Abstract
Objectives: To assess the determinants of Patient’s Global Assessment of Disease Activity (PtGA) and Physician’s Global Assessment of Disease Activity (PhGA) in overall and Japanese patients with rheumatoid arthritis (RA) from two large randomized controlled trials.
Methods: Post hoc analysis of overall and Japanese RA patients who had previous inadequate responses to methotrexate or who had no/minimal previous disease-modifying antirheumatic drug treatment. We examined correlations between PtGA/PhGA and tender joint count in 28 joints (TJC28), swollen joint count in 28 joints (SJC28), inflammatory markers, pain visual analog scale (VAS), and other patient-reported outcomes at baseline, Week 12, and Week 24. Determinants of PtGA/PhGA were identified.
Results: In overall populations, pain VAS was the main determinant of PtGA, whereas TJC28 was the main determinant of PhGA in both studies. In Japanese populations, consistent with overall populations, pain VAS was the main determinant of PtGA in both studies; in contrast to overall populations, pain VAS and SJC28/TJC28 played an important role in PhGA.
Conclusion: Pain was the most important determinant of PtGA, whereas determinants of PhGA varied between populations/studies and were mostly explained by pain/joint counts. Physicians should be aware of patients’ perceptions of disease activity when performing assessments/prescribing treatments.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease characterized by pain in and stiffness of affected joints, with an estimated prevalence of 0.33–1.1% in Northern and Southern Europe, North America, and developing countries [Citation1], and 1% in Japan [Citation2]. The Patient’s Global Assessment of Disease Activity (PtGA) and Physician’s Global Assessment of Disease Activity (PhGA) are important measures in the treatment of RA, but often provide discordant results [Citation3–9]. Both these measures are assessed as part of three commonly used measures of disease activity in clinical trials, the American College of Rheumatology Core Data Set, Simplified Disease Activity Index, and Clinical Disease Activity Index (CDAI) [Citation10]. Patient–physician discordance in global assessment has been widely reported for studies conducted in many countries around the world, including Japan, with PtGA typically being higher than PhGA [Citation3–9,Citation11]. For instance, studies carried out in many countries around the world have reported positive discordance (PtGA > PhGA) ranging from 23.7% to 71.3% and negative discordance (PtGA < PhGA) ranging from 0% to 27.5% [Citation4–9]. In a Japanese study, positive discordance ranged from 32% to 51% and negative discordance ranged from 4% to 10% [Citation3]. Such discordance is concerning, since patient views regarding their priorities and treatment choices are important, particularly in the ‘treat-to-target’ approach.
The reasons underlying patient–physician discordance are presumably due to patients and physicians focusing on different aspects of the disease in their assessments. Specifically, current evidence suggests that pain is the most dominant driver of PtGA [Citation5,Citation7,Citation9,Citation12–14], and to a lesser extent functional disability [Citation13] and fatigue [Citation7]. In contrast, inflammation appears to be the main driver of PhGA, as indicated by measures such as swollen and tender joint counts [Citation7,Citation12–14], C-reactive protein (CRP) [Citation12], and erythrocyte sedimentation rate (ESR) [Citation7]. A better understanding of the determinants of discordance between patients’ and physicians’ global assessments would be helpful to physicians when sharing treatment decisions with patients who have RA.
To date, studies examining discordance and the determinants of discordance between PtGA and PhGA have been restricted to observational studies, with few such studies involving Japanese patients with RA. Of the studies that have been reported, two were large-scale, multinational studies [Citation7,Citation9], one assessed discordance over time [Citation13], and two were cross-sectional studies [Citation3,Citation12]. Moreover, relatively few patient-reported outcomes were included in these analyses. Importantly, no reports to date have provided data from large-scale, multinational, randomized controlled trials on the determinants of PtGA and PhGA.
RA-BEAM [Citation15] and RA-BEGIN [Citation16] were large-scale, multinational, randomized, controlled phase 3 trials in patients with RA. Both studies included patients from Japan. The objective of this post hoc analysis of data from RA-BEAM and RA-BEGIN was to assess the determinants of PtGA and PhGA in overall and Japanese patients with RA. Results for Japanese patients were also descriptively compared with results for the overall study populations of these studies.
Patients and methods
Study design
This post hoc analysis was of overall and Japanese data from two 52-week, multinational, randomized, double-blind, placebo/active-controlled phase 3 trials of baricitinib for the treatment of RA; RA-BEAM (carried out in 26 countries, ClinicalTrials.gov registration: NCT01710358) and RA-BEGIN (carried out in 18 countries, ClinicalTrials.gov registration: NCT01711359). Details of the individual trials are described in the primary publications [Citation15,Citation16]. All trial protocols were approved by the institutional review boards of all sites and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent before participating in any study-related procedures.
Study population
The modified intention-to-treat (mITT) populations of RA-BEGIN and RA-BEAM were included in these post hoc analyses. The mITT included all patients who had undergone randomization and were treated with at least one dose of the study drug.
All patients had moderate to severe RA. RA-BEAM included patients who had a previous inadequate response to methotrexate (MTX), whereas RA-BEGIN included patients who had no or minimal previous conventional synthetic disease-modifying antirheumatic drug (csDMARD) treatment. The inclusion and exclusion criteria for these studies have been reported elsewhere [Citation15,Citation16].
Subgroup analyses were carried out for Japanese patients in both studies.
Additional subgroup analyses of overall populations were carried out by gender (male versus female), age (≥65 years versus <65 years), baseline disease activity (higher baseline disease activity: CDAI >median versus lower baseline disease activity: CDAI ≤median), and disease duration from RA diagnosis (higher disease duration: >median versus lower disease duration: ≤median).
Assessments
Data from assessments made at baseline, Week 12, and Week 24 were used for this post hoc analysis.
PtGA and PhGA
PtGA and PhGA were measured on a visual analog scale (VAS) ranging from 0 (no arthritis activity) to 100 mm (extremely active arthritis). PtGA was assessed by answers to the question, ‘How do you assess your current arthritis disease activity?’, whereas PhGA was assessed by answers to the question, ‘How do you assess your patient’s current arthritis disease activity?’
Assessments performed by physician or joint assessor
Tender joint count of 28 joints examined (TJC28) and swollen joint count of 28 joints examined (SJC28) were performed by a joint assessor who was not the physician responsible for PhGA. Both the joint assessor and the physician who assessed PhGA were blinded to the patient’s high-sensitivity CRP (hsCRP) results.
ESR and hsCRP
ESR was measured in the local laboratories, whereas hsCRP was measured in the central laboratory.
Patient-reported outcomes (PROs)
PROs included the patient’s assessment of pain (0–100 mm VAS; lower scores indicate less pain), Health Assessment Questionnaire-Disability Index (HAQ-DI; lower scores indicate less disability), Functional Assessment of Chronic Illness Therapy-Fatigue scale (FACIT-F; higher scores indicate less fatigue), Medical Outcomes Study Short-Form 36 Health Survey version 2 (SF-36), Mental Component Score (MCS), and Physical Component Score (PCS) (higher scores indicate better outcomes), European Quality of Life-5 Dimensions VAS (EQ-5D VAS; patient’s rating of current health state, 0–100 mm; higher scores indicate better quality of life), and Quick Inventory of Depressive Symptomatology Self-Rated-16 (QIDS-SR16; lower scores indicate less depression).
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics.
Pearson’s correlation coefficients were calculated to identify variables that correlated with PtGA and PhGA at baseline, Week 12, and Week 24. Correlations between changes from baseline to Week 12 and Week 24 in PtGA/PhGA and other clinical measures in the overall populations were also examined. The significant variables identified from this univariate correlation were tested by linear stepwise multiple regression to determine their contribution to PtGA and PhGA. Variables were selected using a cut-off point of p = .05 for entry into and removal from the models. The proportion of variability of each outcome explained by each predictor was expressed by the partial R2. The proportions that could not be explained by the variables included in the models, but may have been explained by variables not measured in the studies, are presented as ‘unexplained’. Analyses were performed separately for baseline, Week 12, and Week 24.
Statistical analyses were conducted using SAS® version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
The overall population included 1305 patients in RA-BEAM and 584 patients in RA-BEGIN. The Japanese population included 249 patients in RA-BEAM and 104 patients in RA-BEGIN.
Baseline demographics, disease characteristics, and clinical measures of disease activity were generally similar between studies and between overall and Japanese populations () [Citation15,Citation16]. The duration of RA was longer for patients in RA-BEAM than in RA-BEGIN in both overall and Japanese patient populations, and mTSS were higher for patients in RA-BEAM than in RA-BEGIN in both overall and Japanese patient populations. PtGA, PhGA, and other clinical measures are shown at baseline, Week 12, and Week 24 in Supplementary Tables S1 and S2. Note: the baseline characteristics in are also disclosed in a related manuscript [Citation17] describing the results of a separate post hoc analysis of data from RA-BEAM and RA-BEGIN.
Table 1. Baseline characteristics for overall and Japanese patient populations in Studies RA-BEAM and RA-BEGIN.
Correlations
PtGA was strongly and consistently correlated with pain, whereas PhGA correlations differed by study and patient population ().
Figure 1. Correlations between PtGA and PhGA and clinical measures at baseline, Week 12, and Week 24 for overall and Japanese patient populations in Studies RA-BEAM (a) and RA-BEGIN (b). All variables were significantly correlated with PtGA and PhGA (p < .05) except for:RA-BEAM: • SJC28 and PtGA at baseline in the Japanese patient population • MCS and PtGA at Week 24 in the Japanese patient population • MCS and PhGA at baseline, Week 12, and Week 24 in the Japanese patient population • QIDS-SR16 and PtGA at Week 24 in the Japanese patient population • QIDS-SR16 and PhGA at baseline and Week 24 in the Japanese patient populationRA-BEGIN: • hsCRP/SJC28/TCJ28 and PtGA at baseline in the Japanese patient population • ESR and PtGA at baseline, Week 12, and Week 24 in the Japanese patient population • ESR/SJC28/TJC28 and PhGA at baseline in the Japanese patient population • MCS and PhGA at baseline in the Japanese patient populationEQ-5D VAS: European Quality of Life-5 Dimensions VAS; ESR: erythrocyte sedimentation rate; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue Scale; HAQ-DI: Health Assessment Questionnaire-Disability Index; hsCRP: high-sensitivity C-reactive protein; MCS: Mental Component Score; PCS: Physical Component Score; PhGA: Physician’s Global Assessment of Disease Activity; PtGA: Patient’s Global Assessment of Disease Activity; QIDS-SR16: Quick Inventory of Depressive Symptomatology-Self-Rated (16 items); SJC28: swollen joint count in 28 joints; TJC28: tender joint count in 28 joints; VAS: visual analog scale.
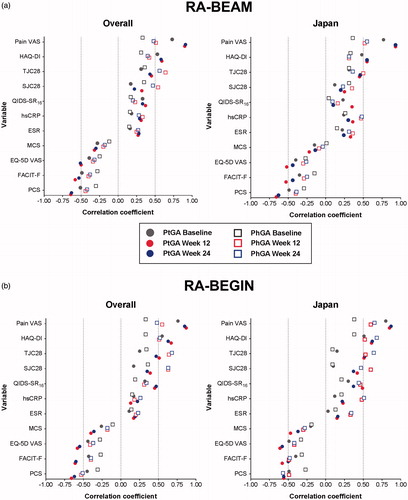
In the overall populations, pain VAS clearly had the strongest correlation (Pearson’s correlation coefficients: 0.73–0.91) with PtGA in both studies. The same was true for the Japanese populations (Pearson’s correlation coefficients: 0.77–0.93).
In the overall populations, TJC28, SJC28, and pain VAS had the strongest correlation (Pearson’s correlation coefficients: 0.33–0.68) with PhGA in both studies.
In the Japanese populations, pain VAS, TJC28, and hsCRP had the strongest correlations (Pearson’s correlation coefficients: 0.26–0.55) with PhGA in RA-BEAM, whereas pain VAS and HAQ-DI had the strongest correlations (Pearson’s correlation coefficients: 0.38–0.68) with PhGA in RA-BEGIN.
Baseline, Week 12, and Week 24 results were generally similar for all correlations.
In the overall populations, the changes from baseline in PtGA/PhGA significantly correlated with changes from baseline in other clinical measures assessed. The magnitude of the correlation for each measure was consistent, regardless of actual scores at Weeks 12 and 24 or the change in scores from baseline to Weeks 12 and 24 (data not shown).
Determinants of PtGA and PhGA
Overall analysis
The main determinants of PtGA and PhGA were different ().
Figure 2. Contribution of variables to PtGA and PhGA at baseline, Week 12, and Week 24 for Japanese and overall patient populations in Studies RA-BEAM (a) and RA-BEGIN (b). EQ-5D VAS: European Quality of Life-5 Dimensions VAS; ESR: erythrocyte sedimentation rate; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue Scale; HAQ-DI: Health Assessment Questionnaire-Disability Index; hsCRP: high-sensitivity C-reactive protein; MCS: Mental Component Score; PCS: Physical Component Score; PhGA: Physician’s Global Assessment of Disease Activity; PtGA: Patient’s Global Assessment of Disease Activity; RA: rheumatoid arthritis; SJC28: swollen joint count in 28 joints; TJC28: tender joint count in 28 joints; VAS: visual analog scale.
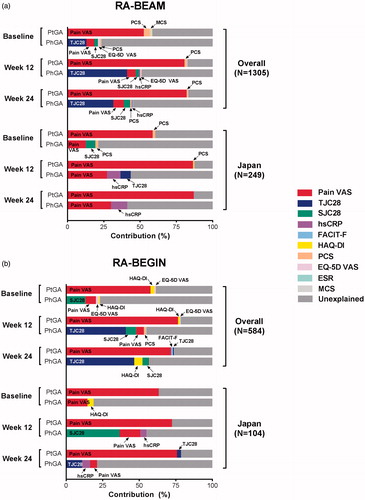
In the overall populations, pain VAS (53–82% contribution) was clearly the main determinant of PtGA in both studies, and at baseline, Week 12, and Week 24. The same was true for the Japanese populations (59–87% contribution).
In overall and Japanese populations, the main, explained, determinants of PhGA differed between populations and studies.
In the overall populations, TJC28 (13–47% contribution) was generally the main determinant of PhGA in both studies, and at baseline, Week 12, and Week 24 (an exception was baseline in RA-BEGIN, where the main determinant of PhGA was SJC28 [13% contribution]).
In the Japanese populations, pain VAS (13–30% contribution) was the main determinant of PhGA in RA-BEAM at baseline, Week 12, and Week 24, whereas in RA-BEGIN, pain VAS (15% contribution), SJC28 (37% contribution), and TJC28 (11% contribution) were the main determinants of PhGA at baseline, Week 12, and Week 24, respectively.
Subgroup analyses
In RA-BEAM and RA-BEGIN, the findings of the subgroup analyses (by age, gender, baseline disease activity, and disease duration from RA diagnosis) of overall populations were generally consistent with those of the overall global population (Supplementary Figures 1 and 2).
Age
Pain VAS (age <65 years: 52–81% contribution; age ≥65 years: 47–86% contribution) was the main determinant of PtGA in both studies, and at baseline, Week 12, and Week 24.
TJC28 (age <65 years: 0–48% contribution; age ≥65 years: 0–40% contribution) was generally the main determinant of PhGA in both studies, and at baseline, Week 12, and Week 24.
Gender
Pain VAS (male: 47–81% contribution; female: 53–82% contribution) was the main determinant of PtGA in both studies, and at baseline, Week 12, and Week 24.
TJC28 (male: 0–40% contribution; female: 0–49% contribution) was generally the main determinant of PhGA in both studies, and at baseline, Week 12, and Week 24.
Baseline disease activity
Pain VAS (low disease activity: 48–79% contribution; high disease activity: 46–85% contribution) was the main determinant of PtGA in both studies, and at baseline, Week 12, and Week 24.
TJC28 (low disease activity: 0–36% contribution; high disease activity: 0–57% contribution) was generally the main determinant of PhGA in both studies, and at baseline, Week 12, and Week 24.
Disease duration
Pain VAS (short duration: 58–80% contribution; long duration: 44–84% contribution) was the main determinant of PtGA in both studies, and at baseline, Week 12, and Week 24.
TJC28 (short duration: 0–46% contribution; long duration: 1–47% contribution) was generally the main determinant of PhGA in both studies, and at baseline, Week 12, and Week 24.
Discussion
This is the first post hoc analysis of two large, multinational, randomized, controlled phase 3 trials to examine determinants of PtGA and PhGA in overall and Japanese patient populations with RA. We found that pain was the most important determinant of PtGA, whereas determinants of PhGA varied somewhat between populations. These findings indicate that different factors underlie patient and physician assessments. Patients’ perceptions of disease activity were similar between overall and Japanese populations, but physicians’ perceptions of disease activity differed between the two populations.
In both studies, we found that pain was the most important determinant of PtGA (53–87% contribution) in overall and Japanese patient populations. These results are consistent with results from studies conducted in Europe, Russia, East Asia, Australia, Latin America, and North America that have assessed determinants of PtGA in patients with RA [Citation5,Citation7,Citation9,Citation12–14]. To our knowledge, no other Japanese data have been reported; however, our results are in keeping with those from a pilot, multicenter, international study that included Japanese patients, in which the clinical expectations of patients were focused primarily on pain control (63.7%) [Citation18]. Our findings indicate that, like patients elsewhere, Japanese patients with RA clearly base their assessments of disease activity on subjective experience with the disease, that is, pain [Citation18].
In contrast to PtGA, much of the determination of PhGA was TJC28. This was especially true for overall patient populations across both studies. Interestingly, pain and, to a lesser extent, hsCRP appeared to be more important contributors to PhGA in Japanese than in overall populations. Pain also appeared to be a more important determinant of PhGA for the Japanese population in RA-BEAM than in RA-BEGIN. This finding is supported by the result of a survey involving 301 rheumatologists in Japan, in which 83.4% rheumatologists considered relief of joint pain as their treatment targets when treating patients with RA [Citation19]. Other observational studies (carried out in multiple countries, including Europe, Russia, Latin America, and North America) have also identified joint counts [Citation7,Citation11–Citation14] and CRP [Citation12] as important determinants of PhGA in patients with RA. In contrast, pain was not found to be a determinant of PhGA in studies examining discordance, and was not an important determinant of PhGA in an Austrian observational study of patients with RA [Citation14], suggesting this may be a characteristic specific to Japanese physicians. Specifically, Japanese physicians may place more emphasis on subjective factors (i.e. patient pain) in their evaluation of patient disease activity than physicians from some other countries.
Another finding of our analysis, as previously noted, is that a considerable proportion of PhGA remained unexplained (42–81%), particularly at baseline and in the Japanese patient population. In contrast, only 33% of PhGA remained unexplained in an observational study of 646 patients with RA [Citation14]. The results from this study showed that physicians’ assessment of disease activity cannot be completely explained by the variables included in our analyses. The proportion of PtGA that remained unexplained was much lower than that of PhGA and was consistent with findings from a previous study [Citation14]. Factors not assessed by the variables included in our analysis (e.g. patient experience with RA, dynamics of the patient–physician relationship, cultural factors, random factors, and health literacy [Citation7,Citation20]) may also have contributed to patient perceptions of disease activity. In both studies, a higher proportion of variability in PhGA remained unexplained at baseline (77–81%) compared with at Weeks 12 and 24 (42–79%) in overall and Japanese patient populations. This difference suggests that physicians may have had a clearer understanding of the factors underlying patient disease as disease activity improved.
Our analysis has several strengths. First, the analysis included data obtained from two large-scale, randomized controlled trials, ensuring that all evaluations were performed in a consistent manner compared with observational studies. Second, we used identical and current-arthritis-disease-activity–focused questions for patients and physicians that helped to avoid issues related to interpretation, which can arise from inconsistencies in wording/phrasing [Citation21]. Third, the analysis included a large number of candidate variables. Last, the use of a ‘dual assessor’ approach (i.e. joint assessor completed joint counts [with no access to PhGA, PRO, or safety data], physician completed PhGA [with access to efficacy and safety data]) and blinding of the assessors to patients’ hsCRP results may have helped to reduce potential bias. Our analysis does, however, have some limitations, including the small number of Japanese patients, which may have contributed to the disparate PhGA results between populations. Other potential limitations include the post hoc nature of the analysis, and the fact that results obtained under controlled trial conditions may not reflect clinical practice. It should also be noted that other factors that were not assessed may have contributed to discordance, for example, environmental and cultural factors, and health expectations.
In conclusion, we found that the main determinants of PtGA and PhGA differed in this post hoc analysis of data from two large-scale, randomized controlled trials. Specifically, pain was the major determinant of PtGA in both overall and Japanese populations; however, the main determinants of PhGA between overall and Japanese populations were different. TJC28 was the major determinant of PhGA in overall populations, whereas pain or joint counts tended to be the main determinants of PhGA in the Japanese populations. Further, pain was the main determinant of PtGA in both studies (RA-BEAM and RA-BEGIN) included in the analyses, which indicates that, regardless of disease duration, pain is the most important determinant of PtGA. From a clinical perspective, we believe the results of the current study are important in that they will help physicians to understand the reasons underlying discordance between patient and physician assessments of disease activity, and address patients’ needs in the management of RA.
Conflict of interest
ZC, MS, KA, CG, BZ, and JG are employees of Eli Lilly and Company. MS, CG and BZ own shares in Eli Lilly and Company. YK has received consulting fees, speaking fees, and/or honoraria from AbbVie, Astellas Pharma, Ayumi Pharmaceutical, Bristol-Myers K.K., Chugai Pharmaceutical, EA Pharma, Eisai, Eli Lilly and Company, Janssen, Mitsubishi Tanabe Pharma, Pfizer Japan, Taisho-Toyama, Takeda Pharmaceutical, and UCB. TT has received grants, consulting fees, and speaking fees from AbbVie, Asahikasei Pharma, Astellas Pharma, Astra Zeneca K.K., Ayumi Pharmaceutical, Bristol–Myers K.K., Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Nipponkayaku, Novartis Pharma K.K., Pfizer Japan, Taiho Pharmaceutical, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, and Teijin Pharma. YT has received consulting fees, speaking fees, honoraria, and/or research grants from AbbVie, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi-Sankyo, Eisai, Eli Lilly and Company, Janssen, Kyowa-Kirin, Mitsubishi Tanabe Pharma, MSD, Ono, Pfizer, Sanofi, Takeda, UCB, and YL Biologics.
IMOR_1422304_supplementary_material.docx
Download MS Word (1.8 MB)Acknowledgments
Medical writing assistance was provided by Thao Le, MD, PhD, and Luke Carey, PhD of ProScribe – Envision Pharma Group, and was funded by Eli Lilly Japan K.K. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
RA-BEAM and RA-BEGIN were supported by Eli Lilly and Company and Incyte Corporation. Eli Lilly and Company was involved in the data collection, data analysis, and preparation of the manuscript. The study was designed by Eli Lilly and Company in consultation with an academic advisory board and Incyte Corporation.
Additional information
Funding
References
- Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35:10–14.
- Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24:33–40.
- Kaneko Y, Kuwana M, Kondo H, Takeuchi T. Discordance in global assessments between patient and estimator in patients with newly diagnosed rheumatoid arthritis: associations with progressive joint destruction and functional impairment. J Rheumatol. 2014;41:1061–6.
- Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient–physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res. 2010;62:857–64.
- Cho SK, Sung YK, Choi CB, Bang SY, Cha HS, Choe JY, et al. What factors affect discordance between physicians and patients in the global assessment of disease activity in rheumatoid arthritis? Mod Rheumatol. 2017;27:35–41.
- Egholm CL, Krogh NS, Pincus T, Dreyer L, Ellingsen T, Glintborg B, et al. Discordance of global assessments by patient and physician is higher in female than in male patients regardless of the physician’s sex: data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO registry. J Rheumatol. 2015;42:1781–5.
- Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C, et al. Determinants of discordance in patients' and physicians' rating of rheumatoid arthritis disease activity. Arthritis Care Res. 2012;64:206–14.
- Nicolau G, Yogui MM, Vallochi TL, Gianini RJ, Laurindo IM, Novaes GS. Sources of discrepancy in patient and physician global assessments of rheumatoid arthritis disease activity. J Rheumatol. 2004;31:1293–6.
- Smolen JS, Strand V, Koenig AS, Szumski A, Kotak S, Jones TV. Discordance between patient and physician assessments of global disease activity in rheumatoid arthritis and association with work productivity. Arthritis Res Ther. 2016;18:114.
- Hobbs KF, Cohen MD. Rheumatoid arthritis disease measurement: a new old idea. Rheumatology. 2012;51(Suppl6):vi21–7.
- Desthieux C, Hermet A, Granger B, Fautrel B, Gossec L. Patient–physician discordance in global assessment in rheumatoid arthritis: a systematic literature review with meta-analysis. Arthritis Care Res. 2016;68:1767–73.
- Furu M, Hashimoto M, Ito H, Fujii T, Terao C, Yamakawa N, et al. Discordance and accordance between patient's and physician's assessments in rheumatoid arthritis. Scand J Rheumatol. 2014;43:291–5.
- Markenson JA, Koenig AS, Feng JY, Chaudhari S, Zack DJ, Collier D, et al. Comparison of physician and patient global assessments over time in patients with rheumatoid arthritis: a retrospective analysis from the RADIUS cohort. J Clin Rheumatol. 2013;19:317–23.
- Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum. 2012;64:2814–23.
- Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Reyes Gonzaga J, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652–62.
- Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69:506–17.
- Ishiguro N, Dougados M, Cai Z, Zhu B, Ishida M, Sato M, et al. Relationship between disease activity and patient-reported outcomes in rheumatoid arthritis: Post hoc analyses of overall and Japanese results from two phase 3 clinical trials. Mod Rheumatol. 2017. [Epub ahead of print]. doi: 10.1080/14397595.2017.1422232
- Wen H, Ralph Schumacher H, Li X, Gu J, Ma L, Wei H, et al. Comparison of expectations of physicians and patients with rheumatoid arthritis for rheumatology clinic visits: a pilot, multicenter, international study. Int J Rheum Dis. 2012;15:380–9.
- Kaneko Y, Koike T, Oda H, Yamamoto K, Miyasaka N, Harigai M, et al. Obstacles to the implementation of the treat-to-target strategy for rheumatoid arthritis in clinical practice in Japan. Mod Rheumatol. 2015;25:43–9.
- Hirsh JM, Boyle DJ, Collier DH, Oxenfeld AJ, Caplan L. Health literacy predicts the discrepancy between patient and provider global assessments of rheumatoid arthritis activity at a public urban rheumatology clinic. J Rheumatol. 2010;37:961–6.
- Nikiphorou E, Radner H, Chatzidionysiou K, Desthieux C, Zabalan C, van Eijk-Hustings Y, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther. 2016;18:251.
