Abstract
Objectives: S-flurbiprofen plaster (SFPP) is a novel non-steroidal anti-inflammatory drug (NSAID) patch, intended for topical treatment for musculoskeletal diseases. This trial was conducted to examine the effectiveness of SFPP using active comparator, flurbiprofen (FP) patch, on knee osteoarthritis (OA) symptoms.
Methods: This was a phase III, multi-center, randomized, adequate, and well-controlled trial, both investigators and patients were blinded to the assigned treatment. Enrolled 633 knee OA patients were treated with either SFPP or FP patch for two weeks. The primary endpoint was improvement in knee pain on rising from the chair as assessed by visual analogue scale (rVAS). Safety was evaluated through adverse events (AEs).
Results: The change in rVAS was 40.9 mm in SFPP group and 30.6 mm in FP patch group (p < 0.001). The incidence of drug-related AEs at the application site was 9.5% (32 AEs, 29 mild and 3 moderate) in SFPP and 1.6% in FP patch (p < 0.001). Withdrawals due to AE were five in SFPP and one in FP patch.
Conclusions: The superiority of SFPP in efficacy was demonstrated. Most of AEs were mild and few AEs led to treatment discontinuation. Therefore, SFPP provides an additional option for knee OA therapy.
Introduction
Knee osteoarthritis (OA) is a common type of arthritis seen in the middle-aged and elderly population [Citation1,Citation2] that is accompanied by chronic pain, inflammation, and impaired motor function, leading to deterioration of quality of life [Citation3].
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for conservative therapy aimed at relieving symptoms of knee OA [Citation4]. Since oral use of NSAIDs has high risk of gastrointestinal disorders, topical NSAIDs are increasingly used as they are safer and better tolerated because of avoiding the direct action of NSAIDs on gastrointestinal tract [Citation5–7]. From an efficacy perspective, most topical NSAIDs are considered as less effective than its oral formulation, due to lower concentration at affected site than oral formulation [Citation8,Citation9].
S-flurbiprofen plaster (SFPP) is an innovative NSAID patch intended for topical treatment of OA. Its active ingredient is S-flurbiprofen, an enantiomer of racemic flurbiprofen (FP) and a strong COX inhibitor [Citation10]. SFPP achieves superior penetration of the active ingredient into the synovial tissue in comparison with the existing FP patch [Citation11], which has been widely used in the treatment of musculoskeletal pain in OA and rheumatoid arthritis patients [Citation12,Citation13]. Therefore, SFPP was considered to be the agent that indicates a strong efficacy while avoiding direct gastrointestinal disorders.
In a previous placebo-controlled dose-finding study conducted in knee OA patients, SFPP (containing 40 mg S-flurbiprofen) significantly improved symptoms of knee OA in comparison with placebo, VAS change in knee pain from baseline to trial end was dose-dependent, least squares mean was 29.5, 31.5, 32.0, and 35.6 mm in placebo, SFPP 10, 20, and 40 mg, respectively (p = 0.001 in 40 mg vs. Placebo). There was no significant difference in the incidence of drug related AEs among four groups. Based on these results, 40 mg was determined as the clinical dosing regimen of SFPP.
The present study was aimed at investigating the efficacy and safety of SFPP by comparing with conventional FP patch.
Patients and methods
Trial design
This was a phase III, multi-center, randomized, active-controlled, adequate, and well-controlled trial in which both investigators and patients were blinded to the assigned treatment of each patient. In the planning of the study method, we referred to the guidelines on clinical study proposals of the European Medicines Agency and of the US Food and Drug Administration [Citation14,Citation15]. The study was conducted at 70 study sites in Japan from November 2011 to October 2013, according to the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol and the informed consent form were approved by the relevant institutional review boards. The investigator explained the study details to all patients using a document and obtained written informed consent from all patients prior to study participation. This study was registered with the JAPIC Clinical Trials Information Center (JapicCTI-111693).
Patients
A total of 869 patients underwent screening, 236 were excluded, 633 of whom were randomly assigned to study treatment groups (316 to SFPP group and 317 to FP patch group). The baseline characteristics of the patients in SFPP and FP patch treatment groups were similar (). The percentage of women was 83.9% in both groups, average age were 67.1 in SFPP group and 67.6 in FP patch group.
Table 1. Baseline characteristics of the study population.
The study enrolled knee OA patients aged ≥20 years who had unilateral knee pain and were classified as Kellgren–Lawrence grade [Citation16] II or III (knee for evaluation, the opposite knee was the same or lower grade) via central assessment of X-ray images. Patients using oral and/or topical NSAIDs continuously to treat the knee for at least three weeks prior to the first visit were selected. In addition, the following inclusion criteria was added: through washout of prior NSAIDs, worsening of pain on rising from the chair with an increase in 100 mm-visual analogue scale score (rVAS) by at least 15 mm and in the investigator's assessment of pain (pain on ascending or descending stairs) by at least one point. Furthermore, rVAS before washout was <80 mm, rVAS after washout was ≥40 mm, and pain in the knee for evaluation was stronger than that in the opposite knee were also included.
Patients were excluded who had complication of rheumatoid arthritis, history of knee surgery, malignant tumor, neuropsychiatric disease, or serious disease.
Study protocol
SFPP is an ochre-colored tape-type patch containing 40 mg of S-flurbiprofen in each patch. FP patch is a commercially available, white-colored gel-type patch containing 40 mg of FP (20 mg of S-flurbiprofen) in each patch. Both were supplied at a size of 10 × 14 cm from Tokuhon Corporation (Tokyo, Japan).
Patients were treated with either SFPP or FP patch for two weeks. The applied SFPP and FP patches were replaced every 24 and 12 h, respectively, corresponding to a daily S-flurbiprofen dose of 40 mg.
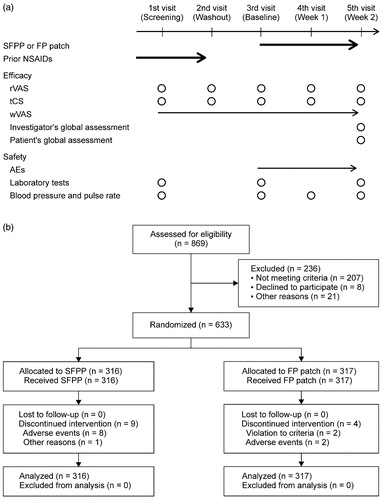
Each patient made five weekly visits to the study site, and the investigator assessed the efficacy and the safety according to the schedule shown in .
Figure 1. Study schedule (a) and flow chart of patients throughout the trial (b). Bold arrow = treatment; arrow = daily assessment; circle = assessment at the visit; rVAS = visual analogue scale on rising from the chair; tCS = total clinical symptoms score; wVAS = visual analogue scale on walking.
The following drugs were not allowed during the treatment period: NSAIDs, opioid analgesics, corticosteroids (except topical corticosteroids), intraarticular knee injections (hyaluronic acid, local anesthetics), antiulcer and gastrointestinal agents, and agents known to interact with FP (such as lithium).
Exercise and physical therapy of the lower legs and hips was allowed only if the patient had already been in rehabilitation for at least two weeks prior to the first visit.
Randomization and concealment
The randomization table, SFPP or FP patch groups at a ratio of 1:1, was constructed by an independent contract research organization using SAS®9.1 (Carry, NC) and concealed from any other participant of the trial. The study was conducted by keeping patients and investigators blinded to the treatment assignments, while the clinical research coordinators were unblinded, as follows. Patients were randomly assigned to either study drug independently of investigators or clinical research coordinators at baseline. Blinding of patients was made by keeping them ignorant of different regimen and appearance of SFPP and FP patch. Packaged study drugs were not distinguishable from appearance including size and weight which confirmed by third party. After assignment, the clinical research coordinator opened the package, recognized the key and explained only the regimen (once or twice daily, but never informed the key) to the patient in absence of the investigator. Prior to the investigator's examination at week 1 and 2, each patient removed the study drug patch from the affected site, investigator was kept away from the study drugs.
Assessments
The primary efficacy endpoint was pain in the knee on rising up evaluated by the change in rVAS from baseline to the end of the trial (ΔrVAS). rVAS was self-assessed by each patient. The assessment was performed following the standardized procedure and in the presence of the clinical research coordinator. To standardize, all study sites used the same chair and adjusted the seat height according to the patient's height for a sitting knee angle of 110°, in order to place similar load on the knee on rising up for all patients. Each patient was instructed to sit on the chair, rest for 5 min, and rise up.
The secondary efficacy endpoints were the total clinical symptoms score (change from baseline to the end of the trial, ΔtCS) and investigator's global assessment. Clinical symptoms were assessed by the investigator for the nine components on a 4-point scale (0 = no symptom, 1 = mild, 2 = moderate, and 3 = severe) and the total score was calculated (0 − 27). Components of pain were passive exercise, ascending or descending stairs, rest, and tenderness, those of inflammation were swelling and ballottement, and those of disability of active daily living were sitting down, rising up, and walking. Investigator's global assessment of improvement were performed using a 5-point scale (marked, moderate, mild, not change, and worse).
Other endpoints included each patient's global assessment and pain of knee on walking. Patient's global assessment of improvement were rated by same scale as investigator's global assessment. For the assessment of pain on walking, each patient self-assessed every day by VAS using a diary, and change from baseline to the end of the trial (ΔwVAS) was calculated.
For the safety assessment, the investigator carefully examined adverse events (AEs) throughout the study period and determined their causal relationships to the study drug. In addition, the investigator rated the severity of each AE on a 3-point scale (mild = treatment not required or daily living not affected, moderate = some treatment required, severe = particular emergency treatment required).
For laboratory tests, blood pressure, and pulse rate data, the investigator assessed each parameter for the presence of abnormal variation by considering whether the change from baseline was clinically significant. Abnormal parameter variations were reported as AEs.
Statistical methods
Analyses indicated that 304 patients per treatment group would be necessary to confirm the superiority of SFPP to FP patch with a difference in ΔrVAS of 4.1 mm between the groups, a common standard deviation of 18 mm, a significance level of 2.5% (one-sided), and a power of 80%. Because of the assumption that some patients will not complete the study, 310 patients were needed in each treatment group.
All analyses were carried out according to the pre-specified statistical analysis plan by using SAS®9.2 (Carry, NC). The significance levels were 2.5% (one-sided) at the primary analysis. Missing data at the end of the study were imputed using the last observation carried forward (LOCF) method. Missing data at other time-points were not imputed. Normal probability plot and Shapiro-Wilk test were used to evaluate whether the assumptions of normality and homogeneity of variance were reasonable. No interim analysis was performed in this study.
For efficacy analyses, the primary population was the full analysis set (FAS), which comprised all patients who had received the study drug at least once and from which one efficacy data had been obtained at least after drug application. The primary outcome and other continuous outcomes were analyzed using analysis of covariance (ANCOVA) with the treatment group as a fixed effect and baseline as a covariate. Categorical outcomes were analyzed using Wilcoxon's rank sum test.
Safety analyses were based on the safety population, which comprised all patients who had been applied the study drug at least once. The number and percentage of patients who developed AE were summarized using the Medical Dictionary for Regulatory Activities (MedDRA/J ver.16.1, McLean, VA) terminologies (system organ class and preferred term). The incidence proportions of AE and drug-related AE were analyzed using the chi-square test without continuity correction. Since SFPP and FP patch are topical formulation, AEs were summarized separately for local AEs at the application sites (skin symptoms) and systemic AEs. Continuous outcomes in laboratory tests and vital signs were analyzed using Student's t-test.
Results
About 620 of 633 subjects (97.9%) completed the study and 13 discontinued study participation ().
Efficacy
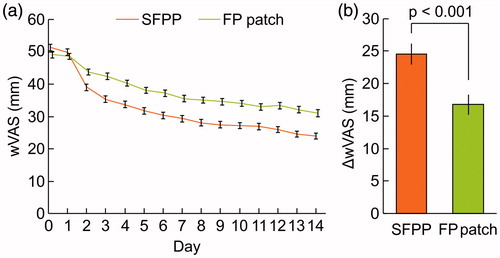
The least squares mean (95% confidence interval) of ΔrVAS were 40.9 (39.3–42.6) mm in SFPP group and 30.6 (28.9−32.2) mm in FP patch group, SFPP group showed significantly reduced pain in comparison with that of FP patch group (p < 0.001, , and Supplementary Table 1). The least squares mean of ΔtCS were 7.3 (6.9–7.6) mm in SFPP group and 5.3 (5.0–5.6) mm in FP patch group (p < 0.001, , and Supplementary Table 2). In the investigator's global assessment, 43.4% were “marked” and 36.7% were “moderate” in SFPP group, while 14.8% were “marked” and 29.0% were “moderate” in FP patch group (p < 0.001, ). In the patient's global assessment, 31.3% “marked” and 51.6% were “moderate” in SFPP group, and 12.0% were “marked” and 33.4% were “moderate” in FP patch group (p < 0.001, ). The least squares mean of ΔwVAS was 24.5 (22.9–26.1) mm in SFPP group and 16.8 (15.2–18.3) mm in FP patch group (p < 0.001, , and Supplementary Table 3).
Figure 2. Time courses and changes in rVAS and tCS, n = 315 in SFPP group and n = 317 in FP group. rVAS = visual analogue scale on rising from the chair; tCS = total clinical symptoms score. In (a) and (c), mean and standard error. In (b) and (d), least squares mean and 95% confidence interval of change from baseline to the end of the trial.
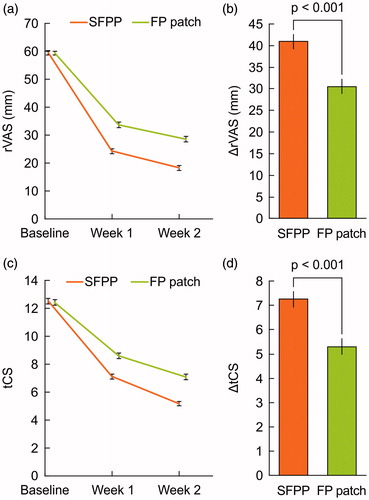
Figure 3. Investigator's global assessment (a) and patient's global assessment (b) for efficacy, n = 316 in SFPP group and n = 317 in FP group.
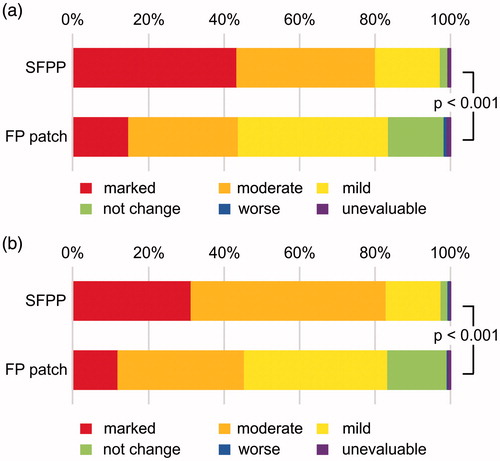
Figure 4. Time courses and changes in wVAS. wVAS = visual analogue scale on walking. In (a), mean and standard error, by observed case data. Treatment with the study drug was initiated on day 1. In (b), least squares mean and 95% confidence interval of the change from baseline to the end of the trial, n = 315 in SFPP group and n = 317 in FP group.
One subject in the SFPP group was excluded because of a lack of data at the end of the trial in rVAS, tCS, and wVAS.
Safety
The incidence rates of AEs were 16.5% in SFPP group and 12.0% in FP patch group (). Treatment discontinuation owing to drug-related AE was reported in five subjects in SFPP group and one subject in FP patch group. Details were one subject with vertigo and others with application site AEs. Vertigo was observed as drug-related serious AE in SFPP group.
Table 2. Adverse events (AEs) by treatment group.
The incidence rates of drug-related AEs at the application site were 9.5% in the SFPP and 1.6% in FP patch (p < 0.001). In the SFPP group, 30 subjects developed 32 drug-related AEs at the application site, including 29 mild and three moderate AEs. In FP patch group, five subjects developed AEs at the application site, which were all mild in severity. An incidence of 0.6% (2 of 316 subjects) of drug-related gastrointestinal AEs and no cardiovascular AEs were reported in SFPP group.
In laboratory test and pulse rate, minimal difference with statistical significance was observed between two groups (Supplementary Table 4).
Discussion
Validity
Complete double-blinding was not possible because SFPP and FP patch were different in appearance (color and touch) and administered on different regimens (once or twice daily). In addition, making a double-dummy study design was infeasible, too, because SFPP and FP patch are formulation which acts on the local site and needed to be applied to the site of pain. In view of the size of the knee and the patch (10 × 14 cm), it is impossible to apply both patches on one knee. Therefore, we conducted this study by keeping patients and investigators blinded to the treatment assignments, while the clinical research coordinators unblinded. We made various procedures for that, and achieved the present study as adequate and well-controlled study which leads to the highly reliable results.
Efficacy
We demonstrated the superiority of SFPP over FP patch in the knee pain as the primary endpoint, ΔrVAS. The rVAS was 59.5 mm at baseline and markedly improved to 18.5 mm after two weeks of treatment with SFPP. The improvement achieved by SFPP was 10.4 mm greater than that produced by FP patch. In all other endpoints, SFPP also showed an improvement with statistical significance in comparison with FP patch, both in patient's self-assessments (ΔrVAS, ΔwVAS, and global assessments) and investigator's assessments (clinical symptoms and global assessment). These results indicated the robust efficacy of SFPP.
In the tCS component, significant intergroup differences were observed in pain (e.g. pain ascending or descending stairs), inflammation (e.g. swelling), and disability in active daily living (e.g. squatting motion, walking) (data not shown), showing that SFPP provided global improvement in OA-specific symptoms.
Furthermore, SFPP acted rapidly in comparison with FP patch, with a significant improvement in rVAS and clinical symptoms observed at week 1. Surprisingly, greater improvement in wVAS was also observed for SFPP in comparison with FP patch from the day after treatment initiation. We have demonstrated that SFPP showed 15 times higher synovial tissue concentration of S-flurbiprofen compared to FP patch, after 12 h application in knee OA patients [Citation11]. Dramatically improved absorption of SFPP seemed to be attributable to the early onset of analgesic efficacy.
Safety
In the safety assessment, most of AEs at the application site of SFPP (29 of 32 AEs) were mild in severity, SFPP treatment was associated with more AEs at the application site than FP patch. Few AEs led to treatment discontinuation or were considered clinically significant. SFPP and FP patch have different characteristics; SFPP is stronger in both adhesive and peeling forces than FP patch. In a previous placebo-controlled study with the same SFPP formulation, the incidence of AEs at the application site across SFPP and placebo groups was not statistically significant, 10.4% (14/134 subjects) in SFPP 40 mg group and 5.5% (7/127 subjects) in placebo group. Therefore, the difference observed in the present study may be attributed to the differing adhesive and peeling forces of the two patches.
Although SFPP was concerned about systemic AEs from its higher systemic exposure than FP patch [Citation11], no significant clinical safety issues were identified by two weeks application. The incidence rate of gastrointestinal disorders was 0.6% (2 of 316 subjects) with SFPP, which was markedly lower than that of patients treated with oral FP (approximately 9%, in premarketing trials) [Citation17]. We consider that it is due to the difference in the route of administration. Increased blood creatinine and blood urea were observed, but the increment were not clinically significant, indicating that SFPP did not lead renal impairment. In addition, no AEs of the cardiovascular system were reported with SFPP. NSAIDs bearing benzophenone in chemical structure like ketoprofen is known to cause photosensitivity and related AEs [Citation18,Citation19]. S-flurbiprofen is free from benzophenone and no subjects in the present study experienced photosensitivity.
Limitation
SFPP was evaluated by continuous application for two weeks based on EMA guideline [Citation14], but the safety and efficacy of SFPP in long-term treatment should be investigated in future studies, because knee OA is a chronic disease that requires treatment for a long period. Besides, this study was not complete double-blinding.
Conclusion
This study demonstrated the potent and immediate analgesic effect of SFPP over FP patch. The strong analgesic effect and rapid onset of SFPP facilitated relief of patients with knee OA by alleviating pain associated with walking and ascending or descending stairs, as well as other disabilities. It was also indicated the safety of SFPP was comparable to FP patch. Therefore, SFPP might be one of the most innovative topical NSAID patch products, a class of medications whose value is being increasingly recognized.
Conflict of interest
I. Yataba, N. Otsuka, and I. Matsushita are employees of Taisho Pharmaceutical Co., Ltd. H. Matsumoto has received a consultant fee from Taisho Pharmaceutical Co., Ltd. Y. Hoshino had received a consultant fee from Taisho Pharmaceutical Co., Ltd. while at the author's previous affiliation.
This study was sponsored by Taisho Pharmaceutical Co., Ltd.
Supplementary material available online
Supplementary Table 4
Download MS Word (55.5 KB)Supplementary Table 3
Download MS Word (35.5 KB)Supplementary Table 2
Download MS Word (41.5 KB)Supplementary Table 1
Download MS Word (42.5 KB)Acknowledgements
We would like to thank the SFPP investigators who contributed to this study (correct at the time of the clinical trial):
Daisuke Kawamura, Susumu Asano, Takumi Takakuwa, and Yoshimitsu Aoki (Hokkaido); Sadafumi Kato (Iwate); Shigeru Mori and Tomomaro Kawamata (Miyagi); Kunio Kamatani, Susumu Maehara, and Yasuo Kobuna (Gunma); Akira Kobayashi, Ruriko Ozawa, Ryoichi Yamazaki, and Yasuhiro Nemoto (Saitama); Hidenori Honda, Masashi Kimoto, Minoru Irahara, Yasutomo Matsubayashi, and Yoshinori Nakata (Chiba); Eijiro Okumura, Fumitoshi Omura, Hideaki Yoshida, Hiroaki Kataoka, Hisayuki Izaki, Ko Matsumoto, Koichi Tanaka, Masahiro Shibasaki, Masakazu Sekiguchi, Masayoshi Kihara, Minako Murata, Ryuji Ikeda, Sanshiro Hashimoto, Shigeru Tsukahara, Takashi Yokoyama, Takeshi Inoue, Toshiyuki Wakabayashi, Yu Miyazaki, and Yutaka Suzuki (Tokyo); Akihito Tomonaga, Hideyuki Yamakawa, Hiroaki Shibata, Keita Watanabe, Kuniaki Katayama, Masato Kasuga, Masayuki Akiyama, and Mitsuru Ikeda (Kanagawa); Motoaki Fujimori (Yamanashi); Chisato Kato (Aichi); Ichiro Yokoyama, Satoshi Sobajima, and Takumi Mori (Osaka); Hideo Watanabe, and Shuichi Ohta (Hyogo); Takeshi Tokito (Yamaguchi); Masahiro Otani, Mitsuru Kajitani, and Norio Yamanaka (Kochi); Hiroshi Nomiyama, Ichiro Hoshiko, Keita Miyanishi, Koichi Kamihirakawa, Mitsuyoshi Kanbara, Nobuyuki Matsuguchi, Shinichiro Nakayama, Shiro Hiroshima, Takaaki Yoshimoto, Takahide Kozuma, Takahiko Sannomiya, and Yosuke Kanazawa (Fukuoka); Hiizu Hara and Kei Shibayama (Saga); and Takanori Nagamine (Kagoshima).
The authors would like to thank Taeko Hosaka for her assistance with drafting and revising the article.
The clinical study registration number: JapicCTI-111693
References
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
- Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–69.
- Michaud CM, McKenna MT, Begg S, Tomijima N, Majmudar M, Bulzacchelli MT, et al. The burden of disease and injury in the United States 1996. Popul Health Metr. 2006;4:11.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54.
- Tomisato W, Tsutsumi S, Hoshino T, Hwang HJ, Mio M, Tsuchiya T, et al. Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem Pharmacol. 2004;67:575–85.
- Tomisato W, Tanaka K, Katsu T, Kakuta H, Sasaki K, Tsutsumi S, et al. Membrane permeabilization by non-steroidal anti-inflammatory drugs. Biochem Biophys Res Commun. 2004;323:1032–9.
- Sinha M, Gautam L, Shukla PK, Kaur P, Sharma S, Singh TP. Current perspectives in NSAID-induced gastropathy. Mediators Inflamm. 2013;2013:258209.
- Rolf C, Engström B, Beauchard C, Jacobs LD, Le Liboux A. Intra-articular absorption and distribution of ketoprofen after topical plaster application and oral intake in 100 patients undergoing knee arthroscopy. Rheumatology (Oxford). 1999;38(6):564–7.
- Miyatake S, Ichiyama H, Kondo E, Yasuda K. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br J Clin Pharmacol. 2009;67(1):125–9.
- Carabaza A, Cabré F, Rotllan E, Gómez M, Gutiérrez M, García ML, Mauleón D. Stereoselective inhibition of inducible cyclooxygenase by chiral nonsteroidal antiinflammatory drugs. J Clin Pharmacol. 1996;36(6):505–12.
- Yataba I, Otsuka N, Matsushita I, Kamezawa M, Yamada I, Sasaki S, et al. Plasma pharmacokinetics and synovial concentrations of S-flurbiprofen plaster in humans. Eur J Clin Pharmacol. 2016;72(1):53–9.
- Coetzer R. Patches for pain relief. S Afr Pharm J. 2013;80:12–15.
- Martens M. Efficacy and tolerability of a topical NSAID patch (local action transcutaneous flurbiprofen) and oral diclofenac in the treatment of soft-tissue rheumatism. Clin Rheumatol. 1997;16(1):25–31.
- European Medicines Agency. Committee for medicinal products for human use, guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis. 2010. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003440.pdf [last accessed 20 Apr 2016].
- U.S. Department Of Health And Human Services Food and Drug Administration. Guidance for industry: clinical development programs for drugs, devices, and biological products intended for the treatment of osteoarthritis (OA), draft guidance. 1999. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071577.pdf [last accessed 20 Apr 2016].
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
- Medicine Interview Form of Froben® (in Japanese). 2012; Version 8. Available from: http://www.info.pmda.go.jp/go/interview/1/200022_1149011D1032_1_080_1F [last accessed 20 Apr 2016].
- Nakazawa T, Shimo T, Chikamatsu N, Igarashi T, Nagata O, Yamamoto M. Study on the mechanism of photosensitive dermatitis caused by ketoprofen in the guinea pig. Arch Toxicol. 2006;80(7):442–8.
- Seto Y, Ohtake H, Kato M, Onoue S. Phototoxic risk assessments on benzophenone derivatives: photobiochemical assessments and dermal cassette-dosing pharmacokinetic study. J Pharmacol Exp Ther. 2015;354(2):195–202.
