Abstract
Background. It remains equivocal if acute type A aortic dissection (ATAAD) surgical outcomes are improving. We analyzed current outcome trends to evaluate improvements and to identify predicting variables. Methods. From 2015 to 2020, 204 patients underwent surgery for ATAAD and were divided into recent (n = 102) and earlier (n = 102) groups. Uni- and multivariable statistical analysis was performed to identify predictors of 30-day mortality. Results. Thirty-day mortality decreased significantly in the recent group (3.9% vs 14.6%, p = .014). Prevalence of neurological insult also decreased significantly (13% vs 25%, p = .028). Other major complications remained unchanged. There was no statistically significant difference in overall 30-day mortality between low-volume vs high-volume surgeons (12.3% vs 7.3%, p = .21). The number of surgeons performing ATAAD procedures decreased from nine in 2015 to five in 2020. Preoperative lactate (OR 1.24, 95%CI 1.03–1.51), dissection of any arch vessel (OR 14.2, 95%CI 1.79–113), non-normal left ventricular ejection fraction (OR 12.5, 95%CI 2.54–61.6), biological composite graft (OR 19.1, 95%CI 2.75–133), concomitant coronary artery bypass grafting (OR 38.8, 95%CI 2.91–517) and intraoperative adverse event (OR 9.5, 95%CI 2.22–40.9) were statistically significant independent predictors of mortality. Conclusions. Early outcomes after ATAAD improved in the most recent experience. Part of the explanation may be fewer surgeons performing more procedures annually, a relatively conservative approach to the extent of aortic resection and ensuring adequate cerebral protection. Major complications remain prevalent and require attention to be further reduced.
Introduction
Acute type A aortic dissection (ATAAD) is a cardiovascular emergency with substantial mortality and morbidity. Early studies reported 30–60% postoperative mortality when repair for ATAAD was first introduced [Citation1,Citation2]. Contemporary studies report considerably lower mortality, but postoperative complications remain common [Citation3,Citation4]. The German Registry for Acute Aortic Dissection type A (GERAADA) showed an overall 30-day mortality of 16.9% between 2006 and 2010 [Citation5]. The International Registry of Acute Aortic Dissection (IRAD) showed a decrease in operative mortality over the 1995–2013 period from 25% to 18.4% [Citation3]. A limited number of studies report excellent outcomes with single digit operative mortality, however often with few patients and over limited time [Citation6–8]. There are conflicting reports about improvement of outcomes after surgery for ATAAD over time. Recent studies have shown either nonsignificant downward trends in mortality or a decrease in mortality for subgroups of ATAAD patients while some studies show a significant decrease in mortality over time and others show no statistically significant difference in postoperative outcomes [Citation9–11].
It has been suggested that outcomes improve if aortic/high-volume surgeons perform the ATAAD surgery [Citation12–15]. However, even if excellent in-hospital survival rates can be achieved, either through improvement with time, the implementation of subspecialized surgeons or a combination of the two, patients undergoing surgery for ATAAD still suffer a substantial rate of adverse events. We, therefore, sought to analyze current trends in mortality as well as major postoperative complications during the most recent period in order to further optimize early outcomes.
Materials and methods
Study population
All consecutive patients who underwent surgical repair for ATAAD from January 2015 to December 2020 at our institution were included. The study population was split into halves and grouped as recent (n = 102) and earlier (n = 102) to give an adequate comparison to the most recent surgical experience. No substantial differences in perioperative management were introduced during the study period. Detailed pre-, per- and postoperative data were collected by medical chart review. Patients with chronic type A dissection (≥14 days from onset of symptoms, or unknown time of onset in the absence of symptoms) were not included. The study was approved by the Swedish Ethical Review Authority (No. 2019-02087), waiving the need to obtain individual written informed consent.
Outcomes
The primary outcome measure was 30-day mortality. Permanent neurological damage was defined as radiologically and/or clinically evident signs of central nervous system damage (including spinal cord) not resolving prior to discharge. Dialysis implied new use of continuous renal replacement therapy (CRRT). Respiratory failure was defined as prolonged mechanical ventilation (>48 h) with or without tracheostomy or, unplanned reintubation driven by respiratory deterioration. Heart failure was defined as requiring inotropic drugs, revascularization or mechanical support or any combination thereof. Multiorgan system failure was defined as failure of two or more organ systems. Major postoperative complication was defined as any of the above. Intraoperative adverse event was defined as unplanned reclamping of the aorta or restart of HCA (due to bleeding, need for additional surgical procedure, malperfusion syndrome).
Perioperative management
Patients were operated with median sternotomy, extracorporeal circulation, cold blood cardioplegic arrest and hypothermic circulatory arrest (HCA) with antegrade cerebral perfusion (ACP). Strategy for arterial cannulation and the conduct of HCA and ACP were at the discretion of the surgeon. Moderate–mild hypothermia (target core temperature of 28–30 °C) was typically employed (). ACP with separate 12 French balloon-inflated perfusion cannulae from the arterial line placed in each cervical vessel directly through the ostia was the standard set-up. The cerebral perfusion was maintained at 20 °C at 500–600 ml/min flow rate with bilateral radial artery pressure as well as bi-frontal near-infrared spectroscopy monitoring. A strict definition of a ‘hemiarch’ as resection of the minor curvature of the aortic arch requiring a beveled anastomosis was used and the procedure otherwise defined as a straight supracoronary graft. Root replacement with either mechanical or biological valve substitute or valve-sparing (David) or partial root-resection (noncoronary sinus resection) was performed ad lib, as was partial or total arch replacement with reimplantation of one or more arch vessels, with or without concomitant elephant trunk procedure.
Table 1. Perfusion and operative characteristics in all patients and groupwise (Recent vs Earlier, Survivors vs Dead).
High-volume surgeon was defined as a surgeon in the tercile that performed most ATAAD repairs during the study period. All other surgeons were classified as low-volume surgeons.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as frequencies and percentages. Groups were compared by Student’s t-test for independent samples (continuous variables) and χ2 test with Pearson’s correction for binary and categorical variables. Logistic regression methods were used for multivariable analysis of independent predictors for the primary endpoint (30-day mortality). For multivariable models, predictors with p < .1 in the univariate analysis were included and stepwise backward elimination employed until remaining predictors were significant at p < .05 level. Final model selection included evaluation of likelihood ratio χ2-test, goodness-of-fit p value and c-statistic. Predictors were expressed as odds ratio (OR) with 95% confidence limits (95%CL). Kaplan–Meier methods were used to estimate survival during follow-up. Groups were compared using the log-rank test. Statistical analysis was performed using Stata v 16 (Stata Corp., College Station, TX).
Results
Study population
In total, 204 patients underwent surgical repair for ATAAD from January 2015 to December 2020. From January 2015 to December 2017, 95 patients underwent ATAAD repair and from January 2018–December 2020, 109 patients underwent ATAAD repair. Patient characteristics are detailed in . There were no statistically significant differences in patient characteristics between the earlier and recent period. Preoperative shock and cardiopulmonary resuscitation were more common in nonsurvivors. Preoperative lactate was higher in nonsurvivors. Preoperative left ventricular ejection fraction was worse in nonsurvivors. Operative risk assessed with The European System for Cardiac Operative Risk Evaluation (EuroSCORE II) was significantly and four-fold higher in nonsurvivors.
Table 2. Preoperative characteristics in all patients and groupwise (Recent vs Earlier, Survivors vs Dead).
Penn class [Citation16] Abc (general and localized end-organ ischemia combined) was more common in nonsurvivors while Penn Class Aa (neither general nor localized end-organ ischemia) was more common in survivors. Penn class distribution remained unchanged over time, mortality in Penn class Abc decreased significantly in the recent group, 2/16 (12%) vs 8/21 (38%), p=.041 (Supplement Figure 1). Anatomical features and extent of dissection are detailed in Supplement Table 1. Arch vessel dissection and dissection in the superior mesenteric artery were more common in nonsurvivors.
Procedural characteristics
Procedural characteristics are detailed in . Almost all patients underwent surgical repair during HCA with bilateral ACP or perfusion of all three neck vessels. Cardiopulmonary bypass time was significantly shorter in the recent time period and significantly longer in nonsurvivors. Aortic occlusion time and HCA time were also significantly longer in nonsurvivors. The use of ACP with perfusion of all three neck vessels increased in the recent group. Root replacement was more common in nonsurvivors. Concomitant (nonaortic) procedures were exclusively performed in the earlier period and were also significantly more common in nonsurvivors. Restart of cardiopulmonary bypass and repeat HCA were more common in nonsurvivors.
Overall, 55 patients (27%) underwent root replacement, 30 (54% of roots) mechanical composite graft, 12 (22%) biological composite graft and 13 (24%) valve-sparing procedure. There was no significant difference in root replacements between the two time periods. 30-day mortality in patients undergoing root replacement with biological composite graft was 58.3% and in patients where the indication for the procedure was destruction by dissection, mortality was 85.7%.
Primary outcome measure
Overall 30-day mortality was 9.3%. A significant decrease in mortality was observed over time (earlier 30-day mortality 14.7% vs recent 30-day mortality 3.9%, p = .008), detailed in .
Table 3. Postoperative outcomes in all patients and groupwise (Recent vs Earlier, Survivors vs Dead).
Independent predictors of 30-day mortality (detailed in Supplement Table 2) were preoperative lactate, dissection of any arch vessel, non-normal left ventricular ejection fraction, biological composite graft, concomitant coronary artery bypass grafting (CABG) and intraoperative adverse event, i.e. reclamping of the aorta or need for a second period of HCA.
Major complications
A significant decrease between the two time periods was seen in central neurological insult (25% vs 13%, p = .028). There was no statistically significant difference in prevalence of other major postoperative complications (reexploration for bleeding, renal insufficiency, respiratory failure, cardiac failure or multiorgan system failure). All major complications were significantly more common in nonsurvivors ().
Low-volume vs high-volume surgeons
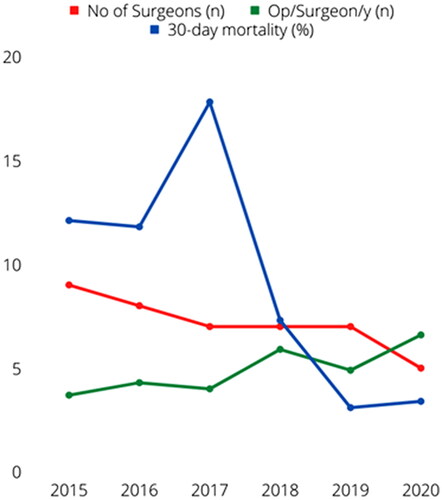
High-volume surgeons operated a median of 7.5 cases per year (range 5–12) vs 3 (1–9) for low-volume surgeons, p<.001. The number of ATAAD surgeons gradually decreased over the study period from nine to five, while the overall median number of annual procedures increased from four to nine, paralleling decreasing annual mortality rates (). As detailed in Supplement Table 3, aortic cross-clamp, cardiopulmonary bypass and HCA durations were significantly shorter for high-volume surgeons, despite more root procedures. In addition, high-volume surgeons more often used ACP with perfusion of all three neck vessels and less often needed a second period of HCA. There were overall no statistically significant differences in early mortality.
Follow-up survival
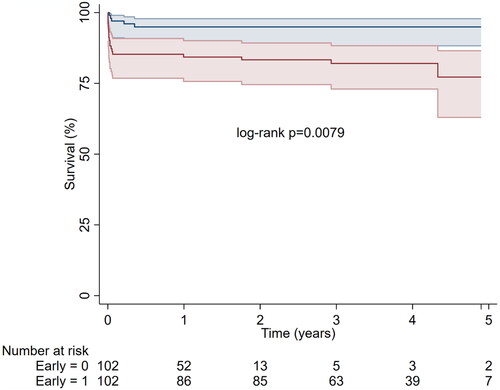
The significant differences in 30-day mortality persisted during a median of 1.9 (range 0–5.7) years follow-up (). Estimated overall 3-year survival was 86% (95%CL 80–91%). In addition to 19 deaths within 30 days, there were a total of six deaths during a total of 449 person-years of follow-up.
Discussion
Previous studies that have investigated if outcomes after surgery for ATAAD change over time have found either a gradual decrease over time, nonstatistically significant downward trends in mortality or an unchanged mortality but with increasing patient risk factors [Citation3,Citation9–11]. To our knowledge, few if any studies show such a distinct decrease in early ATAAD mortality as the present one. In this study, no statistically significant differences in age or comorbidities were observed, possibly due to the limited study period. Furthermore, there was no difference in Penn class distribution (Supplement Figure 1), or incidence of preoperative cardiac tamponade or cardiopulmonary resuscitation between the two time periods, and time from symptom onset to surgery remained unchanged (). Therefore, it is unlikely that the improvement in outcomes were explained by more elaborate patient selection in the later period.
The only notable change occurring during the study period was the decrease in number of surgeons performing the repairs, with increased surgeon annual case-load (). This development was not driven by individual surgeon performance or other structural considerations but remains an important observation. The impact of high-volume surgeons and aortic specialists performing the ATAAD repair has previously been investigated. A recent study showed that aortic specialists had significantly better 30-day mortality compared to nonaortic specialists (10% vs 30%) [Citation12]. However, nonaortic surgeons only performed a total of 46 ATAAD surgeries over the course of 15 years [Citation12]. Another study showed decreased in-hospital mortality for surgeons with a mean annual volume ≥4 ATAAD repairs [Citation4]. A study from Yale found that aortic specialist-status was an independent predictor for improved two-year survival and that aortic specialists performed more complex repairs and more frequently used cerebral perfusion during HCA [Citation13]. As described above, high-volume surgeons did not have statistically significant lower 30-day mortality in the present study. In the multivariable analysis, intraoperative adverse event, defined as the need for an additional period of HCA or re-clamping the aorta, was independently associated with 30-day mortality. High-volume surgeons did less frequently need a second period of HCA. The exact mechanisms of the advent, avoidance and management of intraoperative adverse events warrant further exploration.
We found that dissection of any arch vessel was associated with a higher risk of early mortality, probably reflecting the strong association between cerebral malperfusion, neurological damage and early mortality. A previous IRAD report showed that patients with ATAAD and major brain injury carry a two- to three-fold higher mortality and another study investigating in-hospital mortality in Penn Class Aa ATAAD patients found that neurological damage was the most common cause of death [Citation15,Citation17]. In the present study, neurological damage occurred in 61% of nonsurvivors compared to 15% in survivors. Preoperative cerebral malperfusion was present in 42% of nonsurvivors compared to 15% in survivors. HCA time was also significantly longer in nonsurvivors. This shows the importance of ensuring adequate cerebral protection and avoiding the need for a second period of HCA in all ATAAD patients, and particularly in patients with preoperative cerebral malperfusion. There was no difference in prevalence of arch vessel dissection or cerebral malperfusion between the two eras, however, we did observe a significantly lower prevalence of neurological damage in the recent group. HCA time and cerebral perfusion time did not differ between the two groups, but the use of ACP with perfusion of all three neck vessels was more common in the recent time period. To our knowledge there are no studies that have evaluated ACP with perfusion of all three neck vessels vs bilateral ACP, but bilateral ACP have previously been shown to be a safe method and remains the method of choice for cerebral protection during HCA at many centers [Citation18,Citation19]. It is possible that the more frequent use of ACP with perfusion of all three neck vessels in the recent era have contributed to the decrease in neurological damage and in turn, the observed decrease in early mortality.
Procedures
Total arch repairs were uncommon, 11 in the earlier group and 3 in the recent group. Only four patients underwent frozen elephant trunk, all in the earlier era. Thirty-day mortality in the total arch and frozen elephant trunk subgroup was 30% and 50%, respectively. A total arch repair in the ATAAD patient is a challenging procedure. Even if previous studies have shown excellent outcomes, most studies are from high-volume centers [Citation20,Citation21]. Possibly, the more conservative approach to the extent of aortic resection in the recent era also contributed to the improved outcomes. Concomitant CABG was significantly more common in non-survivors and exclusively performed in the earlier era. This either reflects an extensive dissection affecting the coronary arteries or some intraoperative adverse event forcing the need for an additional procedure and since there was no significant difference in prevalence of cardiac malperfusion between the two groups it is more likely that it represents a higher occurrence of intraoperative events in the earlier era. As discussed, intraoperative adverse events could be more common with less experienced surgeons, and it is possible that the decrease in number of surgeons contributed to the decreased need for concomitant procedures in the recent group. Notably, several variables reached statistical significance in multivariable analysis despite relatively few outcomes. In consequence, the statistical model is overfitted and may not be representative for independent predictors in general, but on the other hand explaining the findings of the present study with a high degree of confidence.
Thirty-day mortality in patients who underwent biological composite graft root replacement was high and it was an independent predictor for early mortality. Mortality was particularly high if the indication for the root procedure was root destruction caused by the dissection. In contrast, mortality in patients undergoing root replacement with either mechanical composite graft or valve-sparing root replacement was 3.3% and zero, respectively. Extensive repairs in older patients seemed to increase the risk of early mortality, and cautious intra- and postoperative care is of particular importance in this patient group.
Study limitations
The present study was single-center, retrospective and limited in size. Interpretations must be cautious and findings may not be widely generalizable. However, a very recent experience is reported, reflecting current practice, and generating hypotheses for further studies, with special emphasis on modifiable factors (both organizational and surgical) associated with improved outcomes.
Conclusions
The results of the present study indicate that the explanation for the improved outcomes is a combination of fewer surgeons performing more procedures, a relatively conservative approach to the extent of aortic resection, meticulous surgical technique with a decreased need for concomitant procedures and ensuring adequate cerebral protection during HCA. Major postoperative adverse events remained common and further measures and studies are needed to continue improving outcomes following surgery for ATAAD.
Supplemental Material
Download MS Word (73.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Miller DC, Stinson EB, Oyer PE, et al. Operative treatment of aortic dissections. Experience with 125 patients over a sixteen-year period. J Thorac Cardiovasc Surg. 1979;78:365–382.
- d‘Allaines C, Blondeau P, Piwnica A, et al. Surgery for aortic dissection: 53 operated cases with 32 in the acute phase. J Cardiovasc Surg. 1977;18:261–266.
- Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-Year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860.
- Bashir M, Harky A, Fok M, et al. Acute type A aortic dissection in the United Kingdom: surgeon volume-outcome relation. J Thorac Cardiovasc Surg. 2017;154(2):398–406.e1.
- Conzelmann LO, Weigang E, Mehlhorn U, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg. 2016;49(2):e44–52–e52.
- Shiono M, Hata M, Sezai A, et al. Validity of a limited ascending and hemiarch replacement for acute type A aortic dissection. Ann Thorac Surg. 2006;82(5):1665–1669.
- Chen LW, Lu L, Dai XF, et al. Total arch repair with open triple-branched stent graft placement for acute type A aortic dissection: experience with 122 patients. J Thorac Cardiovasc Surg. 2014;148(2):521–528.
- Andersen ND, Ganapathi AM, Hanna JM, et al. Outcomes of acute type a dissection repair before and after implementation of a multidisciplinary thoracic aortic surgery program. J Am Coll Cardiol. 2014;63(17):1796–1803.
- Abe T, Yamamoto H, Miyata H, et al. Patient trends and outcomes of surgery for type A acute aortic dissection in Japan: an analysis of more than 10 000 patients from the Japan Cardiovascular Surgery Database. Eur J Cardiothorac Surg. 2020;57(4):660–667.
- Olsson C, Ahlsson A, Fuglsang S, et al. Medium-term survival after surgery for acute type A aortic dissection is improving. Eur J Cardiothorac Surg. 2017;52(5):852–857.
- Narayan P, Rogers CA, Davies I, et al. Type A aortic dissection: has surgical outcome improved with time? J Thorac Cardiovasc Surg. 2008;136(5):1172–1177.
- Khan H, Hussain A, Chaubey S, et al. Acute aortic dissection type A: impact of aortic specialists on short and long term outcomes. J Card Surg. 2021;36(3):952–958.
- Bin Mahmood SU, Mori M, Geirsson A, et al. Acute type A aortic dissection surgery performed by aortic specialists improves 2-Year outcomes. Aorta (Stamford). 2019;7(1):1–6.
- Buonocore M, Amarelli C, Scardone M, et al. Cerebral perfusion issues in acute type A aortic dissection without preoperative malperfusion: how do surgical factors affect outcomes? Eur J Cardiothorac Surg. 2016;50(4):652–659.
- Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S213–21.e1.
- Augoustides JG, Geirsson A, Szeto WY, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the penn classification. Nat Clin Pract Cardiovasc Med. 2009;6(2):140–146. Feb
- Olsson C. Modifiable risk factors for early mortality in low-risk penn class aa acute type A aortic dissection Patients – a descriptive study. Aorta (Stamford). 2017;5(4):117–123. 1
- Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation. 2011;124(4):434–443.
- Angleitner P, Stelzmueller ME, Mahr S, et al. Bilateral or unilateral antegrade cerebral perfusion during surgery for acute type A dissection. J Thorac Cardiovasc Surg. 2020;159(6):2159–2167.e2.
- Uchida N, Katayama A, Tamura K, et al. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothorac Surg. 2011;40:1066–1071.
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation. 2011;123(9):971–978.