorac Surg. 2014;97(4):1220–1225.
- Barili F, Pacini D, Rosato F, et al. In-hospital mortality risk assessment in elective and non-elective cardiac surgery: a comparison between EuroSCORE II and age, creatinine, ejection fraction score. Eur J Cardiothorac Surg. 2014; 46(1):44–48.
- Vogt A, Grube E, Glunz HG, et al. Determinants of mortality after cardiac surgery: results of the Registry of the Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte (ALKK) on 10 525 patients. Eur Heart J. 2000; 21(1):28–32.
- Sanagou M, Wolfe R, Forbes A, et al. Hospital-level associations with 30-day patient mortality after cardiac surgery: a tutorial on the application and interpretation of marginal and multilevel logistic regression. BMC Med Res Methodol. 2012;12:28.
- Siregar S, Groenwold RH, de Mol BA, et al. Evaluation of cardiac surgery mortality rates: 30-day mortality or longer follow-up? Eur J Cardiothorac Surg. 2013;44(5):875–883.
- Nashef SAM, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg. 2012;41(4):734–745.
- Shahian DM, O'Brien SM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1):S2–S22.
- O’Brien SM, Shahian DM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Annals Thorac Surg. 2009;88(1):S23–S42.
- Shahian DM, O’Brien SM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: part 3—valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1):S43–S62.
- Ranucci M, Pistuddi V, Scolletta S, et al. The ACEF II risk score for cardiac surgery: updated but still parsimonious. Eur Heart J. 2018;39(23):2183–2189.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254.
- Moorthy V, Liu W, Chan SP, et al. Elucidation of the novel role of ethnicity and diabetes in poorer outcomes after cardiac surgery in a multiethnic Southeast Asian cohort. J Diabetes. 2020;12(1):58–65.
- Saxena A, Kapoor J, Dinh DT, et al. Preoperative atrial fibrillation is an independent predictor of worse early and late outcomes after isolated coronary artery bypass graft surgery. J Cardiol. 2015;65(3):224–229.
- Bove T, Monaco F, Covello RD, et al. Acute renal failure and cardiac surgery. HSR Proc Intensive Care Cardiovasc Anesth. 2009;1(3):13–21.
- Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047.
- Cywinski JB, Mascha EJ, Kurz A, et al. Estimated glomerular filtration rate better predicts 30-day mortality after non-cardiac surgery than serum creatinine: a retrospective analysis of 92,888 patients. Can J Anesth. 2015;62(7):745–752.
- Mooney JF, Croal BL, Cassidy S, et al. Relative value of cystatin C and creatinine-based estimates of glomerular filtration rate in predicting long-term mortality after cardiac surgery: a cohort study. BMJ Open. 2019;9(9):e029379.
- Mooney JF, Ranasinghe I, Chow CK, et al. Preoperative estimates of glomerular filtration rate as predictors of outcome after surgery: a systematic review and meta-analysis. Anesthesiology. 2013;118(4):809–824.
- Zindrou D, Taylor KM, Bagger JP. Preoperative haemoglobin concentration and mortality rate after coronary artery bypass surgery. Lancet. 2002;359(9319):1747–1748.
- Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation. 2007;116(5):471–479.
- Fowler AJ, Ahmad T, Phull MK, et al. Meta‐analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102(11):1314–1324.
- Padmanabhan H, Siau K, Curtis J, et al. Preoperative anemia and outcomes in cardiovascular surgery: systematic review and meta-analysis. Ann Thorac Surg. 2019;108(6):1840–1848.
- Reisman AM, Thomas AT, Boateng P, et al. Predictors of 30-day outcomes following mitral valve repair. Ann Med Surg (Lond). 2019;47:5–12.
- Böning A, Diegeler A, Hilker M, et al. Preoperative atrial fibrillation and outcome in patients undergoing on-pump or off-pump coronary bypass surgery: lessons learned from the GOPCABE trial. Interact Cardiovasc Thorac Surg. 2015;20(1):74–78.
- Kvidal P, Bergström R, Malm T, et al. Long-term follow-up of morbidity and mortality after aortic valve replacement with a mechanical valve prosthesis. Eur Heart J. 2000;21(13):1099–1111.
- Attaran S, Shaw M, Bond L, et al. A comparison of outcome in patients with preoperative atrial fibrillation and patients in sinus rhythm. Ann Thorac Surg. 2011;92(4):1391–1395.
- Ngaage DL, Schaff HV, Mullany CJ, et al. Does preoperative atrial fibrillation influence early and late outcomes of coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2007;133(1):182–189.
