Abstract
Objectives: To assess the efficacy and safety of intravenous (IV) belimumab plus standard systemic lupus erythematosus (SLE) therapy standard of care (SoC) in Japanese patients with SLE.
Methods: A Phase 3, multicenter, double-blind, placebo-controlled, 52-week study (BEL 113750; NCT01345253) in patients with SLE, randomized 2:1 to belimumab 10 mg/kg plus SoC or placebo plus SoC to Week 48.
Results: Sixty of 707 randomized patients were enrolled from study centers in Japan (belimumab, n = 39; placebo, n = 21). In this cohort, more patients achieved SLE Responder Index 4 response at Week 52 in the belimumab group compared with placebo (46.2% [18/39] vs. 25.0% [5/20]; odds ratio, 2.57 [95% confidence interval: 0.78, 8.47]; p=.1204). Fewer patients receiving belimumab experienced a severe flare through Week 52, with longer median time to flare compared with placebo. More patients with baseline prednisone dose >7.5 mg/d receiving belimumab had a dose reduction of ≥25% from baseline to ≤7.5 mg/d during Weeks 40–52, compared with placebo. No new safety issues were identified within the Japanese cohort.
Conclusion: In Japanese patients with SLE, belimumab improved disease activity, with efficacy and safety results similar and consistent to the pivotal Phase 3 trials, suggesting that belimumab is a potential treatment option in this population.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease that affects multiple systems of the body [Citation1,Citation2]. It is associated with B cell hyperactivity, increased concentrations of B lymphocyte stimulator (BLyS) and the presence of autoantibodies [Citation2–5]. These self-reactive antibodies can deposit in tissues throughout the body, triggering an inflammatory response that may cause irreversible tissue damage [Citation2].
Symptoms of SLE, most frequently affecting the skin, nervous system, joints, muscles and the kidneys, can occur at any stage of the disease and do not always occur simultaneously [Citation2,Citation6]. Fever, fatigue and arthralgia are also frequently occurring non-specific symptoms [Citation6]. SLE is a chronic, relapsing-remitting disease and can severely affect the patient’s health-related quality of life [Citation2,Citation7,Citation8].
Conventional management of SLE may include non-steroidal anti-inflammatory drugs and antimalarials such as hydroxyquinoline [Citation9]. Corticosteroids, such as high-dose prednisone, and immunosuppressive agents, such as cyclophosphamide, azathioprine and mycophenolate mofetil, can be prescribed for severe refractory disease [Citation9].
Use of corticosteroids, particularly over a prolonged period, has been associated with significant side effects and organ damage accrual [Citation10]. For those with high disease activity, there is little evidence of substantial benefits with currently available treatments. Given the complexity of SLE and the limited effective treatment options, there remains an unmet need for therapeutic alternatives.
SLE is associated with mortality rates higher than those seen in the general population [Citation11]. Estimates of incidence and prevalence vary across global populations; however, SLE is thought to be more prevalent and more severe in non-Caucasian populations [Citation12]. This suggests a potential for genetic predisposition to SLE, as well as a possible association with environmental factors.
In Japan, SLE is thought to affect 4.3–37.7 people per 100,000 [Citation10,Citation13]. Globally, SLE is known to be more common among females compared with males and this disparity is equally visible in Japan; one study suggests a female:male ratio of 8.2:1, with 20–39 years and 15–44 years in females and males, respectively, representing the most susceptible age range for patients with SLE [Citation14]. This disparity between the sexes could be indicative of hormonal factors such as estrogen acting as an SLE-related trigger [Citation12].
Belimumab is a human, immunoglobulin (IgG1λ) monoclonal antibody directed against BLyS [Citation15]. Belimumab is approved in the US and Europe for the treatment of adults with active, autoantibody-positive SLE receiving standard SLE therapy standard of care (SoC) [Citation16,Citation17]. Belimumab was approved by the Japanese Ministry of Health, Labor and Welfare for the treatment of adult patients with SLE who are inadequate responders to existing therapies, in September 2017 [Citation18]. Japan-specific guidelines relating to SLE treatment remain under development.
The safety and efficacy of intravenous (IV) belimumab 10 mg/kg plus SoC in patients with SLE were demonstrated in two large Phase 3 studies (BLISS-52, NCT00424476 and BLISS-76, NCT00410384) [Citation3,Citation19]; however, the proportion of patients from North-East Asia in these studies was small. Consequently, a Phase 3, randomized, double-blind, study (BEL113750; NCT01345253) was conducted in North-East Asia (China, Japan and South Korea) to assess the efficacy and safety of belimumab as add-on to SoC compared with placebo plus SoC in this population [Citation20]. In this North-East Asia study, the primary endpoint, SLE Responder Index 4 (SRI4) response rate at Week 52, was met: SRI4 response rate was higher with belimumab vs. placebo (53.8 vs. 40.1%; odds ratio [OR]: 1.99 [95% confidence interval (CI): 1.40, 2.82; p=.0001]). This article presents a subgroup analysis of the Japanese cohort within the North-East Asian study, to assess the efficacy and safety of belimumab specifically in Japanese patients with SLE.
Materials and methods
Study design
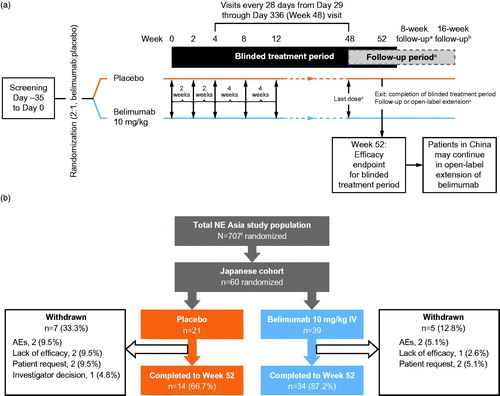
Of 49 participating centers in the North-East Asia study, 16 centers were in Japan. The study design has been previously published [Citation20]. Briefly, patients completed a screening period of up to 5 weeks, following which all eligible patients were randomized 2:1 to receive either belimumab 10 mg/kg IV or placebo, in addition to SoC, on Days 0, 14 and 28 and then every 28 days until Week 48, with a final assessment at Week 52 (). Randomization was stratified according to screening Safety of Estrogen in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) (≤9 vs. ≥10), complement (C) levels (low C3 [<0.9 g/L] and/or C4 [as <0.10 g/L] vs. no low C3 or C4), and country of origin. All patients provided written informed consent prior to the performance of any study-specific procedures. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation on Good Clinical Practice, and the applicable country-specific regulatory requirements [Citation21].
Figure 1. Study design (a) and patient disposition (b). aAll patients who withdrew early or did not enter the open-label period of the study were asked to return as least 6 months after the last dose of investigational product to repeat the immunogenicity test; bpatients who withdrew early or who did not enter the open-label period of the study; cfollow-up period for those not entering the open label period of the study; dlast dose for those not entering the open-label period of the study; eexit/complete blinded treatment for those not entering the open-label period of the study; f707 patients were randomized to receive belimumab or placebo; however, two patients were identified as ineligible for inclusion after randomization and did not receive the first dose of the study drug. This is the Day 0 visit or first dose for those entering the open-label period of the study. AEs: adverse events; IV: intravenous; SoC: standard of care.
Patients
Details of patient inclusion and exclusion criteria have been published previously. Eligible patients were ≥18 years of age with a clinical diagnosis of SLE according to the American College of Rheumatology criteria and a SELENA-SLEDAI score of ≥8 at screening. Patients were receiving stable SLE treatment for at least 30 d prior to Day 0.
Endpoints and assessments
Efficacy
The primary endpoint was the SRI4 response rate at Week 52 (SRI4: ≥4-point reduction in SELENA-SLEDAI score; no worsening [<0.3 increase from baseline] in Physician’s Global Assessment [PGA]; no new British Isles Lupus Assessment Group [BILAG] a domain score or 2 new BILAG B domain scores vs. baseline). Key secondary endpoints included percentage of patients with a ≥ 4-point reduction from baseline in SELENA-SLEDAI score at Week 52; SRI7 response rate (modified SRI criteria: ≥7-point reduction from baseline in SELENA-SLEDAI score) at Week 52; time to first severe flare (modified SELENA-SLEDAI Flare Index [SFI]) over 52 weeks; and number of days of prednisone dose ≤7.5 mg/d and/or reduced by 50% from baseline over 52 weeks in patients with baseline dose >7.5 mg/d. SRI4 response over time and BILAG improvement by organ domain in patients with an A and B domain score at baseline were also analyzed.
Additional prednisone assessments included the cumulative prednisone dose over 52 weeks, percentage of patients whose average prednisone dose was reduced by ≥25% from baseline to ≤7.5 mg/d during Weeks 40 through to Week 52, percentage of patients with daily prednisone dose reduced from >7.5 to ≤7.5 mg/d at baseline by visit, and percentage of patients with daily prednisone dose increased from ≤7.5 to >7.5 mg/d at baseline by visit.
Pharmacokinetics (PK) and biomarkers
Serum PK samples were taken at each visit to allow assessment of steady-state peak (Cmax) and trough (Cmin) concentrations. Observations were compared with simulation results from a previously developed population pharmacokinetic model [Citation22] taking into account individual patient characteristics such as body weight. Biomarker assessments included change from baseline in serum Ig (including isotypes IgG, IgM and IgA), autoantibodies (anti-double-stranded DNA [anti-dsDNA]), serum levels (C3 and C4) and B cell subsets.
Safety
Safety was assessed via monitoring of adverse events (AEs), including severe and treatment-related, those leading to permanent discontinuation, and AEs of special interest (AESI), serious AEs (SAEs) and deaths. Changes in laboratory parameters, including hematological and clinical chemistry parameters and urinalysis, were assessed.
Statistical analysis
In the Japanese cohort, the modified intent-to-treat population and safety populations included all randomized patients who received ≥1 dose of study drug. A logistic regression model was used for responder endpoints. The number of days of daily prednisone dose ≤7.5 mg/d (or equivalent) and/or reduced by 50% from baseline over 52 weeks was analyzed using a rank analysis of covariance model with treatment group as an independent variable. Hazard ratios (HR) for the time to severe SFI flare over 52 weeks were derived using a Cox proportional hazards model. All data summaries were performed using Statistical Analysis Software (SAS), version 9.3 (Cary, NC). Data analyses summarized continuous variables, reporting mean, median and standard deviation, 25th and 75th percentile, minimum and maximum values. Categorical variables were summarized with frequency counts and percentages.
As this analysis is limited to a subgroup of the original North-East Asian study, the results presented here are not powered for hypothesis testing and any trends in the subgroup data need to be interpreted with caution.
Results
Study population and patient disposition
Of the 707 patients randomized to treatment in the overall trial, 60 patients were enrolled and randomized (belimumab, n = 39; placebo, n = 21) from study centers in Japan. The PK population comprised the 39 patients in the belimumab group. Overall, 48/60 (80.0%) patients completed the 52-week study (). Reasons for withdrawals were: AEs, n = 4 (6.7%); withdrawal by patient, n = 4 (6.7%); lack of efficacy, n = 3 (5%); and withdrawal by investigator decision, n = 1 (1.7%). Demographic and baseline disease characteristics were similar between treatment groups (). Patients across both groups were all taking concomitant steroid medication and the majority was taking concomitant immunosuppressive/immunomodulatory agents (belimumab, 69.2%; placebo, 90.5%). It should be noted that hydroxyquinoline was not available in Japan at the time of the study.
Table 1. Baseline patient characteristics (mITT population and safety population, as indicated).
Efficacy
In the Japanese cohort, more patients in the belimumab group achieved an SRI4 response at Week 52 (n = 18/39, 46.2%) compared with the placebo group (n = 5/20, 25.0%) (OR 2.57 [95% CI: 0.78, 8.47]; p = .1204) (). The highest SRI4 response rate in the belimumab group was observed at Week 24 (n = 23/39, 59.0%). The duration of Week 52 SRI response was summarized by monthly intervals (≥1 to ≥11 months) (Supplementary Table 1).
Figure 2. SRI4 response and its components by visit: SRI4 response (a), ≥4-point reduction in SELENA-SLEDAI (b), no worsening (<0.3 increase) in PGA (c) and no new BILAG 1A/2B (d). *p<.05; p values are from a logistic regression model for the comparison between belimumab and placebo with only treatment group as an independent variable. BILAG: British Isles Lupus Assessment Group; PGA: Physician’s Global Assessment; SELENA-SLEDAI: Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index; SRI: SLE Responder Index.
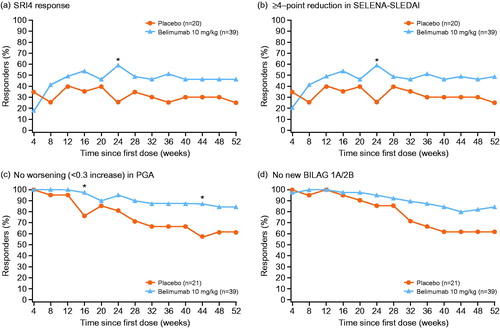
The proportion of patients in the belimumab group achieving a ≥ 4-point reduction in SELENA-SLEDAI score at Week 52 was 48.7% (19/39), compared with 25.0% (5/20) of those receiving placebo (OR 2.85 [95% CI: 0.87, 9.38]; p = .0848) (). The proportion of patients in the belimumab group experiencing no worsening in PGA at Week 52 was 84.6% (33/39), compared with 61.9% (13/21) of those receiving placebo (OR, 3.38 [95% CI: 0.98, 11.67]; p = .0535) (). The proportion of patients in the belimumab group with no new BILAG 1A/2B domain scores at Week 52 was 84.6% (33/39), compared with 61.9% (13/21) of those receiving placebo (OR, 3.38 [95% CI: 0.98, 11.67]; p = .0535) ().
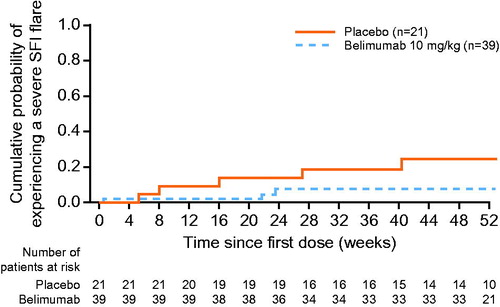
The proportion of patients receiving belimumab achieving an SRI7 response at Week 52 was 13.9% (5/36), compared with 10.0% (2/20) of those receiving placebo (OR, 1.45 [95% CI: 0.25, 8.26]; p = .6747). In total, 7.7% (3/39) of patients in the belimumab group and 23.8% (5/21) of patients in the placebo group had a severe SFI flare at any time post dose (HR, 0.31 [95% CI: 0.07, 1.28]; p = .1047) ().
The cumulative prednisone dose over 52 weeks was lower in the belimumab group vs. placebo (p = .0597) (). In total, 23 patients in the belimumab group and 14 patients in the placebo group had baseline prednisone doses >7.5 mg/d (). Results for the number of days that prednisone equivalent dose was ≤7.5 mg/d and/or reduced by 50% in this subgroup were similar in both treatment groups (). By Week 52, 3 (13.0%) patients within the belimumab group and 2 (14.3%) patients within the placebo group experienced a prednisone reduction to ≤7.5 mg/d.
Table 2. PrednisoneTable Footnotea dose over 52 weeks (mITT population).
More patients with baseline prednisone dose >7.5 mg/d in the belimumab group (n = 2) had a dose reduction of ≥25% from baseline to ≤7.5 mg/d, during Weeks 40–52, compared with those receiving placebo (n = 0).
Among patients with an BILAG A or B domain score at baseline, BILAG improvement at Week 52 in favor of belimumab was seen in the mucocutaneous (p = .0365) organ domain only; however, it is important to note that patient numbers were small (Supplementary Table 2).
Pharmacokinetics
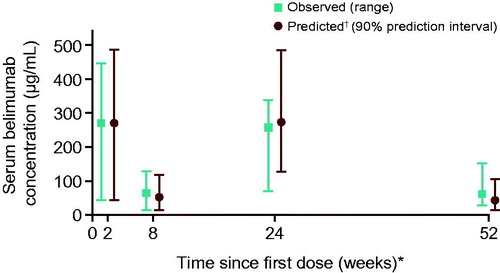
The overall observed median and ranges for the Cmax,n and Cmin,n of belimumab in this Japanese patient population were similar to the predicted values. As a consequence of bi-weekly loading doses, early Cmax/Cmin (Week 2/8) were similar to steady-state Cmax/Cmin (Week 24/52). The observed median peak (Cmax) serum belimumab concentrations were 271 µg/mL (Week 2) and 258 µg/mL (Week 24), whereas the observed median Cmin values were 65 µg/mL (Week 8) and 63 µg/mL (Week 52), with overlapping ranges ().
Figure 4. Observed vs. predicted median serum belimumab concentrations over time (linear scale). *Cmax collected at Week 2 (post dose 2), Cmin at Week 8 (prior to dose 4), Cmax at Week 24 (post dose 8), and Cmin 4 weeks after Week 48 (dose 14). †Predicted concentrations based on 200 replicate simulations for the patients in this study, using the PK population model from previous, global Phase 1 to Phase 3 studies in patients with SLE. PK: pharmacokinetic; SLE: systemic lupus erythematosus.
Biomarkers
Greater mean reductions from baseline in IgG were observed in the belimumab group compared with the placebo group at all visits; treatment differences in favor of belimumab were observed for IgG, IgA and IgM isotypes at Week 52 (). No Japanese patients in this study experienced a > 2-grade worsening shift in IgG. Numerically greater median (25th, 75th percentile) percent reductions in anti-dsDNA antibodies from baseline were observed in favor of the belimumab group at Week 52 (). In patients with low C3 and C4 levels at baseline, numerically greater increases in C3 and C4 levels were observed in patients treated with belimumab compared with placebo (, respectively). At Week 52, the median percent reduction in CD19+ B cells was 51.5% with belimumab treatment compared with 23.0% for placebo (p = .0189). Similar median percent reductions in CD20+ B cells as for CD19+ B cells were observed in both groups. Reductions in naïve B cells (CD19+/CD20+/CD27–) were greater in patients receiving belimumab (68.9%) compared with placebo (21.4%) (p = .0005) at Week 52 and throughout the study. An initial median increase relative to baseline (140.2%, Week 8) in memory B cell concentrations in peripheral blood (CD19+/CD20+/CD27+) were observed in the belimumab group, followed by a subsequent decline to a 28.4% increase over baseline at Week 52.
Figure 5. Box and Whisker Plots of Percentage change from baseline in (a) anti-dsDNA, (b) C3a and (c) C4a (safety population). aFor patients with low values at baseline. Boxes represent the interquartile range, horizontal line is median, with mean represented by + or o symbol. Anti-dsDNA: anti-double-stranded DNA; C3/C4: complement 3/complement 4.
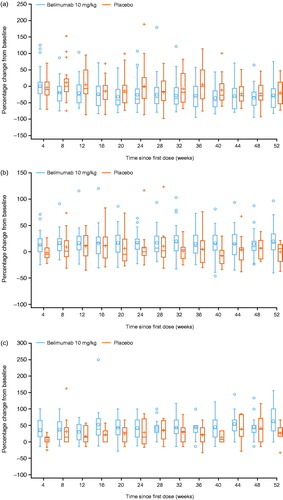
Table 3. Immunoglobulin biomarkers at Week 52 (safety population).
Safety
In the Japanese cohort, a higher incidence of AEs was observed in the belimumab group (100.0%) compared with the placebo group (90.5%) (). Most AEs in both treatment groups were mild (belimumab, 56.4%; placebo, 52.4%) or moderate (belimumab, 25.6%; placebo, 28.6%) in severity. The most common AE reported in both groups was nasopharyngitis (56.4 and 42.9% for belimumab and placebo, respectively). The incidence of SAEs was higher in patients receiving placebo (28.6%) than in those receiving belimumab (23.1%). When considering AESI, the incidence of post-infusion systemic reactions was similar between the belimumab and placebo groups (7.7 and 9.5%, respectively). Infections of special interest were reported in the belimumab group only (7.7%). A higher percentage of patients experienced depression in the belimumab group (7.7%) compared with the placebo group (4.8%) (), no instances of which were serious. No patients within the belimumab or placebo group experienced suicide/self-injury (). There were no completed suicides and no deaths. There were no clinically meaningful differences between treatment groups based on mean absolute values or changes from baseline over time for hematology or liver function parameters (data not shown).
Table 4. Summary of AEs (safety population).
Discussion
This subgroup analysis investigated the safety and efficacy of belimumab IV plus SoC in patients with active, autoantibody-positive SLE in Japan. The results demonstrated that Japanese patients with SLE treated with belimumab have similar baseline characteristics to those in the overall North-East Asia study population and that outcomes in Japanese patients are similar and consistent with those observed in the parent North-East Asia study [Citation20]. Additionally, the observed efficacy results are also consistent with those seen in two global Phase 3 studies of IV [Citation3,Citation19] belimumab and a Phase 3 subcutaneous (SC) belimumab study [Citation23].
This analysis provides valuable information about the efficacy of belimumab treatment in Japanese patients with active, autoantibody-positive SLE. In the Japanese subgroup, more patients achieved an SRI4 response at Week 52 in the belimumab group compared with the placebo group. Differences in SRI4 response in favor of belimumab were seen from the second visit in Week 8. The clinically meaningful improvement demonstrated by SRI4 response with belimumab treatment was further supported by the observation that more patients in the belimumab group achieved a greater magnitude of improvement indicated by SRI7 response compared with placebo.
Reducing the corticosteroid dose is a key treatment goal for SLE [Citation24]. The BLISS studies suggested that belimumab has a steroid-sparing effect [Citation3,Citation19,Citation23] and the North-East Asia study demonstrated that cumulative prednisone use over the 52-week treatment period was significantly lower in patients receiving belimumab, compared with those receiving placebo. This study of the Japanese subgroup demonstrated that fewer patients in the belimumab group experienced an increase in corticosteroid dose compared with placebo and a greater number of patients in the belimumab group with baseline prednisone dose >7.5 mg/d had a dose reduction of ≥25% from baseline to ≤7.5 mg/d, during Weeks 40–52. Furthermore, patients in the belimumab group had a lower cumulative corticosteroid dose over the 52 weeks, compared with patients receiving placebo.
Overall, serum belimumab concentrations observed in this study were similar to those predicted; moreover, the PK profile observed in this study was consistent with previous observations in global and Japanese populations [Citation22,Citation25]. Treatment differences in favor of belimumab were observed for IgG, IgA and IgM isotypes at Week 52. A change in IgG levels has been observed in other clinical trials of belimumab [Citation19,Citation23]. No Japanese patients in this study experienced a >2-grade worsening shift in IgG. Within the parent North-East Asia study, one patient from China receiving belimumab had a Grade 3 low IgG value at Week 24 that was a shift from a baseline Grade of 0; this patient did not experience an infection within 30 d of the date of the Grade 3 IgG value [Citation20]. Reductions in overall B cells and naïve B cells, as well as an initial increase in memory B cells, were seen during the treatment period. These findings are similar to those observed previously [Citation26]. The initial increase in memory B cells may be secondary to a release from disrupted lymphoid germinal centers, as seen in cynomolgus monkeys [Citation27] or caused by inhibition of memory B cell return to germinal centers [Citation28].
No new safety issues were identified in the Japanese cohort and the results were consistent with the established safety profile of belimumab.
The Japanese subpopulation analysis was limited by the small number of patients in the subgroup (n = 60). The study was not powered for subgroup analyses and lacks sufficient statistical power to draw definitive conclusions, consequently, the results should be interpreted with caution. Patients with SLE with active severe lupus nephritis or active central nervous system disease and patients with a SELENA-SLEDAI score <8 at screening were excluded; therefore, belimumab could not be evaluated in these patients. Post-marketing clinical experience will be vital in better understanding the real-world safety profile of belimumab in Japanese patients. Rare safety signals and safety data from the cumulative use of belimumab will be examined in patients treated for longer than 52 weeks.
Conclusion
The results of this subgroup analysis suggest that the benefits of belimumab in Japanese patients with SLE are similar to and consistent with those observed in the overall Phase 3 trial population.
No new safety issues were identified in Japanese patients with SLE. The efficacy and safety results in this study were similar to those observed in previous belimumab (IV and SC) studies, indicating that belimumab as an add-on to SoC is a viable option for Japanese patients with SLE.
Conflict of interest
Damon Bass, Myron Chu, Sally Egginton, Beulah Ji and David Roth are employees of GlaxoSmithKline (GSK) and hold shares in the company. Herbert Struemper is a former employee of GSK and holds shares in the company. Yoshiya Tanaka received consulting fees, speaking fees, and/or honoraria from AbbVie, Chugai, Daiichi-Sankyo, Bristol-Myers Squibb, Mitsubishi-Tanabe, Astellas, Takeda, Pfizer, Asahi-Kasei, YL Biologics, Sanofi, Janssen, Eli Lilly and GSK, and has received research grants from Mitsubishi-Tanabe, Takeda, Daiichi-Sankyo, Chugai, Bristol-Myers Squibb, MSD, Astellas, AbbVie, and Eisai.
Supplemental Material
Download MS Word (15.6 KB)Additional information
Funding
References
- Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039–21.
- Gottschalk TA, Tsantikos E, Hibbs ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front Immunol. 2015;6:550.
- Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31.
- Zhang J, Roschke V, Baker KP, Wang Z, Alarcón GS, Fessler BJ, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166(1):6–10.
- Tanaka Y, Kubo S, Iwata S, Yoshikawa M, Nakayamada S. B cell phenotypes, signaling and their roles in secretion of antibodies in systemic lupus erythematosus. Clin Immunol. 2017;186:21–5
- Kuhn A, Bonsmann G, Anders HJ, Herzer P, Tenbrock K, Schneider M. The diagnosis and treatment of systemic lupus erythematosus. Dtsch Ärztebl Int. 2015;112(25):423.
- Furie R, Petri MA, Strand V, Gladman DD, Zhong ZJ, Freimuth WW. Clinical, laboratory and health-related quality of life correlates of Systemic Lupus Erythematosus Responder Index response: a post hoc analysis of the phase 3 belimumab trials. Lupus Sci Med. 2014;1(1):e000031.
- Strand V, Levy RA, Cervera R, Petri MA, Birch H, Freimuth WW, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis. 2014;73(5):838–44.
- Bertsias G, Ioannidis J, Boletis J, Bombardieri S, Cervera R, Dostal C, et al. EULAR recommendations for the management of systemic lupus erythematosus (SLE) report of a task force of the European standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2007; 67(2):195–205.
- Bruce IN, O'Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74(9):1706–13.
- Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res Ther. 2012;14 (l4):S4.
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15(5):308–18.
- Jakes RW, Bae SC, Louthrenoo W, Mok CC, Navarra SV, Kwon N. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res. 2012;64(2):159–68.
- Ohta A, Nagai M, Nishina M, Tomimitsu H, Kohsaka H. Age at onset and gender distribution of systemic lupus erythematosus, polymyositis/dermatomyositis, and systemic sclerosis in Japan. Mod Rheumatol. 2013;23(4):759–64.
- Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48(11):3253–65.
- European Medicines Agency (EMA). Summary of product characteristics. 2011. Available from:http://www.ema.europa.eu/ema/ [last accessed 06 June 2017].
- Food and Drug Administration (FDA). FDA approves Benlysta to treat lupus. 2011. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm246489.htm [last accessed 06 June 2017].
- GSK. GSK receives approval for Benlysta in Japan for the treatment of systemic lupus erythematosus. 2017. Available from: https://us.gsk.com/en-us/media/press-releases/2017/gsk-receives-approval-for-benlysta-in-japan-for-the-treatment-of-systemic-lupus-erythematosus/ [last accessed 27 September 2017].
- Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–30.
- Zhang F, Bae SC, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77(3):355–363.
- World Medical Association. WMA Declaration of Helsinki - ethical principles for medical research involving human subjects. 2008. Available from: http://www.wma.net/en/30publications/10policies/b3/index.html [last accessed 06 June 2017].
- Struemper H, Chen C, Cai W. Population pharmacokinetics of belimumab following intravenous administration in patients with systemic lupus erythematosus. J Clin Pharmacol. 2013;53(7):711–20.
- Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2017;69(5):1016–27.
- Gatto M, Saccon F, Zen M, Bettio S, Iaccarino L, Punzi L, et al. Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. J Autoimmun. 2016;74:94–105.
- Yamada M, Akita M, Nakagawa T, Takahashi N, Endo A, Yoshida P. Safety, tolerability, pharmacokinetics and pharmacodynamics of belimumab in Japanese patients with mild-to- moderate systemic lupus erythematosus. J Drug Assess. 2013;2(1):40–8.
- Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64(7):2328–37.
- Halpern WG, Lappin P, Zanardi T, Cai W, Corcoran M, Zhong J, et al. Chronic administration of belimumab, a BLyS antagonist, decreases tissue and peripheral blood B-lymphocyte populations in cynomolgus monkeys: pharmacokinetic, pharmacodynamic, and toxicologic effects. Toxicol Sci. 2006;91(2):586–99.
- Badr G, Borhis G, Lefevre EA, Chaoul N, Deshayes F, Dessirier V, et al. BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood. 2008;111(5):2744–54.