Abstract
Objectives
Peripartum cardiomyopathy (PPCM) is a pregnancy-associated and life-threatening cardiac disease. However, the causes and pathogenesis are not fully understood. Accumulating studies show that cardiomyopathy often appears to be associated with elevated levels of β1-adrenoceptor (β1AR) antibodies, indicating a possible involvement of β1AR antibodies in the development of PPCM.
Design
We injected the antigen peptide segment of the β1AR into the postpartum Wistar rats to make the immune models and their cardiac function was detected by echocardiography. Also, the concentration of β1AR antibodies and apoptosis rate of left ventricular myocytes was tested by SA-ELISA, TUNEL, HE staining, qRT-PCR and western blot methods. Finally, the expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and its related proteins were examined by qRT-PCR and western blot methods.
Results
We found that the level of β1AR antibodies in the serum was significantly increased and the postpartum rats exhibited symptoms of PPCM after autoimmunity. Moreover, the expression of peroxisome PGC-1α, which was a master regulator of mitochondrial metabolism, and its downstream transcript vascular endothelial growth factor (VEGF), was decreased in autoimmune perinatal rats. In addition, the expression of the apoptosis factor caspase 3 as well as the apoptosis rate of left ventricular myocytes was significantly increased.
Conclusions
The results suggested that the symptoms of PPCM that appeared in autoimmune perinatal rats may be due to the increase of β1AR antibodies, which inhibited the pathway associated with peroxisome PGC-1α.
Introduction
Peripartum cardiomyopathy (PPCM) is a kind of dilated cardiomyopathy and defined as idiopathic cardiomyopathy. The pregnant women present with heart failure secondary to left ventricular systolic dysfunction at the end of pregnancy or in the months following delivery for unknown reasons [Citation1–3]. The disease is relatively rare, but its incidence is rising [Citation1]. Only 25% of patients with PPCM in developing countries survive up to five years, with associated infant mortality of 50–75% [Citation4]. Because the cause of the disease remains unknown, management of PPCM is mostly limited to the same neurohormonal antagonists used in other forms of cardiomyopathy, and no validated disease-specific therapies yet available [Citation1].
Beta-1-adrenoceptor (β1AR) belongs to the family of G-protein-coupled receptors. In heart, β1AR induce positive chronotropic, inotropic and dromotropic action on myocardial through stimulatory Gs protein [Citation5]. In the past two decades, β1 AR antibodies were found in the serum of healthy people as well as patients with several cardiovascular diseases characterized by heart failure [Citation6–8]. Moreover, it is worth noting that although patients with PPCM have no prior history of heart disease and there are no other known possible causes of heart failure, our previous study found that there was a high correlation between β1AR antibodies and the development of PPCM [Citation9]. However, at present, there is no direct evidence that β1AR antibodies can induce PPCM and the mechanisms that how the β1AR antibodies cause the disease remain completely unclear.
In this study, we injected the antigen peptide segment of the β1AR into the postpartum Wistar rats to induce the animals developing autoimmunity. We found that the level of β1AR antibodies in the serum was significantly increased and the animals showed the PPCM symptoms. Furthermore, a recent study showed that cardiac-specific deletion of proliferator-activated receptor-gamma coactivator-1α (PGC-1α) (a potent regulator of mitochondrial metabolism) develops profound PPCM [Citation10]. Besides, PCG-1α drives the expression of vascular endothelial growth factor (VEGF), the most widely studied angiogenic factor [Citation11]. We also found that the expression of PGC-1α and VEGF were decreased after animal’s autoimmunity. It suggests that the β1AR antibodies may be responsible for PPCM through PGC-1α pathway.
Materials and methods
Animals and active immunity
Twelve postpartum Wistar rats were obtained from the Model Animal Research Center of Nanjing University. Six animals were used to build models and six animals as control. All the animals were maintained in specific pathogen-free (SPF) conditions under a 12 h-light-12h-dark cycle. All experiments were conducted under the guidelines and regulations of the Institutional Animal Care and Use Committee of the Nanjing University. The antigenic peptide segment of β1AR was purchased from GL Biochem Company (Shanghai, China). Although the timing of PPCM was uncertain. Demakis and Rahimtoola [Citation12] originally noted that most cases occur in the initial weeks after delivery, and some others also found that the incidents were higher in the first week after delivery [Citation1,Citation13,Citation14]. We considered that during this period, the mother would be more sensitive to the etiology. Thus, we started modeling on the sixth day after delivery. Modeling time lasts for three weeks. For first immunization, the antigenic peptide segment was dissolved into Na2CO3 solution and mixed this solution with freund’s incomplete adjuvant 1:1. The final concentration of an antigenic peptide segment in the mixture was 1 mg/ml. Then the mixture was injected subcutaneously in the back of each animal with an antigenic peptide segment 0.4 μg/g. After two weeks of first immunization, the immune process was repeated. For the control group, the antigen mixture was replaced by the same amount of Na2CO3 solution while as the experimental scheme was the same as the immunological group. At the end of the third week, all the animals were sacrificed after the ultrasound experiment.
Terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL) of myocardial cells
Left ventricular myocardium TUNEL staining was implemented to recognize apoptotic nuclei. The myocardial tissues were surgically removed, frozen and cut into 10-mm thick sections in a freezing cryostat. A fluorometric TUNEL detection kit (Genecopoeia, Rockerville, MD) was used to detect apoptotic DNA strand breaks. The tissue sections were fixed with 4% neutral buffered formaldehyde in PBS (pH 7.4) at 25 °C for 20 min, permeated with proteinase K at 25 °C for 20 min and incubated with the labeling reaction mixture in a humidified chamber at 37 °C for 2 h. Pre-digestion of tissue section with nuclease served as a positive control. The sections were then processed with a standard immunocytochemical staining procedure to incubate with antibody against DAPI (a cell nucleus marker; Invitrogen, Carlsbad, CA). Finally, a leica fluorescence microscope (DM6000B, Leica, Wetzlar, Germany) was used to capture the images and the ratio of TUNEL positive nuclei in total (DAPI positive nuclei) was computed to express the cardiac myocytes’ apoptosis.
Echocardiography
Transthoracic echocardiography was performed using the Ultra High-Frequency Imaging Platform Vevo 2100 system (FujiFilm, Toronto, Canada) equipped with a 15-mHz transducer on the last day of each week. Rats were anesthetized with 3% sodium pentobarbital and the core temperature (36–37 °C) was continuously monitored. Cardiac geometry and functional parameters, the left ventricular ejection fraction (LVEF), left ventricular shortening fraction (LVFS), left ventricular end-systolic diameter (LVESD) and left ventricular end-diastolic diameter (LVEDD) were measured from images obtained using two-dimensional B-mode and M-mode views.
SA-ELISA
At 0th, 2nd and 3rd weeks after immunization, blood samples were taken from rat tail’s vein and serum was separated and stored in −80 °C refrigerators. To test the serum level of β1AR antibodies, SA- ELISA was performed. Precisely, the antigen peptide segment of β1ARin phosphate Na2CO3 solution (pH 11) was used as coating antigen at the optimal concentration (2.5 μg/50 μl/well). After blocking nonspecific sites, the rat serum (diluted 1:10with PBS; 200 μl/well) was added and incubated for 1 h at 37 °C. The wells were then incubated for 1 h at 37 °C with an antibody labeled with biotin anti-rat monoclonal IgG (1:1,000, 50 μl/well) (BOSTER, Cat#: BA1005). Next, streptomycin with horseradish peroxidase (HRP) (1:500, 50 μl/well) (Bioss, Cat#: bs-0437P-HRP) was added into the wells and incubated for 1 h at 37 °C. Finally, chromogenic tetramethylbenzidine (TMB) solution (50 μl/well) was added into each well and incubated for 10–30 min at 37 °C. Optical density (OD) was measured at 450 nm on a microplate reader (Erba Lisa Scan II, Mannheim, Germany).
HE staining
Thin slices of myocardium tissue for all cases received in our histopathology unit, were fixed in 4% formaldehyde solution (pH 7.0) for time periods not exceeding 24 h. The tissues were processed routinely for paraffin embedding and 4 μm-thick sections were cut and placed on glass slides. Tissue samples were stained with hematoxylin and eosin.
Quantitative real time-PCR (qRT-PCR)
Total RNA of heart tissues was isolated using Ultrapure RNA Kit (Cat#: CW0581S, CWBio, Cambridge, MA). Of 400 ng of RNA was subjected to reverse transcription-PCR with SuperRT cDNA Synthesis Kit (Cat#: CW0741S, CWBio) according to the instruction. Quantitative RT-PCR was performed with PCR primers listed in Supplemental Table. Ultra SYBR Mixture Kit (Cat#: CW2602M, CWBio) was employed to detect mRNA levels of these genes. All reactions were repeated 3 times and GAPDH was used to normalize target.
Western blot analysis
Heart tissues were lysed in RIPA buffer (P0013C, beyond time) containing 1 mM PMSF (ST505, beyond time). Total protein of 30 μg was subjected to electrophoresed on 12–6% SDS-Page gels and transferred to PVDF membranes. Antibodies against β1AR (1:1000; cat. bs-0498R, Bioss, Woburn, MA), VEGF (1:800; cat. AF5131, Affinity), PGC-1α (1:1000, cat. bs-1832R, Bioss), β-arrestin 1/2 (1:1000; cat. bs-0857R, Bioss), P-VASP (1:800; cat. Ab194747, Abcam, Cambridge, UK), VASP (1:800; cat. ab205378, Abcam) and caspase 3 (1:1000; cat. bs-0081R, Bioss) were used as primary antibodies. Rabbit IgG antibodies coupled to HRP were used as secondary antibodies. GAPDH (1:1000; cat. BA2913, Boster, Pleasanton, CA) was used as a loading control. An enhanced chemiluminescence (ECL) system was used for detection of protein bands.
Statistical analysis
All data were presented as mean and SEM. One-way ANOVA was conducted to evaluate the one-way layout data. When a significant difference was observed, Bonferroni’s post-hoc test was conducted to identify groups with significant differences. The relative mRNA levels were calculated using the 2−ΔΔCt method. All analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL). Differences were considered significant with p < .05.
Results
Antigen peptide segment of the β1AR injection elevated level of β1AR antibodies
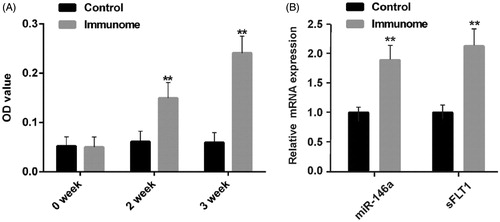
As shown in , compared to the control group, after 2 weeks of active immunity, the OD value of serum β1AR antibodies of the immune group had significantly increased, indicating the elevated level of β1AR antibodies in serum. In addition, with the prolongation of immune time, the rising β1AR antibodies level also increased. In order to verify that our model had the characteristics of PPCM, we also tested two biomarkers of PPCM [Citation2,Citation10,Citation15]. We found that the expression of miR-146a and soluble Fms-like tyrosine kinase 1 (sFlt1), both increased after immunity ().
Figure 1. The SA-ELISA results of the serum level of β1AR antibodies and expression of biomarkers of PPCM. A. The serum level of β1AR antibodies increases with the increase of immune time (n = 6); B. The mRNA expression of two biomarkers of PPCM increased. **p<.01 (n = 6). Group data presented by mean and SEM.
The autoimmune animals had a significant decline in cardiac function
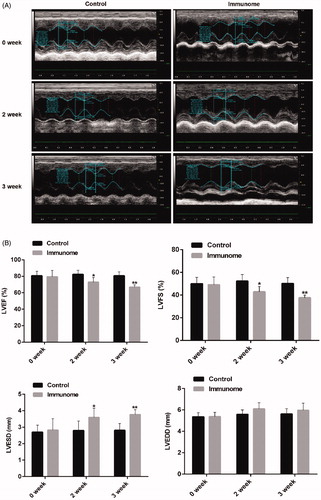
Because PPCM is defined as an idiopathic cardiac failure that occurs in the absence of definable heart disease [Citation1,Citation12], we detected the cardiac function of autoimmune animals. As shown in , raw data of echocardiography detection was used to indicate how to obtain measurement parameters. The LVEF and LVFS decreased in immuno group compared with control group (). Furthermore, at the second week and third week, the LVESD increased (). It suggested that the cardiac function of the immune group was decreased significantly.
Figure 2. The autoimmune animals had a significant decline in cardiac function. A. Examples of raw data of echocardiography detection from one control animal and immunome model. B. Group data of LVEF, LVFS, LVESD and LVEDD. The left ventricular ejection fraction (LVEF) and left ventricular shortening fraction (LVFS) decreased in immuno group compared with control group from 82.4 ± 2.5 to 72.9 ± 2.3% (n = 6, p<.01) and 52.6 ± 2.9 to 42.9 ± 1.9% (n = 6, p<.05) at second week, and 81.8 ± 1.9 to 66.8 ± 1.1% (n = 6, p < .01) and 51.4 ± 2.1 to 37.7 ± 0.9% (n = 6, p < .01) at third week. While, the left ventricular end-systolic diameter (LVESD) increased compared to the control group from 2.8 ± 0.2 to 3.6 ± 0.2 mm (n = 6, p < .05) at second week and 2.8 ± 0.1 to 3.7 ± 0.1 mm at the third week. *p < .05; **p < .01 (n = 6). Group data presented by mean and SEM.
The apoptosis rate of left ventricular myocytes of autoimmune animals increased
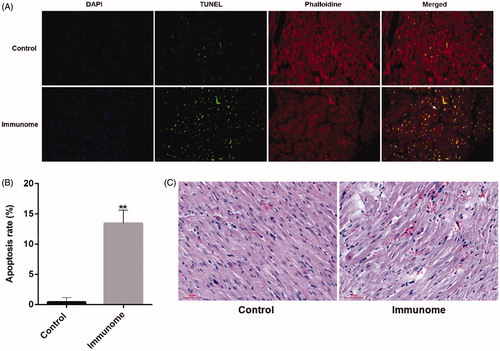
Few TUNEL-positive nuclei were found in control myocardial cells, while the rate of TUNEL-positive nuclei in left ventricular myocardium significantly increased in the immune group (). In addition, the results of HE staining showed that the myocardial cells had lots of focal dissolution, different degrees of particles and vacuolar degeneration and cells’ interspaces noticeably broadened in the immune group (). These results indicated that β1AR antibodies inducing apoptosis of left ventricular myocytes lead to acute tissue damage in the left ventricle.
Figure 3. The apoptosis rate of left ventricular myocytes of autoimmune animals increased. A. TUNEL positive nuclei obtained from left ventricular myocardium visualized by fluorescence microscopy. B. Group data of apoptosis rate. **p<.01 (n = 6). Group data presented by mean and SEM. The rate of TUNEL-positive nuclei in left ventricular myocardium significantly increased in the immune group from 0.4 ± 0.4 to 13.4 ± 1.2%. C. HE staining of left ventricular myocardial tissue. Scale bar, 50µm.
The expression of PGC-1α related signal factor in autoimmune animals decreased
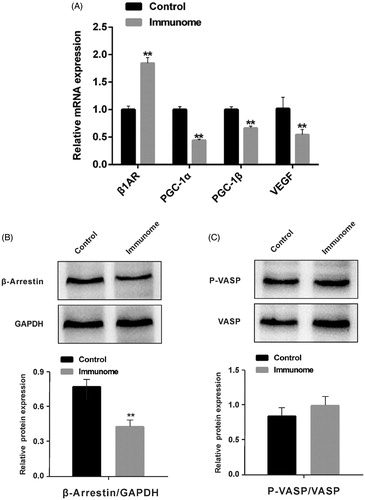
Because cardiac-specific deletion of PGC-1α could lead to PPCM symptoms, on the next stage, we investigated the difference of expression of PGC-1α related signal factor in ventricular myocardium between control and immune group. As shown in , the mRNA level of PGC-1α, PGC-1β and VEGF notably decreased. On the other hand, the mRNA level of β1AR significantly increased in the immune group. We also tested the downstream signals of β1AR. The activation of β1AR could lead to PKA–phosphorylation of target proteins at multiple subcellular localization or activity of the β‐arrestin through non-G protein receptor pathway [Citation16]. We found that P-VASP/VASP had no statistical significance (), while the β‐arrestin 1/2 expression was significantly decreased after immunity ().
Figure 4. The relative mRNA expression of β1AR and PGC-1α related signal factor and protein expression of downstream signal of β1AR. A. The relative mRNA expression of PGC-1α, PGC-1β and VEGF decreased and the relative mRNA expression of β1AR increased in the immune group (n = 6); B. The β-arrestin 1/2 decreased and PKA activity had no significant difference after immunity (n = 3). **p < .01. Group data presented by mean and SEM.
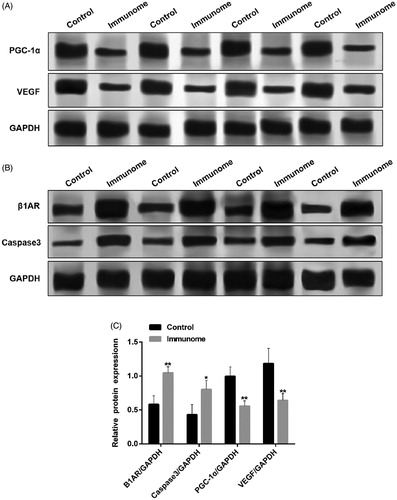
Furthermore, the protein expression of PGC-1α and VEGF considerably decreased (), while the protein expression of β1AR and caspase 3 increased noticeably (). All these results suggested that β1AR antibodies induced myocardial cell apoptosis may be through PGC-1α related pathway.
Figure 5. The relative protein expression of PGC-1α, VEGF, β1AR and caspase 3. A and B. Raw data of WB test of PGC-1α, VEGF, β1AR and caspase 3; C. The relative protein expression of β1AR and caspase 3 increased and PGC-1α and VEGF decreased in the immune group (n = 3). p < .05; **p < .01. Group data presented by mean and SEM.
Discussion
The Heart Failure Association of the European Society of Cardiology Working Group defined PPCM as idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction toward the end of pregnancy or in the months following delivery, where no other cause of heart failure is found [Citation17]. On one hand, 30% patients suffering from dilated cardiomyopathy have circulating β1AR antibodies [Citation18,Citation19]. On the other hand, compared to β1AR antibodies-negative patients, β1AR antibodies positive dilated cardiomyopathy patients exhibited poorer LV function [Citation20,Citation21]. However, there is no direct evidence that β1AR antibodies and PPCM have a direct causal relationship. In this study, first, we selected the animals one week after delivery, which was a period of high PPCM incidence. Second, elevation in the level of β1AR antibodies in these postpartum animals lead to the increase of miR-146a and sFlt1, which are two biomarkers of PPCM. In addition, the results of structural and functional measurements of the left ventricle also showed that these postpartum animals had significant changes in cardiomyopathy under the action of β1AR antibodies. All these results suggested that β1AR antibodies might directly trigger PPCM.
It is well known that apoptosis plays a pathophysiological role in heart failure. In this study, the apoptosis rate of ventricular myocytes in autoimmune perinatal rats was significantly increased. In the light of our results, it has been reported that β1AR antibodies induce apoptosis in cardiomyocytes which were isolated from normal rats [Citation22]. Notably, mitochondria play a major role in apoptosis. Moreover, the progression to heart failure is greatly associated with a decline in the activity of mitochondrial respiratory pathways leading to minimized capacity for ATP production [Citation23]. PGC-1α is a key integrator of transcriptional circuits regulating mitochondrial biogenesis and functions [Citation23]. Activation of the PGC-1α regulatory cascade increases cardiac mitochondrial oxidative capacity in the heart [Citation23] and protects the cells from apoptosis [Citation24]. The most important point to note is that the mice that lack cardiac PGC-1αdevelop profound PPCM and VEGF therapies, which rescue PPCM in these animals [Citation10]. Our results showed that the expression of PGC-1α and its downstream target VEGF were noticeably decreased in autoimmune perinatal rats. Considering that the protein expression of β1AR and apoptosis factor caspase 3 were remarkably increased in the meantime, we suggested that the symptoms of PPCM appeared in autoimmune perinatal rats may be due to the inhibition of PGC-1α related pathway. We also tested the downstream signals of β1AR and found that the expression of β‐arrestin1/2 was decreased. However, PKA activity has no statistical significance. We suggested that the decrease in the expression of β-arrestin may lead to a reduction in ingestion and degradation of β1AR and long-term immunity may cause the desensitization of receptors.
Altogether, our present findings establish that β1AR antibodies directly induce PPCM-like symptoms in postpartum Wistar rats and this effect is accompanied by the suppression of PGC-1α pathway, which had been shown to be the one causes of PPCM [Citation11]. Therefore, our results reveal a previously unknown role of the β1AR antibodies pathway in the etiology of PPCM, and provide a novel potential target for the treatment of PPCM.
Ethical approval
The Institutional Animal Care and Use Committee of the Nanjing University guidelines for the care and use of animals were followed.
Supplemental Material
Download MS Word (14.4 KB)Disclosure statement
Yuan Zhang declares that she has no conflict of interest; Jia Liu declares that she has no conflict of interest. Linying Shi declares that she has no conflict of interest. Mulei Chen declares that he has no conflict of interest. Jiamei Liu declares that she has no conflict of interest.
Y. Zhang and J. Liu performed the experiments. L. Y. Shi and M. L. Chen helped perform statistical analysis of results and wrote related parts of the manuscript. Y. Zhang and J. M. Liu contributed study design and revised the manuscript. J. M. Liu wrote the final manuscript. All authors contributed to the manuscript.
Additional information
Funding
References
- Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133(14):1397–1409.
- Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600.
- Baris L, Cornette J, Johnson MR, et al. Peripartum cardiomyopathy: disease or syndrome? Heart. 2019;105(5):357–362.
- Fett JD, Murphy JG. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Gynaecol Obstet. 2006;94(2):135–136.
- Ishikawa Y, Homcy CJ. The adenylyl cyclases as integrators of transmembrane signal transduction. Circ Res. 1997;80(3):297–304.
- Liu HR, Zhao RR, Zhi JM, et al. Screening of serum autoantibodies to cardiac beta1-adrenoceptors and M2-muscarinic acetylcholine receptors in 408 healthy subjects of varying ages. Autoimmunity. 1999;29(1):43–51.
- Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91(5):1964–1968.
- Sterin-Borda L, Borda GG. E. Chagasic IgG bingding with cardiac muscarinic cholinergic receptors modifies cholinergic-mediated cellular transmembrane signals. Clin Immunol Immunopathol. 1991;61:389–397.
- Liu J, Wang Y, Chen M, et al. The correlation between peripartum cardiomyopathy and autoantibodies against cardiovascular receptors. PLoS One. 2014;9(1):e86770.
- Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–338.
- Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012.
- Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–968.
- Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–2055.
- Pillarisetti J, Kondur A, Alani A, et al. Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol. 2014;63(25 Pt A):2831–2839.
- Halkein J, Tabruyn SP, Ricke-Hoch M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest. 2013;123(5):2143–2154.
- Najafi A, Sequeira V, Kuster DW, et al. β-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest. 2016;46(4):362–374.
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al.; Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778.
- Magnusson Y, Marullo S, Hoyer S, et al. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86(5):1658–1663.
- Fu ML, Hoebeke J, Matsui S, et al. Autoantibodies against cardiac G-protein-coupled receptors define different populations with cardiomyopathies but not with hypertension. Clin Immunol Immunopathol. 1994;72(1):15–20.
- Jahns R, Boivin V, Siegmund C, et al. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99(5):649–654.
- Iwata M, Yoshikawa T, Baba A, et al. Autoantibodies against the second extracellular loop of b1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37(2):418–424.
- Staudt Y, Mobini R, Fu M, et al. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466(1–2):1–6.
- Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115(3):547–555.
- Sen N, Satija YK, Das S. PGC-1α, a key modulator of p53, promotes cell survival upon metabolic stress. Mol Cell. 2011;44(4):621–634.
