ABSTRACTS
Objectives
Electrocardiogram (ECG) and measurement of plasma brain natriuretic peptides (BNP) are established markers of right ventricular dysfunction (RVD) in the setting of acute pulmonary embolism (PE) but their value at long-term follow-up is largely unknown. The purpose of this prospective study was to determine the prevalence of ECG abnormalities, describe levels of N-terminal proBNP (NT-proBNP), and establish their association with dyspnea at long-term follow-up after PE.
Design
All Swedish patients diagnosed with acute PE in 2005 (n = 5793) were identified through the Swedish National Patient Registry. Surviving patients in 2007 (n = 3510) were invited to participate. Of these, 2105 subjects responded to a questionnaire about dyspnea and comorbidities. Subjects with dyspnea or risk factors for development of chronic thromboembolic pulmonary hypertension were included in the study in a secondary step, which involved collection of blood samples and ECG registration.
Results
Altogether 49.3% had a completely normal ECG. The remaining participants had a variety of abnormalities, 7.2% had atrial fibrillation/flutter (AF). ECG with any sign of RVD was found in 7.2% of subjects. Right bundle branch block was the most common RVD sign with a prevalence of 6.4%. An abnormal ECG was associated with dyspnea. AF was associated with dyspnea, whereas ECG signs of RVD were not. 61.2% of subjects had NT-proBNP levels above clinical cut-off (>125 ng/L). The degree of dyspnea did not associate independently with NT-proBNP levels.
Conclusions
We conclude that the value of ECG and NT-proBNP in long term follow-up after PE lies mostly in differential diagnostics.
Introduction
Many studies have investigated the short-term outcomes after an acute episode of pulmonary embolism (PE) [Citation1–5], but less attention has been directed to the long-term effects of an acute PE. Dyspnea and functional limitations are known, frequent long-term complaints after an acute PE, despite adequate anticoagulation [Citation6–8]. Although studies have shown that these complaints are often explained by factors other than the PE itself [Citation9], we recently showed that PE was found to be an independent risk factor for long term dyspnea [Citation10].
Right ventricular dysfunction (RVD), a common finding in acute PE, is associated with dyspnea and is associated with an adverse short-term outcome [Citation11]. RVD also frequently presents at long term follow-up [Citation12]. RVD is commonly diagnosed by echocardiography or by elevated right ventricle/left ventricle ratio on computed tomography but is also associated with electrocardiographic (ECG) abnormalities [Citation13] and elevated levels of plasma brain natriuretic peptides (BNP) [Citation14].
Both BNP and N-terminal proBNP (NT-proBNP) are established reliable markers of RVD [Citation14–18], and elevated levels at admission for an acute PE are associated with a complicated clinical course and increased mortality [Citation19,Citation20]. Additionally, several ECG abnormalities have been associated with PE-associated RVD and short-term mortality in PE [Citation13,Citation21–23]. Promising results have been shown from attempts to incorporate ECG abnormalities and BNP/NT-proBNP levels in diagnostic algorithms for excluding pulmonary hypertension/chronic thromboembolic pulmonary hypertension (CTEPH) [Citation24–26], but the natural course of normalization of these parameters and their overall associaions with PE-related morbidity or mortality has yet to be established.
In this study, we hypothesized that ECG abnormalities and elevated levels of NT-proBNP are associated with dyspnea after an acute PE. We aimed to determine the prevalence of ECG abnormalities and levels of NT-proBNP at long-term follow-up after an acute PE, and to explore their association with dyspnea. As a secondary aim we also wanted to determine predictors of NT-proBNP levels.
Materials and methods
Subjects
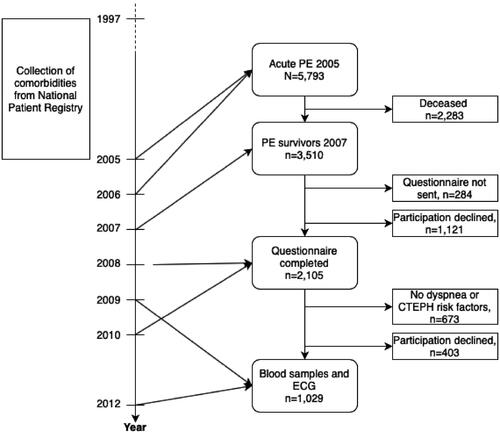
The initial selection of the study subjects and their comorbidities has previously been described [Citation10]. In short, in 2007 all surviving Swedish patients diagnosed with acute PE in 2005 (ICD-10-SE code I26) were invited to participate in the study. The original study was designed to establish the incidence in CTEPH after an acute PE by applying a stepwise selection process starting with a questionnaire and followed by blood sampling and ECG registration in a second step. The selection process is summarized in . The invitation included a questionnaire and a consent form which were to be returned to the study center. Patients that did not respond to a written re-invitation were contacted by telephone by a study nurse and asked questions for a non-responder analysis.
Figure 1. Flow chart illustrating timeline and the selection process for the study population. PE: Pulmonary embolism; CTEPH: chronic thromboembolic pulmonary hypertension; ECG: electrocardiogram.
The questionnaire was designed to compile information regarding symptoms and risk factors for long-term sequel including CTEPH [Citation10]. The questionnaire and the list of conditions that led to inclusion in the current study, as second step building upon the initial study, are shown in the supplementary material. Patients reporting dyspnea that had either worsened or remained after the acute PE, as well as patients with known risk factors for CTEPH [Citation27], including patients with chronic diseases were considered to be at risk for long-term sequel.
Subjects identified as at risk for long-term sequelae received an additional letter in which they were asked to proceed to the next step which involved collection of blood samples and registration of a 12-lead resting ECG.
Two questions from the questionnaire were used in the current study:
1. Do you experience shortness of breath upon physical exertion?
– No, I don’t experience shortness of breath or get physically exhausted
– Yes, upon rigorous exertion (walking up two stairs without rest)
– Yes, upon slight exertion (walking up one stairs)
– Yes, I experience shortness of breath also at rest
2. Did a doctor or other medical staff on any occasion inform you that you have the pulmonary disease COPD (chronic obstructive pulmonary disease)?
– Yes
– No
To achieve a clinically relevant threshold, answers from the dyspnea question (question 1), were dichotomized; the first two answers were clustered to “No, or mild dyspnea” and the last two answers were clustered as “severe dyspnea”.
The second question was used in the study to identify those patients with COPD as reliable diagnosis of COPD cannot be retained from the Swedish National Patient Register, as only diagnoses from hospital in-patient care are reported. All other diagnoses of comorbidities used in the study were obtained from the Swedish National Patient Register as previously described [Citation10].
This study complied with the Declaration of Helsinki and was approved by the Regional Ethics Review Board in Umeå, Sweden (07-074 M). The Ethics Board at the Swedish National Board of Health and Welfare reviewed and approved the extraction of data from the Swedish National Patient Register (34-9322-2007). Written consent was obtained from all participants.
Blood samples
Subjects were provided with two sodium heparin tubes and were asked to go to the nearest health center for blood sampling. Samples were sent overnight to Norrlands University Hospital where they were spun and aliquoted for analysis for NT-proBNP and creatinine.
The analyses of NT-proBNP and creatinine were performed by the clinical chemistry laboratory at Skellefteå hospital (Västerbotten Region, Sweden) according to standard clinical practices using an Immulite machine and the reagent IMMULITE® from Diagnostic Products Corporation, USA, and an IDMS (isotope dilution mass spectrometry) traceable method, respectively.
Electrocardiogram (ECG)
Twelve lead resting ECGs were recorded at the patients nearest health center and sent by mail to Umeå for analysis. All ECGs were analyzed by one experienced physician (C.B.) at the department of clinical physiology, and categorized according to the Minnesota Code [Citation28]. Additional comments were recorded if the ECG was difficult to assess. All ECGs with comments were reviewed by the first author for evaluation according to AHA/ACCF/HRS (American Heart Association; American College of Cardiology Foundation; Heart Rhythm Society) expert consensus documents [Citation29–31].
The ECG was defined as normal in absence of Minnesota codes. Atrial fibrillation/flutter was defined as Minnesota codes 8-3-1 and 8-3-2. Evidence of previous myocardial infarction was defined as Minnesota codes 1–1 and 1–2 [Citation32]. Sinus tachycardia was defined as sinus rhythm with heart rate ≥100 beats per minute (bpm). Patients were defined as having right bundle branch block (RBBB) if coded with Minnesota codes 7-2, 7-3 and 7-8. RV strain pattern was defined as T wave inversion in leads V1-V4 with an amplitude of ≤−0.1 mV. S1Q3T3 pattern was defined as S wave ≥ 0.15 mV in lead I, Q wave ≥ 0.15mV and ≥ 0.03 s in lead III with T wave inversion ≤ 0,1 mV in lead III [Citation33]. Right axis deviation was defined as Minnesota codes 2–2 and 2–3. P pulmonale was defined as Minnesota code 9-2. An aggregate variable consisting of any ECG pattern suggestive of a right-sided heart abnormality (RBBB/RV strain/S1Q3T3/right axis deviation/P pulmonale) was constructed and named “Any right-sided abnormality”.
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and differences between groups were evaluated with Pearson χ2 tests, or Fischer’s exact test when appropriate. Continuous variables were inspected visually for normality on histograms and Q-Q plots, as well as with the Kolmogorov-Smirnov test. Variables with a non-normal distribution are expressed as the median and interquartile range (IQR). Differences between groups were tested with Mann-Whitney U test, and Spearman’s rank order was used for correlation analysis.
Predictors of NT-proBNP levels were analyzed with forced entry multivariable linear regression. Due to skewed distribution of NT-proBNP and creatinine, logarithmic transformation was performed. Age, sex, dyspnea upon exertion, log10 creatinine, self-reported diagnosis of COPD, previous and current smoking, and previous diagnoses of congestive heart failure, cancer, ischemic heart disease, cerebrovascular disease, atrial fibrillation/flutter (AF) or PE were included as independent variables in the model. The regression model was examined for goodness-of-fit (standardized residuals, deviance statistics), independence of errors (Durbin-Watson statistics), multicollinearity (variance inflation factor, tolerance statistics), and influence statistics (Cook’s distance, leverage statistics, and DFbeta).
A clean dataset was used without imputation, and missing data/loss to follow-up data were not addressed in the statistical analyses.
Data was analyzed using IBM SPSS statistics software version 28.
Results
Altogether 5793 subjects with an ICD-10-SE diagnosis of pulmonary embolism (I26) in 2005 were identified, and 3510 (60.6%) of them survived until 2007. Of these, 2105 subjects (60.0%) responded to the questionnaire with a median (IQR) duration between the acute PE and the completion of the questionnaire of 3.4 (3.0–3.7) years. 673 subjects (32.0%) reported improved health condition or absence of dyspnea and of the predefined risk factors for CTEPH, and 403 subjects (19.1%) declined further participation in the study. This resulted in 1029 subjects (48.9%) participating in the second phase of the study. The selection process for the study population is summarized in .
Baseline characteristics of the 1029 study participants and the 407 subjects who declined participation are summarized in . Altogether 1013 subjects had an ECG registered, of which 50.6% of subjects had an abnormal ECG, and 7.2% had atrial fibrillation/flutter. The prevalence of ECG abnormalities suggestive of right-sided heart abnormalities was low.
Table 1. Baseline characteristics with comparisons between study participants and subjects who declined participation.
NT-proBNP was analyzed in 1029 subjects. The median (IQR) NT-proBNP level was 178 (80–431) ng/L and 630 (61.2%) subjects had levels above clinical cut-off (>125 ng/L) [Citation34]. Median (IQR) NT-proBNP levels were higher in women (193 [99–401] ng/L) than in men (143.5 [65–512]), p = 0.01. Older age was associated with higher NT-proBNP levels, p < 0.001.
The associations between ECG findings, NT-proBNP levels and dyspnea are summarized in .
Table 2. ECG findings, NT-proBNP levels and their associations to exertional dyspnea.
Subjects with severe dyspnea (dyspnea upon climbing one stairs or dyspnea at rest) had higher levels of NT-proBNP than subjects with mild dyspnea (no reported dyspnea or dyspnea only upon rigorous exertion), with a median (IQR) NT-proBNP of 214 (95–566) ng/L vs 148 (73–348) ng/L, respectively, p < 0.001.
Multivariable linear regression analysis was carried out to predict log10 ] NT-proBNP levels based on a number of independent variables (). Age, female sex, log10 creatinine and previous diagnoses of congestive heart failure, ischemic heart disease, cerebrovascular disease and AF were found to be significant predictors of logNT-proBNP, whereas dyspnea upon exertion, self-reported diagnosis of COPD, smoking habits and previous diagnoses of cancer and PE were not. The dyspnea variable with the original 4 levels (see above) was tested in a separate regression analysis with almost identical results (data not shown).
Table 3. Multivariable analysis of predictors of log10 NT-proBNP levels.
Discussion
This study is, to our knowledge, the first study to investigate the long-term prevalence of ECG abnormalities and NT-proBNP levels after an acute PE. We found a low prevalence of ECG abnormalities suggestive of right-sided cardiac abnormalities with no associations between these and degree of dyspnea.
In a study on normotensive patients with acute PE, Vanni et al. reported RBBB in 10%, S1Q3T3 in 15% and negative T-wave in V1-V3 in 16%, and these abnormalities were also associated with echocardiographic signs of RVD [Citation13]. Similar findings were reported in a population of elderly patients with acute PE [Citation21]. However, the comparison with prevalence of ECG abnormalities in the acute phase should be made cautiously since these ECG abnormalities are predictive of short-term mortality, and our participants are long-time survivors. One should also bear in mind that the Minnesota classification is strict, and other studies used less strict definitions, such as negative T-wave in leads V1-V3 instead of in leads V1-V4 [Citation13,Citation35], which may lead to a higher prevalence of ECG abnormalities.
A Spanish study on a general population without history of cardiovascular disease (mean age 65.9 years) demonstrated prevalences of complete/incomplete RBBB of 3.2% and 4.6%, respectively [Citation36]. The RBBB prevalence of 6.4% in our study is somewhat higher, but the fact that cardiovascular comorbidity was common in our population suggests that ECG abnormalities indicative of right-sided cardiac abnormalities tend to normalize with time after acute PE.
We found that 49.3% of the study subjects had a normal ECG without any abnormalities and 7.2% had AF. In a Swedish general population screening study for AF in subjects aged 75-76 years, the prevalence of AF was 9.8% [Citation37], which is consistent with the prevalence in this study of 9.6% in the corresponding age-group.
61.2% of study subjects had NT-proBNP levels above normal range, however NT-proBNP levels were not associated with dyspnea in multivariable linear regression. The high prevalence of elevated NT-proBNP levels probably reflects the presence of multiple comorbidities. Our study-design led to an accumulation of subjects with various comorbidities due to the selection of subjects with reported dyspnea or known risk factors for CTEPH.
In the multivariable model, much of the variation in NT-proBNP levels could be attributed to age, female sex, kidney function and previous cardiovascular disease, but apparently dyspnea per se did not contribute, which may have several explanations. Residual confounding indicating unknown factors is possible as the R2 was 0.467. Hemoglobin levels, body mass index (BMI) or other measurements of obesity, obstructive sleep apnea or other pulmonary diseases as well as psychological disability are possible explanatory fators, although not available in our dataset.
Another possibility, supported by our previous findings [Citation10], is that a significant proportion of the subjects with long-term dyspnea after acute PE suffer from post-PE syndrome or chronic thromboembolic pulmonary disease (CTEPD). As proposed in recent years, patients with this condition have PE-related symptoms with persistent dyspnea and functional limitations and/or radiological signs of unresolved thrombus or RVD [Citation6,Citation38–40]. These patients may have normal NT-proBNP levels despite dyspnea, since RVD is not a mandatory criterion for the post-PE syndrome/CTEPD [Citation6,Citation38–40]. Additionally, NT-proBNP levels are usually lower in the presence of RVD compared to left ventricular dysfunction [Citation41].
An obvious limitation in this study is that the subjects were selected on basis of symptoms or risk factors for CTEPH, and that no control group of asymptomatic PE patients was available. Furthermore, dyspnea is a subjective symptom and objective measures of functional limitation was not accessible. Additionally, data regarding PE severity at diagnosis and anticoagulant treatment was not available, both of which could possibly have an influence on long-term ECG abnormalities, symptoms and NT-proBNP levels.
The studied population was a Swedish national population, which probably means that the generalizability of the results is good. Subjects who declined participation were older and had more comorbidity than the studied subjects resulting in potential selection/survival bias reducing generalizeability. Finally, changes in treatment for PE since 2005, when subjects in this study had their PE, might also influence generalizability of the results.
In conclusion, we found ECG abnormalities suggestive of RVD to be uncommon at long-term follow-up post-acute PE and they were not associated with the degree of self-reported dyspnea. We found no association between the degree of dyspnea and levels of NT-proBNP. Overall, this indicates that the value of resting 12-lead ECG and NT-proBNP in the long-term follow-up of an acute PE, probably lies in differential diagnostics.
Ethical approval
This study was approved by the Regional Ethics Review Board in Umeå, Sweden (07-074 M). The Ethics Board at the Swedish National Board of Health and Welfare reviewed and approved the extraction of data from the Swedish National Patient Register (34-9322-2007).
Supplemental Material
Download MS Word (20.4 KB)Acknowledgements
The research behind this paper would not have been possible without the extraordinary work done by late Christer Backman, MD, who interpreted and coded over one thousand ECG:s. We also want to acknowledge Professor Kurt Boman for enabling the analysis of NT-proBNP and creatinine.
Furthermore, we are very grateful to Owe Luhr, Yvette Palm Jensen, and Kristian Broms at Actelion Pharmaceuticals, Sweden, for practical and financial support; to Lucy Fisher, fellow PhD student for greatly appreciated help with language editing; and to Camilla Ring, research nurse, for incredible work with questionnaires and contacting the authorities, clinics, and hundreds of patients with PE.
Disclosure statement
No potential conflict of interest was reported by the author(s). S.S. has participated in advisory boards for Actelion/Jansen, and has received speaker’s honoraria from Actelion/Jansen, for presentations on topics outside the submitted study. T.A. received speaker’s honoraria from AstraZeneca, Vifor Pharma and Boeringer Ingelheim and participated in advisory boards for Vifor Pharma and Pharmacosmos.
Additional information
Funding
References
- Elias A, Mallett S, Daoud-Elias M, et al. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. doi: 10.1136/bmjopen-2015-010324.
- Vedovati MC, Germini F, Agnelli G, et al. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013;11(12):2092–2102. doi: 10.1111/jth.12429.
- Qaddoura A, Digby GC, Kabali C, et al. The value of electrocardiography in prognosticating clinical deterioration and mortality in acute pulmonary embolism: a systematic review and meta-analysis. Clin Cardiol. 2017;40(10):814–824. doi: 10.1002/clc.22742.
- El-Menyar A, Sathian B, Al-Thani H. Elevated serum cardiac troponin and mortality in acute pulmonary embolism: systematic review and meta-analysis. Respir Med. 2019;157:26–35. doi: 10.1016/j.rmed.2019.08.011.
- Prosperi-Porta G, Ronksley P, Kiamanesh O, et al. Prognostic value of echocardiography-derived right ventricular dysfunction in haemodynamically stable pulmonary embolism: a systematic review and meta-analysis. Eur Respir Rev. 2022;31(166):220120. doi: 10.1183/16000617.0120-2022.
- Sista AK, Klok FA. Late outcomes of pulmonary embolism: the post-PE syndrome. Thromb Res. 2017;164:157–162. doi: 10.1016/j.thromres.2017.06.017.
- Alblas H, van Kan C, van Het Westeinde SC, et al. Persistent dyspnea after acute pulmonary embolism is related to perfusion defects and lower long-term quality of life. Thromb Res. 2022;219:89–94. doi: 10.1016/j.thromres.2022.09.008.
- Yuriditsky E, Horowitz JM, Lau JF. Chronic thromboembolic pulmonary hypertension and the post-pulmonary embolism (PE) syndrome. Vasc Med. 2023;28(4):348–360. doi: 10.1177/1358863X231165105.
- Klok FA, van Kralingen KW, van Dijk APJ, et al. Prevalence and potential determinants of exertional dyspnea after acute pulmonary embolism. Respir Med. 2010;104(11):1744–1749. doi: 10.1016/j.rmed.2010.06.006.
- Nilsson LT, Andersson T, Larsen F, et al. Dyspnea after pulmonary embolism: a nation-wide population-based case-control study. Pulm Circ. 2021;11(4):20458940211046831. doi: 10.1177/20458940211046831.
- van der Meer RW, Pattynama PMT, van Strijen MJL, et al. Right ventricular dysfunction and pulmonary obstruction index at helical CT: prediction of clinical outcome during 3-month follow-up in patients with acute pulmonary embolism. Radiology. 2005;235(3):798–803. doi: 10.1148/radiol.2353040593.
- Konstantinides SV, Vicaut E, Danays T, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol. 2017;69(12):1536–1544. doi: 10.1016/j.jacc.2016.12.039.
- Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009;122(3):257–264. doi: 10.1016/j.amjmed.2008.08.031.
- Henzler T, Roeger S, Meyer M, et al. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 2012;39(4):919–926. doi: 10.1183/09031936.00088711.
- Reesink HJ, Tulevski II, Marcus JT, et al. Brain natriuretic peptide as noninvasive marker of the severity of right ventricular dysfunction in chronic thromboembolic pulmonary hypertension. Ann Thorac Surg. 2007;84(2):537–543. doi: 10.1016/j.athoracsur.2007.04.006.
- Gutte H, Mortensen J, Jensen CV, et al. ANP, BNP and D-dimer predict right ventricular dysfunction in patients with acute pulmonary embolism. Clin Physiol Funct Imaging. 2010;30(6):466–472. doi: 10.1111/j.1475-097X.2010.00967.x.
- Yardan T, Altintop L, Baydin A, et al. B-type natriuretic peptide as an indicator of right ventricular dysfunction in acute pulmonary embolism. Int J Clin Pract. 2008;62(8):1177–1182. doi: 10.1111/j.1742-1241.2007.01380.x.
- Cavallazzi R, Nair A, Vasu T, et al. Natriuretic peptides in acute pulmonary embolism: a systematic review. Intensive Care Med. 2008;34(12):2147–2156. doi: 10.1007/s00134-008-1214-5.
- Ohigashi H, Haraguchi G, Yoshikawa S, et al. Comparison of biomarkers for predicting disease severity and long-term respiratory prognosis in patients with acute pulmonary embolism. Int Heart J. 2010;51(6):416–420. doi: 10.1536/ihj.51.416.
- Nithianandan H, Reilly A, Tritschler T, et al. Applying rigorous eligibility criteria to studies evaluating prognostic utility of serum biomarkers in pulmonary embolism: a systematic review and meta-analysis. Thromb Res. 2020;195:195–208. doi: 10.1016/j.thromres.2020.07.037.
- Bolt L, Lauber S, Limacher A, et al. Prognostic value of electrocardiography in elderly patients with acute pulmonary embolism. Am J Med. 2019;132(12):e835–e43. doi: 10.1016/j.amjmed.2019.05.041.
- Thomson D, Kourounis G, Trenear R, et al. ECG in suspected pulmonary embolism. Postgrad Med J. 2019;95(1119):12–17. doi: 10.1136/postgradmedj-2018-136178.
- Digby GC, Kukla P, Zhan Z-Q, et al. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: a consensus paper. Ann Noninvasive Electrocardiol. 2015;20(3):207–223. doi: 10.1111/anec.12278.
- Boon GJAM, Ende-Verhaar YM, Bavalia R, et al. Non-invasive early exclusion of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the InShape II study. Thorax. 2021;76(10):1002–1009. doi: 10.1136/thoraxjnl-2020-216324.
- Klok FA, Surie S, Kempf T, et al. A simple non-invasive diagnostic algorithm for ruling out chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Thromb Res. 2011;128(1):21–26. doi: 10.1016/j.thromres.2011.03.004.
- Bonderman D, Wexberg P, Martischnig AM, et al. A noninvasive algorithm to exclude pre-capillary pulmonary hypertension. Eur Respir J. 2011;37(5):1096–1103. doi: 10.1183/09031936.00089610.
- Kim NH, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2012;21(123):27–31. doi: 10.1183/09059180.00009111.
- Blackburn H, Keys A, Simonson E, et al. The electrocardiogram in population studies. A classification system. Circulation. 1960;21(6):1160–1175. doi: 10.1161/01.cir.21.6.1160.
- Surawicz B, Childers R, Deal BJ, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):976–981. doi: 10.1016/j.jacc.2008.12.013.
- Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e241-50–e250. doi: 10.1161/CIRCULATIONAHA.108.191096.
- Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397.
- Sandler LL, Pinnow EE, Lindsay J. The accuracy of electrocardiographic Q waves for the detection of prior myocardial infarction as assessed by a novel standard of reference. Clin Cardiol. 2004;27(2):97–100. doi: 10.1002/clc.4960270212.
- Punukollu G, Gowda RM, Vasavada BC, et al. Role of electrocardiography in identifying right ventricular dysfunction in acute pulmonary embolism. Am J Cardiol. 2005;96(3):450–452. doi: 10.1016/j.amjcard.2005.03.099.
- Mueller C, McDonald K, de Boer RA, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715–731. doi: 10.1002/ejhf.1494.
- Casazza F, Pacchetti I, Rulli E, et al. Prognostic significance of electrocardiogram at presentation in patients with pulmonary embolism of different severity. Thromb Res. 2018;163:123–127. doi: 10.1016/j.thromres.2018.01.025.
- Alventosa-Zaidin M, Guix Font L, Benitez Camps M, et al. Right bundle branch block: prevalence, incidence, and cardiovascular morbidity and mortality in the general population. Eur J Gen Pract. 2019;25(3):109–115. doi: 10.1080/13814788.2019.1639667.
- Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation. Circulation. 2015;131(25):2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343.
- Klok FA, van der Hulle T, den Exter PL, et al. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28(6):221–226. doi: 10.1016/j.blre.2014.07.003.
- van Kan C, van der Plas MN, Reesink HJ, et al. Hemodynamic and ventilatory responses during exercise in chronic thromboembolic disease. J Thorac Cardiovasc Surg. 2016;152(3):763–771. doi: 10.1016/j.jtcvs.2016.05.058.
- Capone C, Valentini A, Spinillo SL, et al. Radiological differences between chronic thromboembolic pulmonary disease (CTEPD) and chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol. 2021;31(8):6230–6238. doi: 10.1007/s00330-020-07556-4.
- Morrison LK, Harrison A, Krishnaswamy P, et al. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol. 2002;39(2):202–209. doi: 10.1016/s0735-1097(01)01744-2.
