Abstract
Objectives
We report the mid-term outcomes of valve-sparing aortic root replacement (VSRR) in a cohort including patients with bicuspid aortic valve (BAV), connective tissue disorder (CTD), aortic dissection (AD), and congenital heart disease (CHD). Design. From 2005 to 2017, 174 patients underwent VSRR with the reimplantation technique. The mean age was 46 ± 14 years. The mean follow-up time was 4.8 ± 2.8 years. The indication for operation was aortic aneurysm for 127 (73%), aortic insufficiency (AI) for 38 (22%), and AD for 9 patients (5%). Preoperatively, 53 patients (31%) had ≥ moderate AI. BAV, CTD (Marfan or Loyes-Dietz), previous Ross procedure, or CHD was present in 57 (33%), 28 (16%), 7 (4%) and 12 patients (7%), respectively. Concomitant aortic valve repair was performed for 103 patients (59%). Results. Thirty-day mortality was zero. Four patients underwent aortic valve replacement (AVR) during follow-up. Kaplan–Meier estimates for survival, freedom from AVR, and freedom from ≥ moderate AI or reoperation were 96, 98, and 97% at 5 years. There was no difference in survival, freedom from AVR, or freedom from ≥ moderate AI or reoperation in patients with and without BAV, CTD, leaflet repair, or preoperative ≥ moderate AI. In Cox regression analysis, BAV, CTD, aortic valve repair, preoperative ≥ moderate AI, or aortic dimension were not risk factors for reoperation or valve dysfunction. Conclusions. Mid-term outcomes of VSRR for patients with diverse indications in terms of survival, reoperation rate, and valve dysfunction rate were excellent in a center with a limited annual volume of VSSR.
Introduction
Valve-sparing aortic root replacement (VSRR) is an established method for the treatment of aortic root dilatation and aortic valve insufficiency (AI) [Citation1]. Durable mid-term results have also been published with extended indications including patients with acute aortic dissections (ADs), previous surgery for congenital heart disease (CHD), and connective tissue disorders (CTD) [Citation2–4]. Clear benefits of this method are the preservation of the native aortic valve resulting in improved hemodynamics and the avoidance of life long systemic anticoagulation, especially in young patients [Citation5,Citation6].
Risk factors for long term valve durability and valve reoperations are presently unclear. Bicuspid valve aortopathy, aortic leaflet repair, and diameter of aortic root have been reported to associate with late aortic regurgitation and reoperations after VSRR in some series [Citation7,Citation8]. There have been concerns that preserving aortic valve leaflets in patients with CTD might be a risk of repair failure because of tissue weakness [Citation5]. The reported results concerning the reoperation rate after VSRR in comparison to the composite valve graft in patients with CTD have been partly conflicting [Citation9–11], but VSRR is generally accepted as the preferred method for treatment of aortic root dilatation and aortic valve regurgitation in patients with CTD.
The purpose of this study was to report the midterm outcomes of VSRR and aortic valve repair in a cohort that included diverse indications and high-risk patients, and to determine possible risk factors for reoperation and valve dysfunction.
Materials and methods
We retrospectively reviewed 174 consecutive patients who underwent VSRR with or without leaflet repair in the Helsinki University Hospital between 2005 and 2017. The local hospital research council approved the study and waived the need for patient consent. Electronic patient records were reviewed and if the patients were followed at other hospitals, copies of patient records were obtained. Time of death and cause of death was collected from the registry of the National Institute for Health and Welfare, and from death certificates. Demographic data of the patients are presented in . Fifty-seven (33%) patients had a BAV, 28 (16%) patients had CTD, 12 (7%) patients had CHD, and 7 (4%) patients had previous Ross operation.
Table 1. Demographics and preoperative data.
Indications for surgery
The indication for surgery was aortic aneurysm for 127 (73%) patients, aortic insufficiency (AI) for 38 (22%) patients, and type A AD for 9 patients (5%).
Surgical technique
All 174 patients underwent valve-sparing aortic root replacement from standard median sternotomy with the reimplantation technique. David I technique was used for 48 (28%) patients, David IV for one (1%) patient, and David V including the Stanford modification for 116 (67%) patients. Valsalva graft was used in 21 (12%) operations. Aortic valve repair was performed for 103 patients (59%). Aims of the repair were no or trace residual AI and coaptation height of at least 5 mm. Operative data are presented in . Seventy-four concomitant procedures were performed, and details of these procedures are presented in . Details of leaflet repair techniques are presented in .
Table 2. Operative and perioperative data.
Table 3. Details of leaflet repair techniques.
Echocardiographic and clinical follow-up
Clinical data was collected from medical records. Intraoperative echocardiography was performed to evaluate residual AI grade. Residual AI was not reported for 5 (3%) patients. Transthoracic echocardiographic was performed before discharge, after three months, and yearly thereafter. The mean follow-up time was 4.8 ± 2.8 years and follow-up was 95% complete for echocardiography. However, the median follow-up time of patients with BAVs was significantly shorter than the median follow-up time of patients with tricuspid valves (3.8 ± 3.5 vs. 5.1 ± 4.2 years p = .009). There were no differences in the median follow-up times of patients with previous Ross procedure, CHD, aortic diameter > 55 mm, aortic valve repair, or CTD. Also, the median follow-up time of patients with AI as the indication for the operation was significantly shorter than the median follow-up time of patients with either aortic aneurysm or dissection as the indication (2.7 ± 2.4 vs. 5.1 ± 4.1 p < .001). Mortality data was collected from the National Institute for Health and Welfare and was 100% complete.
Statistical analysis
All-cause mortality, valve-related reoperation, and freedom from greater than mild AI or valve reoperation were defined as the primary outcomes. Mann–Whitney U test was used for continuous variables. Change in LVEF and LVEDD during follow-up was analyzed with paired samples t-test. Survival, reoperation, and freedom from greater than mild valve insufficiency or reoperation were analyzed with the Kaplan–Meier estimates and Logrank test. Cox regression analysis was used for multivariable analysis of valve durability. Previous Ross procedure, CHD, aortic diameter > 55 mm, BAV, aortic valve repair technique (no repair, simple cusp plication or more complex), indication for operation, and CTD were covariates in the multivariable model. Selection of covariates was based on reported risk factors for recurrent AI in the literature [Citation5,Citation7,Citation8]. Statistical analyses were performed with the SPSS, version 24 (Chicago, IL). P values <0.05 were considered significant and all p-values were two-tailed.
Results
Mortality and complications
The 30-day mortality was 0 (0%). Five (3%) patients died during follow-up. Six (3%) patients underwent reoperation for bleeding and nine (5%) patients needed drainage for pericardial effusion (four subxiphoid fenestrations and five re-sternotomies). The indication for drainage was pericardial tamponation in one patient and pre-tamponation or large pericardial effusion in eight patients. Five (3%) patients had post-pericardiotomy syndrome, two (1%) patients had stroke, four (2%) patients had pulmonary embolism, two (1%) had renal failure, and four (2%) needed a permanent pacemaker. There were three (2%) early reoperations not related to the aortic valve. One patient underwent early tricuspid valve repair for severe regurgitation, one patient underwent closure of an iatrogenic ventricular septal defect, and one patient underwent mitral valve repair for severe regurgitation. In addition, there were two (1%) late reoperations not related to the aortic valve. One patient underwent reconstruction of the descending thoracic aorta for dilatation of dissected aorta and one patient underwent mitral valve repair for severe regurgitation. Mean stay in the intensive care unit was 2 ± 7 days and mean stay in hospital was 8 ± 10 days. Overall survival was 96.1 ± 2.0% at 5 years. Patients with tricuspid aortic valve had 95 ± 3% survival and patients with bicuspid aortic valve (BAV) had 100% survival at 5 years (Logrank p = .209). In age-adjusted Cox regression analysis, there was no difference in survival of patients with bicuspid in comparison to patients with tricuspid valves (p = .734). Patients with CTD had 94 ± 6% survival and patients without connective disorder had 97 ± 2% survival at 5 years (Logrank p = .920).
Valve durability and echocardiographic follow-up
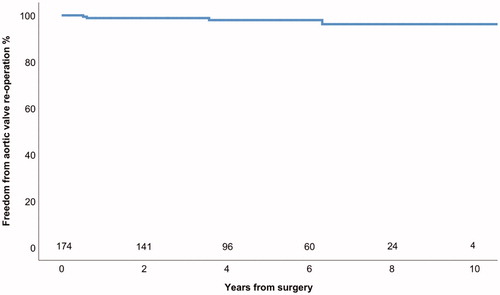
Four (2%) patients underwent aortic valve replacement (AVR) due to severe AI, in addition 2 patients (1%) had greater than mild AI during follow-up. All aortic valve reoperations were AVRs. Actuarial freedom from AVR was 98 ± 1% at 5 years for all patients (). The mechanism of valve failure and severe AI in all of the reoperated patients was new valve prolapse (). Patients with tricuspid aortic valve had 97 ± 2% and patients with BAV had 98 ± 2% actuarial freedom from AVR at 5 years (Logrank p = .914) (). Patients with CTD had 95 ± 5% and patients without connective disorder had 99 ± 1% actuarial freedom from AVR at 5 years (Logrank p = .070) (). Patients with moderate or severe preoperative AI had 98 ± 2% and patients with less severe preoperative AI or without AI had 98 ± 1% freedom from AVR at 5 years (Logrank p = .877). Patients who underwent aortic valve leaflet repair had 99 ± 1% freedom from AVR at 5 years and those who did not undergo aortic valve leaflet repair had 97 ± 3% freedom from AVR at 5 years (Logrank p = .209) (). In patients with ascending aortic diameter >55 mm freedom from aortic valve reoperation was 98 ± 2% and in patients with aortic diameter of 55 mm or less 98 ± 2% at 5 years (Logrank p = .554).
Figure 2. Kaplan–Meier estimates of freedom from aortic valve reoperation in patients with bicuspid and tricuspid aortic valves.
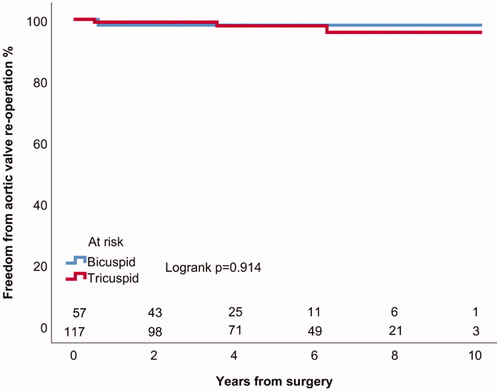
Figure 3. Kaplan–Meier estimates of freedom from aortic valve reoperation in patients with and without connective tissue disorder.
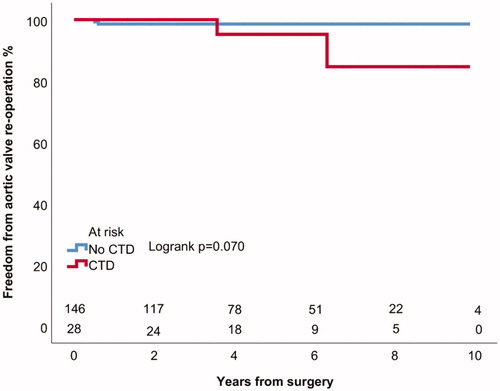
Figure 4. Kaplan–Meier estimates of freedom from aortic valve reoperation in patients with and without aortic leaflet repair.
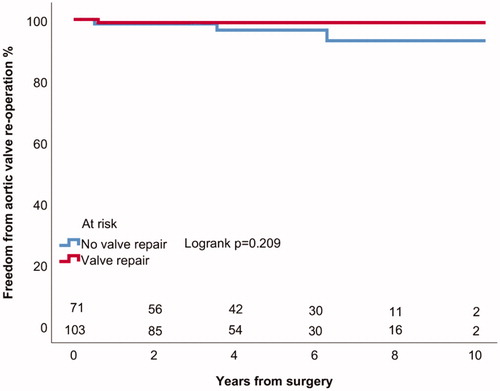
Table 4. Details of aortic valve reoperations.
During follow-up 144 (83%) patients had no or trace aortic regurgitation, 24 (14%) patients had mild aortic regurgitation, 2 (1%) patients had moderate regurgitation and 4 (2%) patients had severe regurgitation. All patients with severe aortic regurgitation underwent AVR. Freedom from greater than mild AI or AVR was 97 ± 2% at 5 years for all patients. Patients with tricuspid aortic valve had 98 ± 2% and patients with BAV had 95 ± 4% freedom from greater than mild AI or AVR at 5 years (Logrank p = .715). Patients with CTD had 95 ± 5% and patients without connective disorder had 98 ± 1% freedom from greater than mild AI or AVR at 5 years (Logrank p = .170). Patients with moderate or severe preoperative AI had 94 ± 5% and patients with less severe preoperative AI or without AI had 97 ± 3% freedom from greater than mild AI or AVR at 5 years (Logrank p = .680). Patients who underwent aortic valve leaflet repair during VSRR had 98% and patients who did not undergo leaflet repair had 97 ± 3% freedom from greater than mild AI or AVR at 5 years (Logrank p = .278). In patients with aortic diameter >55 mm freedom from AVR or greater than mild AI was 98 ± 2% and patients with aortic diameter of 55 mm or less freedom from AVR or greater than mild AI was 96 ± 2% at 5 years (Logrank p = .377). In the latest echocardiography, mean left ventricle ejection fraction was 59 ± 8% and left ventricle end-diastolic dimension (LVEDD) was 53 ± 7 mm (significant remodeling compared to preoperative LVEDD 60 ± 8 mm p < .001).
In multivariable analysis, BAV, CTD, aortic diameter >55 mm, aortic valve leaflet repair technique, indication for operation, CHD, or previous Ross procedure were not associated with aortic valve reoperation or the composite endpoint of greater than mild AI or aortic valve reoperation.
Discussion
Our data demonstrate excellent overall mid-term outcomes of VSRR in a cohort including patients with extended indications. Perioperative mortality was zero, and late mortality, late aortic valve insufficiency rate, and reoperation rate were low. Overall survival was 96% at 5 years, which is excellent considering the inclusion of high-risk patients with ADs, reoperations after previous Ross procedure, and patients with CHD. BAV morphology, CTD, aortic valve repair, preoperative AI grade or aortic dimension did not have an impact on outcomes.
The overall freedom from AVR was 98% at 5 years and the freedom from AVR or more than mild AI was 97% at 5 years. These results are comparable with some previous outstanding results [Citation1,Citation8,Citation12,Citation13]. The David reimplantation procedure stabilizes the aortic annulus and therefore all operations in our series were reimplantation operations. Our series included different modifications of the David procedure and a number of procedures with the Valsalva graft, as described previously [Citation14]. However, currently, we do not believe that different modifications of the David technique have a significant impact on outcomes, although more physiological function of the reimplanted valve and theoretically improved outcomes have been suggested with the David V modification or with the Valsalva graft [Citation15]. In our more recent experience with aortic valve repair our aim has been to reduce the size of the annulus to normal, most commonly to under 25 mm, depending on patient size, and to plicate the valve cusps in order to achieve an effective height of at least 9 mm as suggested by the Homburg group [Citation16]. In BAV repair, we believe that a repair with 180 to 180-degree commissural orientation is reproducible and allows for exact adjustment of coaptation and effective height. In general, bicuspid valves with commissural orientation >160° and pliable leaflets after possible shaving were considered feasible for repair.
In our series, the reason for reoperation was severe AI due to new valve cusp prolapse in every case. Even though the exact mechanism of failure is difficult to ascertain in a retrospective series, it is probable that lack of proper annular stabilization with migration or incomplete fixation of the prosthesis contributed to these failures. We therefore believe that meticulous dissection of the aortic root and positioning of the prosthesis as close to the level of the virtual annular ring as possible especially in the area of the right coronary cusp is important in order to achieve proper annular stabilization. In fact, in the last 85 operations in our institution, there have been no failures so far.
Bicuspid aortic valve has been suggested to be a risk factor for late valve dysfunction after VSRR [Citation7,Citation8]. In our data, patients with bicuspid valve had comparable results in comparison to patients with tricuspid valves. Some other groups have also reported similar midterm VSRR outcomes between tricuspid and bicuspid valve [Citation13,Citation17]. Also, in the midterm, outcomes of VSRR and the Bentall procedure for bicuspid valves have been comparable [Citation17]. Unfortunately, it is possible that bicuspid valves will degenerate later after more than a decade, as recently reported by Klotz et al. and longer follow-up is needed to confirm this finding [Citation18].
Connective tissue weakness in aortic valve patients with CTD (fibrillin-1 mutation in Marfan syndrome and tissue growth factor beta pathway gene mutations in Loyes-Dietz) and risk of repair failure and reoperations has been a concern in VSRR [Citation5]. In our data, no difference was observed between patients with and without CTD. Furthermore, comparable results have been reported earlier in many series in patients with Marfan syndrome [Citation1,Citation5,Citation7,Citation19]. Patel reported a high reoperation rate in patients with Loyes–Dietz syndrome after aortic root surgery, but the reoperations were not related to the aortic valve [Citation20]. Reports comparing the risk of reoperation of Marfan patients after VSRR and root replacement with a composite graft have been conflicting, some favoring VSRR, some favoring the composite graft, and others not being able to demonstrate a difference between the two. A recent meta-analysis did not detect a difference in the reoperation risk [Citation5]. We analyzed the Marfan and Loyes–Dietz patients together, because of the low number of reoperations and the small number of patients with Loyes–Dietz. Furthermore, due to the low overall rate of reoperations in this series and patients with CTD results must be interpreted with caution.
In the present series, 58% of the patients underwent aortic valve leaflet repair during VSRR and the indication for the operation was significant AI for 20% of the patients. Furthermore, preoperative AI was at least moderate in 28% of the patients. In these cases, complex valve repair that addresses root pathology at all of the levels of the aortic root is necessary. In a recent report, preoperative AI severity did not impact valve durability [Citation12] and our finding was similar. In our data, valve repair was not a risk factor for valve reoperation. Conflicting results have been published earlier [Citation8], but our data indicates that, with the techniques used complex aortic valve repairs can be performed with good midterm results. However, we did not use pericardial patch repairs to reconstruct severely damaged valve cusps since these repairs tend to fail due to calcification [Citation21].
Aortic root dimension greater than 55 mm, has been suggested to be a risk factor for late valve dysfunction after VSRR [Citation7]. In this study, aortic dimension was not a risk factor for late aortic valve reoperation. However, in our data, the greatest aortic dimension of the ascending aorta was recorded, which was not necessarily the dimension of the aortic root.
Study limitations
There are a number of limitations in this study. First of all, it is a retrospective report from a single center and selection bias of patients and confounding by indication is possible. Due to the retrospective nature of the study, it was impossible to determine which patients received VSRR or aortic valve repair and which patients received root replacement with a composite graft or AVR. During the first 3 years of the study period, the volume of VSRR operations was low and increased throughout the study period. Measurement of AI grade by echocardiography may be challenging, and thus not fully accurate. All follow-up data was not 100% complete for every patient. In our data, overall reoperation rate was low which makes analysis of risk factors for reoperations and valve dysfunction challenging due to limited statistical power. Larger numbers of operated patients with longer follow-up and a higher number of events in the future will need further evaluation.
Conclusions
Overall mid-term outcomes of VSRR and aortic valve repair in terms of survival, valve dysfunction, and reoperation rate were excellent in a patient cohort including patients with BAV, severe aortic valve insufficiency, CHD, and type A AD. Bicuspid aortic valve, preoperative AI grade, aortic valve repair, CTD, or aortic dimension did not increase the risk of reoperation or valve dysfunction. We conclude that herein we report excellent midterm outcomes of VSRR for patients with diverse indications, in a center with a limited annual volume of VSSR.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- David TE, Feindel CM, David CM, et al. A quarter of a century of experience with aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2014;148(3):872–879.
- Baliulis G, Ropponen JO, Salmon TP, et al. Valve-sparing aortic root replacement in adult patients previously operated for congenital heart defects: an initial experience. Eur J Cardiothorac Surg. 2016;50(1):155–159.
- Valo J, Jokinen JJ, Kaarne M, et al. Expanding indications for valve-sparing aortic root reconstruction: early and midterm results. Ann Thorac Surg. 2013;95(2):579–585.
- Kvitting JP, Kari FA, Fischbein MP, et al. David valve-sparing aortic root replacement: equivalent mid-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg. 2013;145(1):117–126.
- Flynn CD, Tian DH, Wilson-Smith A, et al. Systematic review and meta-analysis of surgical outcomes in marfan patients undergoing aortic root surgery by composite-valve graft or valve sparing root replacement. Ann Cardiothorac Surg. 2017;6(6):570–581.
- Price J, De Kerchove L, Glineur D, et al. Risk of valve-related events after aortic valve repair. Ann Thorac Surg. 2013;95(2):606–612.
- Esaki J, Leshnower BG, Binongo JN, et al. Risk factors for late aortic valve dysfunction after the David V valve-sparing root replacement. Ann.Thorac.Surg. 2017;104(5):1479–1487.
- Settepani F, Cappai A, Basciu A, et al. Impact of Cusp repair on reoperation risk after the David procedure. Ann.Thorac.Surg. 2016;102(5):1503–1511.
- Gaudino M, Lau C, Munjal M, et al. Contemporary outcomes of surgery for aortic root aneurysms: a propensity-matched comparison of valve-sparing and composite valve graft replacement. J Thorac Cardiovasc Surg. 2015;150(5):1120–1129.e1.
- Martens A, Beckmann E, Kaufeld T, et al. Shrestha, valve-sparing aortic root replacement (David I Procedure) in Marfan disease: single-centre 20-year experience in more than 100 patients. Eur J Cardiothorac Surg. 2019;55(3):476–483.
- Zehr KJ, Matloobi A, Connolly HM, et al. Surgical management of the aortic root in patients with Marfan syndrome. J Heart Valve Dis. 2005;14:121–128.
- Keeling WB, Leshnower BG, Binongo J, et al. Severity of preoperative aortic regurgitation does not impact valve durability of aortic valve repair following the David V valve sparing aortic root replacement. Ann Thorac Surg. 2017;103(3):756–763.
- Bavaria JE, Desai N, Szeto WY, et al. Valve-sparing root reimplantation and leaflet repair in a bicuspid aortic valve: comparison with the 3-Cusp David procedure. J Thorac Cardiovasc Surg. 2015;149(2):S22–S228.
- de Kerchove L, Boodhwani M, Glineur D, et al. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg. 2011;92(2):749–751.
- Demers P, Miller DC. Simple modification of "T. David-V" valve-sparing aortic root replacement to create graft pseudosinuses. Ann Thorac Surg. 2004;78(4):1479–1481.
- Aicher D, Kunihara T, Issa OA, et al. Schafers, valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123(2):178–185.
- Vallabhajosyula P, Szeto WY, Habertheuer A, et al. Bicuspid aortic insufficiency with aortic root aneurysm: root reimplantation versus bentall root replacement. Ann Thorac Surg. 2016;102(4):1221–1228.
- Klotz S, Stock S, Sievers HH, et al. Survival and reoperation pattern after 20 years of experience with aortic valve-sparing root replacement in patients with tricuspid and bicuspid valves. J Thorac Cardiovasc Surg. 2018;155(4):1403.e1–1411.e1.
- David TE, David CM, Manlhiot C, et al. Outcomes of aortic valve-sparing operations in Marfan syndrome. J Am Coll Cardiol. 2015;66(13):1445–1453.
- Patel ND, Crawford T, Magruder JT, et al. Cardiovascular operations for Loeys-Dietz syndrome: intermediate-term results. J Thorac Cardiovasc Surg. 2017;153(2):406–412.
- Ram E, Moshkovitz Y, Shinfeld A, et al. Pericardial patch augmentation is associated with a higher risk of recurrent aortic insufficiency. Ann Thorac Surg. 2018;106(4):1171–1177.