Abstract
It is estimated that electronic and electrical equipment discarded in 2021 weigh more than 57 million tonnes, and less than one-fifth of this electronic waste (e-waste) is recycled. The Printed Circuit Boards (PCBs) are the integral component of any electronic equipment, and this is a useful type of waste due to their nearly 30% metal content. This research aims to explore the hydrometallurgy technique to recover precious metals from e-waste, and to transform the materials into wearable jewellery through developing new ways of utilising this material, specifically working with metals in solution.
Background
With the rapid development of industrialisation, the usage and sales of electronic products have been increasing but the lifespan of these products is decreasing. According to United Nations (UN) statistics, by 2019, each person produces 7.3 kg of e-waste in their lifetime, but only 1.7 kg of e-waste will be recycled. Most of these unused electronic products are kept in drawers or simply disposed of – often ending up in landfill sites. Such waste has nearly 30% metal content (Ghosh Citation2015). It is estimated that recycling one tonne of mobile phones could produce on average 130 kg of copper, 3.5 kg of silver, 0.34 kg of gold and 0.14 kg of Palladium (Petter Citation2014), meaning that, according to some estimates, by 2080 the largest metal reserves will not be underground but in circulation as existing products. However, we are currently throwing away, burning and burying the same valuable materials that we have gone to such great lengths to excavate, to the extent that copper can now be found in higher concentrations in the ash leftover from the incineration of than in traditionally mined ore (Treggiden Citation2020).
Methods of recovering metals from e-waste include pyrometallurgy, hydrometallurgy and/or bio metallurgy. Pyrometallurgy is traditionally used to recover copper and precious metals from waste printed circuit boards (WPCB). However, pyrometallurgy requires industrial-scale production equipment, which means material needs to be transported over large distances (Wilson Citation2020). Hydrometallurgy uses liquid chemical reagents to selectively recover precious metals from e-waste under laboratory conditions, a process which can be utilised on a small scale at local sites, offering lower capital investment routes that limit the discharge of organic chemicals into the environment (Rao Citation2020). This method of working with metals in solution also opens up new opportunities to create jewellery through techniques such as electroforming and electroplating as well as electro-displacement plating and metal patination.As the World Gold Council states on their website, jewellery manufacturers are the largest group that use non-renewable resources, such as precious metals (approx. 52%). It is now imperative to find new ways to obtain and reuse these significant resources. Recycling and converting existing metal products provides a more environmentally friendly method than mining and extracting virgin mineral resources (Zeng, Mathews, and Li Citation2018). Jewellery, as a wearable object, is close to people’s daily lives and could play an essential role in drawing public attention to e-waste recycling. However, existing design outcomes made from electronic waste often only uses this kind of material in expected ways instead of seizing the opportunity to tell stories about it. In Emotionally Durable Design: Objects, Experiences and Empathy (2015), Jonathan Chapman provides a beyond-materiality perspective on repurposing such waste materials, including creating an emotional association with that material (Chapman Citation2015).
General objectives of research
This research explores how jewellery design that utilises precious metals recovered from e-waste both in theory and in practice can potentially enhance e-waste recycling and reduce the environmental impact of current recycling methods. The goal of this project is to explore a workflow and evaluation system, from e-waste to jewellery, to contribute towards achieving the UN Sustainable Development Goals – particularly Goals 11, 12 and 13, which are concerned with sustainable consumption, production patterns and making cities and human settlements safe and sustainable.
The aims of my research are twofold: addressing technical problems associated with recycling metal from electronic waste and researching design methods when using recycled material from e-waste.
Research questions
The research questions identified are as follows:
What opportunities are there in a hydrometallurgy process that recovers precious metals from e-waste for creating new jewellery from precious metals that have been recovered?
What is the emotional association of recycled materials to new products? How can designers tell the story about the transformation of e-waste to the jewellery to make this material chain more accepf to the audience and consumers?
Methodology
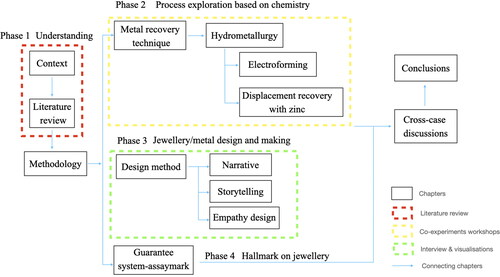
This report is part of a practice-based PhD study, that adopts a combined approach of practical experimentation and theoretical research. The experiments include utilising copper sulphate solution from the hydrometallurgy metal recovery method and how this can be applied as a new material in the creation of precious metal jewellery. Considering how metal that has been created through different process from copper sulphate solution recovered from e-waste can be applied to jewellery design is a novel methodology, and so I am also employing the method of emotional association research to consider if these techniques can promote and improve the acceptability of such a material that has been recovered from waste to the public. The research strategy will unfold in four phases as detailed in .
The first phase is about understanding the main principles of hydrometallurgy and the meaning of applying these in the jewellery workshop. This is achieved through conducting a literature review about the current practices of waste printed circuit board recycling to develop a methodological framework on how to shape the jewellery maker’s position on this new material development. This is important to focus on developing techniques for working with recovered metals for jewellery design.
Phase 2 is about conducting experiments with two main jewellery techniques that could work with metals in solution from the hydrometallurgy recovery process: electroforming and electro-displacement plating (extracting metal by a chemical displacement reaction). This is important as both experiments are relatively small-scale and have low-cost equipment requirements, so they can also be implemented in small scale jewellery workshops. One of the solutions that arises from this process is copper sulphate. This is often a concentrated solution as copper is one of the main metals included in an electronic device.
Phase 3 is about researching the emotional association of waste materials to new objects from the perspective of narrative storytelling, based on six working stages (Koppen and Meinel Citation2012) which represents a user-centred design thinking methodology that seeks to improve the acceptability of metals recovered from e-waste from the design perspective.
Phase 4 is about researching and establishing a provenance system, for example creating a chain of custody mark much like an assay mark on jewellery that will guarantee where the metal has come from. This will be achieved by exploring current hallmarks of jewellery based in the United Kingdom and China, including a workflow system, hallmark, and case study.
Process
In terms of addressing research question 1, which is aiming to test the techniques for recovering metal from e-waste liquid based on hydrometallurgy, there are two main experiments described in sections below. To address research question 2 which is about the emotional association between jewellery and e-waste metal, a particular design method (empathy design), is discussed in the last section.
Experiment 1: electroforming
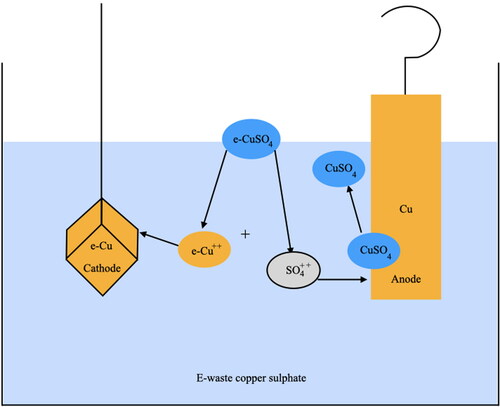
Electroforming is one of three different methods of electrodeposition, which describes the manufacturing process in which a layer of metal thick enough to be self-supporting is deposited on a surface made of another material. Electroforming is not a new process, it has been used for over 200 years since its first recorded use by Jacobi in 1811. In 1841 this was used for commercial purposes by Elkington’s in Birmingham (Curtis Citation2004). The main chemical solution used in this method is copper sulphate. When copper sulphate is added to water, it disassociates to release copper particles and sulphate particles. An electrical current is passed through the solution, causing the copper particles to deposit as a metallic lattice layer over the object’s surface which has been prepared to be conductive in advance. The copper sulphate used in my research is sourced from leaching e-waste. The leaching process provides efficient dissolution of metals found within e-waste and delivers them in a suitable form for the following step ().
Experiment 2: recovering solid copper from e-waste solution with zinc
The other experiment involved in this project will extract copper from the copper sulphate solution from e-waste using the principle of displacement reaction. Within the periodic table, a metal positioned further to the left is more active than those further to the right and can displace the metal in the latter position within the solution (Lister and McBryde Citation2019). Zinc is positioned before copper, so the addition of zinc can displace the copper in solution.After adding solid zinc pellets into the solution for a few minutes, a red solid begins to appear in the solution. From the point of view of the reaction principle, the red solid is presumed to be copper. However, the outcome is too soft to be used directly. After heating for a few minutes, the soft copper hardens into a solid nugget ().
Designing with metal from e-waste
Besides the experiments related to material research, another essential part that will be involved in this practice-based PhD project is researching the design methods suitable for working with this material. The first is empathy design, linking the material with people’s emotions, or attachments, to the original electronic objects to the new jewellery. Empathy design is a methodological approach that can support design teams in building a creative understanding of users and their everyday lives (Suri Citation2003). Based on Koppen’s research (Koppen and Meinel Citation2012), there will be six working stages: Understand, Observe, Synthesize, Conceive, Prototype and Test.The person who throws out e-waste could also be the person who buys jewellery. In the ‘understanding’ and ‘observation’ phases, the purpose is to collect facts about this group of people to better understand their behaviour and thoughts at key moments, such as when dropping off electronic waste (i.e. mobile phones or laptops) at recycling centres, or when buying important pieces of jewellery (i.e. wedding rings).
According to emotional attachment theory, some of the thoughts will reflect to a certain extent on daily objects around people (Keller-Aviram Citation2021). Based on this the electroforming attempts has been started with the objects collected from the daily life. At present there are not too many restrictions on the selection of daily objects, whose materials could include both organic and inorganic types. The purpose of doing this, initially is to test the application of the electroforming technique on different materials; secondly is to explore how to repurpose daily objects to tell the story of materials made from e-waste ().
The findings of the initial phases will then be synthesised. I will consider an ‘ideal user’ who is characteristic of the audience’s needs and expectations to test subsequent designs. Considering the e-waste involved in this project are mainly small electronic devices, such as phones and laptops, which are strongly associated with personal experience and are private, the synthesising phase will take place through combining the context from in-depth interviews to explore the storytelling from the personal perspective. The remaining three stages are ‘Ideation’, ‘Prototyping’ and ‘Testing’. They are about generating ideas expressed through prototypes to test them with users, which are close to the characteristic user.
Results and discussion
There are millions of old mobile phones, tablets, laptops etc. that are discarded in drawers. However, the raw materials of this end-of-life electronic equipment will offer an important recycling potential for the secondary supply of precious metals, which has been termed ‘urban mining’ (Hagelüken and Corti Citation2010). Hydrometallurgy is the main ‘urban mining’ method in this research. Compared to other methods, the whole process of hydrometallurgy can be viewed directly, offering the audience a chance to view how their electronic products are transformed into new metal objects, to make the workflow more transparent.
Based on hydrometallurgy, the focus of this project is the electroforming process and precious metal recovery with zinc, both of which are using copper sulphate solution. Different from existing research, this project aims to utilize copper sulphate leached from e-waste, and currently these processes are being reviewed from a theoretical perspective. From the results of the test pieces, copper recovery from solution can be achieved to a certain extent, but the concentration and purity of the copper needs to be investigated further. The use of e-waste copper sulphate is at a very early stage and the remaining challenges of this process will form the focus for the further development of this project, including how to make the metal layer of the coating stronger and improve the purity of recovered metals.Most of the existing designs that use waste materials only focus on the materials themselves, rather than exploring the meaning of it. This could be a reason why recycled material is more easily rejected by the audience. For the 2020 Tokyo Olympics, the medals were made of metal recovered from e-waste. In the official video showing the process, different reactions appeared in the comments section. Some people think that this is conducive to sustainable development by commenting ‘It comes from garbage, it seems to have a connection to my life,’ however, a considerable number of people tend to reject this approach with some concerns from a cultural perspective, which is expressed through several comments: ‘Olympic medals should have been made by some precious metals,’ ‘What will those athletes think if they know they were biting rubbish…’. This paper has referred to the empathy design theories proposed by Chapman and attempts to reframe the waste material from a narrative perspective, which make this research differ from existing research. Further exploration will keep focusing on revising the design methodology so that it can be applied to using recycled metal to transform the emotional impact from disused objects to the newly created objects. Current e-waste recycling methods may cause health problems for practitioners due to the manual processes and hazardous gases that are involved in the recycling process. In Phase 4, further development is needed to consider the necessity of establishing a chain of custody mark. When these recycled metals become jewellery and are sold to consumers, they will be informed of the source of the metal and that it is safe for both workers and the environment. This will demonstrate the contribution to sustainable development made by these processes and objects.
Conclusions
My research explores techniques that can utilise copper sulphate recovered metal from e-waste. The initial stage of the research is concerned with designing with more empathic methods. The techniques effectively apply hydrometallurgy in jewellery to collect and reuse metal from abandoned equipment. This application expands sustainable development through design.
This research contributes to an attempt at design-driven wasted material handling, offering two practical approaches that jewellers can use for metalwork: electroforming and recovering copper from e-waste solutions. Regarding the design phase, the approach of designing from a storytelling perspective will be explored to improve the acceptability of the materials coming from abandoned waste.
Since the research aims to focus on both metalwork techniques using e-waste solutions and design practice, further research is needed to evaluate the techniques applied and particular design processes suited to creating a material circular economy within the jewellery manufacturing industry.
Acknowledgments
The author gratefully acknowledges the support of her supervisors Prof. Sandra Wilson and Dr Katharina Vones from the University of Dundee, Duncan of Jordanstone College of Art & Design.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Notes on contributors
Kunning Ding
Kunning Ding received the MFA degree in Jewellery from The University of Edinburgh, United Kingdom, in 2021. She is currently working toward the PhD degree in Art & Design with The University of Dundee. Her research interests include contemporary jewellery design, material and craft.
References
- Chapman, Jonathan. 2015. Emotionally Durable Design: Objects, Experiences and Empathy. London: Routledge.
- Curtis, Leslie. 2004. Electroforming. London: A. C. Black.
- Ghosh, B., M. K. Ghosh, P. Parhi, P. S. Mukherjee, and B. K. Mishra. 2015. “Waste Printed Circuit Boards Recycling: An Extensive Assessment of Current Status.” Journal of Cleaner Production 94: 5–19. doi:10.1016/j.jclepro.2015.02.024.
- Hagelüken, Christian, and Christopher. W. Corti. 2010. “Recycling of Gold from Electronics: Cost-Effective Use through ‘Design for Recycling.” Gold Bulletin 43 (3): 209–220. doi:10.1007/BF03214988.
- Keller-Aviram, Danielle. 2021. “Traceability, Sustainability, and Circularity as Mechanism in the Luxury Jewelry Industry Creating Emotional Added Value.” In Environmental Footprints and Eco-Design of Products and Processes, 87–115. Singapore: Springer Singapore.
- Koppen, Eva, and Christoph Meinel. 2012. “Knowing People: The Empathetic Designer.” Design Philosophy Papers 10 (1): 35–51. doi:10.2752/089279312X13968781797553.
- Lister, M. W., and W. A. E. McBryde. 2019. Elementary Experimental Chemistry. Toronto: University of Toronto Press, doi:10.3138/9781487583026.
- Petter, P. M. H., H. M. Veit, and A. M. Bernardes. 2014. “Evaluation of Gold and Silver Leaching from Printed Circuit Board of Cellphones.” Waste Management (New York, N.Y.) 34 (2): 475–482. doi:10.1016/j.wasman.2013.10.032.
- Rao, Mudila Dhanunjaya, Kamalesh. K. Singh, Carole. A. Morrison, and Jason. B. Love. 2020. “Challenges and Opportunities in the Recovery of Gold from Electronic Waste.” RSC Advances 10 (8): 4300–4309. doi:10.1039/c9ra07607g.
- Suri, Jane Fulton. 2003. “The Experience of Evolution: Developments in Design Practice.” The Design Journal 6 (2): 39–48. doi:10.2752/146069203789355471.
- Treggiden, Katie. 2020. Wasted: When Trash Becomes Treasure. London: Ludion.
- Wilson, Sandra. 2020. “The New Gold Rush: Precious Metals Mined from Electronic Waste.” Metalsmith Magazine Vol 40 No 3 2020, Society of North American Goldsmiths (SNAG) USA pp38 – 45. ISSN 0270–1146.
- Zeng, Xianlai, John. A. Mathews, and Jinhui Li. 2018. “Urban Mining of E-Waste is Becoming More Cost-Effective than Virgin Mining.” Environmental Science & Technology 52 (8): 4835–4841. doi:10.1021/acs.est.7b04909.