ABSTRACT
This study analyses zooarchaeological material recovered from the late precolumbian site of El Flaco (AD 990–1450), northern Dominican Republic. The faunal assemblage from this inland settlement demonstrates terrestrially focused modes of faunal exploitation but with some resources obtained from coastal ecosystems, such as mangrove forests, sandy-bottom, and reefs, which are located approximately 20 km to the northwest. This study establishes last occurrence dates for extinct taxa; examines the spatial distribution of fauna; explores modes of food procurement; and the effects of agricultural activities on local fauna by investigating animal remains from three excavation units. A diachronic study of animal remains from one artificial mound demonstrates changing patterns in resource exploitation, such as an increasing consumption of land crab over a roughly 100-year period. This study follows previous research that examined the isotope ecology of endemic species from El Flaco that indicates some hutias were possibly scavenging or being fed cultivated plants. Palaeoenvironmental data suggest that Indigenous landscape transformations led to the creation of mosaic environments, which may have attracted and supported certain species, implying that the inhabitants of El Flaco likely did not have to venture far to trap or hunt many of the animals upon which they relied.
Introduction
Zooarchaeological investigations in the insular Caribbean indicate that late precolumbian societies interacted with a wide range of animals, reflecting the high degree of species biodiversity and endemism within this tropical oceanic island system. Only two domesticated animals coexisted with Indigenous peoples, guinea pigs (Cavia porcellus) and dogs (Canis familiaris), both of which have been studied extensively (Grouard, Perdikaris, and Debue Citation2013; Kimura et al. Citation2016; LeFebvre and deFrance Citation2014; Newsom and Wing Citation2004; Oswald et al. Citation2020). These animals form minor components of assemblages at most sites, suggesting that people relied mainly upon ‘wild’ animals which were usually hunted or trapped within a range of 3–5 km from settlements (Grouard Citation2002; Giovas Citation2013; Wing and Wing Citation2001). At coastal sites in the Greater Antilles, and on the relatively smaller islands of the Lesser Antilles, a demonstrably greater importance was placed on the exploitation of coastal habitats, with marine fauna generally constituting around 70% of the minimum number of individuals (MNI) at many sites (Carder and Crock Citation2012; Carder, Reitz, and Crock Citation2007; Giovas Citation2016, Citation2013; Grouard Citation2001; LeFebvre Citation2007; LeFebvre and Giovas Citation2009; Newsom and Wing Citation2004; Steadman and Jones Citation2006). In contrast, inland sites often demonstrate greater dependence on terrestrial animals (Grouard Citation2010; Newsom and Wing Citation2004; Scudder Citation1991).
This paper presents the findings from the study of faunal remains recovered from the late precolumbian settlement of El Flaco, Dominican Republic (Hofman and Hoogland Citation2015) with the aim to reconstruct aspects of its food culture through time and to investigate the impact of agricultural activities on the local fauna. Located approximately 20 km from the coast and nestled in the leeward foothills of the Cordillera Septentrional, El Flaco overlooks the fertile Cibao Valley in Hispaniola’s northern interior. The site was regularly occupied between AD 990 and 1452, with a latest radiocarbon date of AD 1490, suggesting possible contemporaneity with the arrival of Columbus (Hofman and Ulloa Hung Citation2019; Hofman and Hoogland Citation2015; Hofman et al. Citation2018; Hofman, Valcárcel Rojas, and Ulloa Hung Citation2020). This small settlement is situated at the terminus of the theorised ‘ruta de Colón’, a pass through the Cordillera Septentrional that Christopher Columbus traversed during the seminal European expedition into inland Hispaniola in AD 1494 (Guerrero and Veloz Maggiolo Citation1988; Hofman et al. Citation2018; Citation2020; Ortega Citation1988).
Human Niche Construction and Garden Hunting in the Neotropics
The abundance of certain animal species within archaeological assemblages may be explained as being the result of human niche construction activities (Odling-Smee, Laland, and Feldman Citation1996; Boivin et al. Citation2016). This is because human alterations to natural plant communities by deforestation or the planting of economically important species result in changes in ecosystems, which in turn create adaptive pressures on animals inhabiting them (Smith Citation2001, Citation2007, Citation2011; Boivin et al. Citation2016; Zeder Citation2015). Evidence from the humid Neotropics indicates that some animals, mostly dietary generalists or synanthropic in behaviour, might benefit from these landscape changes. Farming can attract certain animals to altered environments due to an increased food supply from domestic crops but also as a by-product of landscape changes, which create beneficial mosaic environments (Linares Citation1976; Arce-Peña et al. Citation2019; Loiselle and Blake Citation1992; Ramírez-Barajas and Calmé Citation2015; Smith Citation2005). These attracted animals may in turn be targeted by people tending farming plots, a practice known as ‘garden hunting’ (Linares Citation1976). Isotopic studies of ‘wild’ animal remains in the Neotropics indicate varying degrees of human influence on the diets of animals, which may point to scavenging from farming plots, particularly of cultivated maize (Zea mays). This may explain relatively higher carbon (δ13C) isotope values for deer and birds at precolumbian mainland sites in Panama (Sugiyama et al. Citation2020), and for hutias in the Bahamas (LeFebvre et al. Citation2019) and in Hispaniola (Shev, Laffoon, and Hofman Citation2021).
Following Zeder (Citation2015), a process in which an animal species exists within or moves into anthropogenic landscapes and forms a mutually beneficial but asymmetric relationships with humans could be considered as a ‘commensal pathway’ towards domestication. It is important to note that ‘commensal pathways’ do not necessarily lead towards domestication, or even to direct management of animal populations by humans. We define ‘garden hunting’ as the activity of hunting or trapping animals from within, or nearby horticultural plots or settlements to which they are attracted to, which may subscribe to Zeder’s (Citation2015) definition of a ‘commensal pathway’ (LeFebvre and deFrance Citation2018).
To examine the effects of agricultural activities on animals represented in archaeological assemblages, knowledge of the palaeoenvironment is required. For our study area, starch grain analysis has established which plant species, domestic and wild, are found within archaeological deposits at El Flaco (Pagán-Jiménez et al. Citation2020), and sediment cores provide pollen, fungal and charcoal records demonstrating evidence for the impact of landscape modification on the flora within the Cibao Valley (Castilla-Beltrán et al. Citation2020, Citation2018; Hooghiemstra et al. Citation2018).
El Flaco: Site Overview
Excavations at El Flaco took place between 2013 and 2016 as part of the ERC-funded NEXUS-1492 project. The site consists of several artificially levelled areas containing the circular arrangements of postholes indicative of household spaces. Six earthen mounds with multi-functional purposes were identified comprising layers of accumulated waste forming thick strata of fine ash from cooking activities, dark soils probably used for horticulture, and human burials (Hofman and Hoogland Citation2015; Hofman, Valcárcel Rojas, and Ulloa Hung Citation2020; Keegan and Hofman Citation2017; Pagán-Jiménez et al. Citation2020). The palaeobotanical study of the southernmost mound at the site revealed preserved starch grains of maize (Zea mays), gourd (Curcurbita sp.), and arrowroot (Maranta sp.), which were likely cultivated at or nearby the site (Pagán-Jiménez et al. Citation2020). El Flaco overlooks the fertile Cibao Valley, an area that the early fifteenth century chronicler, Bartolomé de las Casas, noted for its intensive agricultural usage, human use of fire in the landscape, and prolific hutias inhabiting its grasslands (Las Casas Citation1875).
Archaeological surveys in Puerto Plata province to the north and Montecristi province to the northwest identified a multitude of Indigenous sites containing Meillacoid, Chicoid or multicomponent ceramics located in coastal habitats (Herrera Malatesta Citation2018; Herrera Malatesta and Hofman Citation2019; Ulloa Hung Citation2014). It is important to note that El Flaco is located in the southern foothills of the Cordillera Septentrional, and therefore access to these coastal environments and settlements would have been restricted by the rugged terrain of this dividing mountain range (Hofman and Hoogland Citation2015).
Biogeography of the Area Surrounding El Flaco
El Flaco is situated between two significant geological features in the northern Dominican Republic, the Cordillera Septentrional and the Cibao Valley. The Cordillera was formed during the Tertiary Period by the deposition of sea sediments along the coast, while the agriculturally fertile Cibao Valley to its south was mostly formed by alluvial silting during the Quaternary period. The Cordillera Septentrional divides the Cibao Valley from the Atlantic coast, following a northwest-southeast axis from Montecristi and to Gran Estero (Carmona, Ramírez, and Cano-Ortiz Citation2010; Mollatt et al. Citation2004). El Flaco straddles two overlapping ecological zones, the Central Subprovince and the Caribbean-Atlantic Subprovince, and the local area is populated by semiarid-humid plant communities including cloudy broad-leafed forest, ombrophilous forest, dry forest and grasslands (Carmona, Ramírez, and Cano-Ortiz Citation2010). The location of El Flaco provided the inhabitants of the site with access to a plethora of exploitable environments, from montane rainforest immediately to the north to the agriculturally fertile valley to its south.
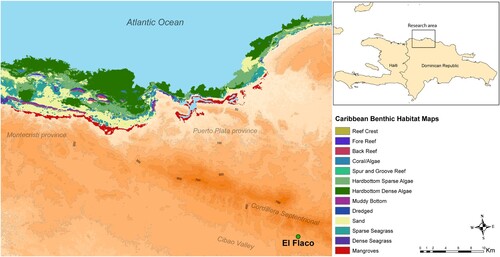
Around 20–30 km northwest of El Flaco in western Puerto Plata and eastern Monte Cristi provinces, much of the northern coastline is comprised of a low gentle slope to the sea from the Cordillera Septentrional (). This coastal area mainly consists of sandy-bottomed environments and contiguous pockets of mangrove forests that stretch between 5 and 10 km in length, and is interspersed with offshore coral reefs and seagrass beds in shallow waters located close to the shoreline (Geraldes Citation2003; Herrera et al. Citation2011; Spalding Citation2010). In this study, we define coastal habitats as areas where marine processes such as storm surges, erosion and deposition and terrestrial processes intermix (Ford Citation2013), whereas coastal sites are defined as existing within these habitats, unless access to the sea is restricted by topography.
Figure 1. Elevation map showing the location of El Flaco in relation to important geographical features discussed in this paper. Includes benthic and coastal environments located to the northwest of the site. Mangrove distribution data obtained from Spalding et al. Citation2010, and benthic habitat data obtained from The Nature Conservancy (Schill et al. Citation2021).
Palaeoenvironmental studies indicate that Indigenous landscape changes likely impacted the composition of floral communities in the region surrounding El Flaco. Sediment coring from Laguna Biajaca in the Cibao Valley, 12 km southwest of El Flaco, suggests a decrease in diversity of palm and hardwood flora leading up to cal AD 1250. An increase in charcoal particles indicates that fire usage intensified between cal AD 1150 and 1450, likely due to an increase in slash-and-burn agricultural activities. The abundance of certain pollen taxa and a decrease in charcoal particles suggest a notable decrease in burning activities and an expansion of high-canopy plants between cal AD 1500 and AD 1800, which may be accounted for by the decimation of Indigenous communities and a cessation of their environmental practices (Castilla-Beltrán et al. Citation2020, Citation2018). Sediment coring from Los Indios, located adjacent to the Yacque del Norte River in the Cibao Valley 47 km to the west of El Flaco, also indicates that farming practices formed a mosaic of natural and human-altered vegetation communities between cal AD 200 and 1525, with evidence of increasing fire usage around cal AD 1410 (Hooghiemstra et al. Citation2018).
The insular Caribbean has high degrees of endemism in terrestrial flora and fauna, with the highest taxonomic diversities in the Greater Antillean islands of Cuba, Jamaica, Hispaniola, and Puerto Rico that together constitute roughly 90% of the land area of the insular Caribbean (Grouard Citation2010; Kier et al. Citation2009; Newsom and Wing Citation2004). Hispaniola alone is home to approximately 2050 endemic plant species (Meijía Citation2006) and is characterised by high diversity in its numerous endemic reptiles, endemic and migratory birds, and mammals. Herpetofauna are the most diverse with high degrees of endemism including 147 reptile and 62 frog species found nowhere else on earth (Hedges Citation2007, 18; Powell Citation2012).
As part of the greater insular Caribbean region, Hispaniola is unique in belonging to one of the few oceanic island systems settled by terrestrial mammals. European colonisation led to the mass extinctions of many endemic species (Crosby Citation2003; Turvey et al. Citation2007, Citation2017) with Hispaniolan hutia (Plagiodontia aedium) and solenodon (Solenodon paradoxus) being the sole surviving endemic mammals (Mcfarlane et al. Citation2000; Whidden and Asher Citation2001). Post-AD 1500 extinctions of endemic mammals include all West Indies shrews, all but one species of hutia and all edible rats (Hansford et al. Citation2012; Mcfarlane et al. Citation2000; Macphee, Flemming, and Lunde Citation1999).
The potential for Indigenous management of certain hutia species has long been speculated by some researchers (Carlson and Steadman Citation2009; Colten and Worthington Citation2014; LeFebvre and deFrance Citation2018; LeFebvre et al. Citation2019; Oswald et al. Citation2020; Wilkins Citation2001; Wing Citation2008a, Citation2012). One species, Puerto Rican hutia (Isolobodon portoricensis), a misnomer as it likely evolved in Hispaniola and was introduced by humans to Puerto Rico and the Virgin Islands, often comprises significant proportions of archaeological assemblages (Flemming and MacPhee Citation1999; Morgan et al. Citation2019; Wing Citation2012, Citation2008b; Wing, Ray, and Kozuch Citation2002). As determined from isotopic analysis, some hutia species likely had human-influenced diets, with a study on I. portoricensis collagen revealing that 53.3% (n = 8) of individuals at El Flaco had high dietary carbon (∂13C) values that were within the range of domestic dogs from the same site, but distinct from other examined endemic fauna (Shev, Laffoon, and Hofman Citation2021). However, high carbon isotope values in some individuals does not necessarily indicate their systematic management and can be associated with other factors, such as intensive farming which may have encouraged scavenging behaviours of the hutia due to an increase in the availability of their food sources (LeFebvre et al. Citation2019; Shev, Laffoon, and Hofman Citation2021).
Materials and Methods
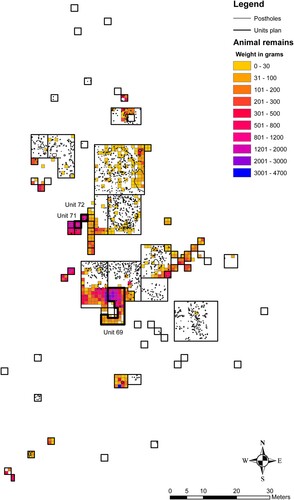
During excavation, all earth was passed through a 4 mm or 2 mm mesh. Animal bone was separated during sieving and later sorted by taxonomic class. Faunal material was counted and weighed, allowing distribution mapping of broad faunal categories across the entire excavated area (). Zooarchaeological analysis, involving the identification of taxa beyond broad faunal categories, was conducted on faunal remains from three excavation units: 69, 71 and 72. We chose these three units due to the high amount of faunal material they contained, and the presence of features such as hearths and burials. Unit 69, in the south of the site, was multifunctional and used for refuse disposal, horticulture, and for burials (Hofman and Hoogland Citation2015; Keegan and Hofman Citation2017). The excavated surface area of Unit 69 is 42m2, while a volume of 40.5m3 was excavated at varying depths given the sloping nature of the mound. Units 71 and 72 (both 2 × 2 m) were connected in one corner, included domestic refuse and hearth features and did not contain burials. Units 71 and 72 are located within an artificial mound at the western part of the excavated site, which likely served as a refuse area for the large housing structure located adjacent to the east. Unit 71 contained 4.4m3 and Unit 72 5.2 m3 of excavated earth.
Figure 2. Horizontal distribution map of the weights (g) of recorded faunal remains across all excavated units at El Flaco. Demonstrates the location of Units 69, 71 and 72 and of postholes indicating dwellings. Stratigraphic layers are combined. (Map by Simone Casale, copyright NEXUS1492)
We conducted a diachronic study of fauna from Units 71 and 72, quantifying material by excavation layer and interpreting this according to stratigraphic profiles, archaeological features, and radiocarbon dating.
We took three radiocarbon samples from Unit 71 (n = 1) and Unit 72 (n = 2) to assess the date range of the western mound which contains these excavation units. We calibrated the conventional radiocarbon dates in the OrAU OxCal software (v4.4) using the IntCal20 calibration curve (Reimer et al. Citation2020) to produce probable calendar ages (in BC/AD) at both 95.4% and 68.3% confidence. Additionally, to provide reliable temporal boundaries for this context using the available data we produced Bayesian start and end dates for this deposit by constructing a simple single-phase Bayesian model using the OrAU OxCal software (v4.4) (SI Figure 1). The Bayesian start and end models are reported at 68.3% confidence, and a statistical median is provided for each model.
We identified terrestrial vertebrates in collaboration with Sr Juan Almonte and Dr Carlos Suriel at El Museo Nacional de Historia Natural ‘Prof. Eugenio de Jesús Marcano’ in Santo Domingo. Rodent identifications were based on dental morphology, as post-crania of caviomorph rodents are exceedingly difficult to accurately identify (Hermanson and Woods Citation2012, 187). Reptiles were identified using select diagnostic elements, such as the dentaries, mandibles, frontals, and some postcrania. Avian remains were few and largely undiagnostic due to their high degree of fragmentation and poor preservation.
Fish remains were identified using a reference collection currently housed at the NEXUS1492 field unit at Cruce de Guayacanes, Valverde Province, Dominican Republic. Fish skeleton pictorial guides were used: the online fish skeleton pictorial guides of Osteobase (http://www.osteobase.mnhn.fr/), FishBase (http://fishbase.org/), and the Florida Museum of Natural History Pictorial Skeletal Atlas of Fishes (http://www.floridamuseum.ufl.edu/fishatlas/). Due to the lack of an extensive, locally available, physical reference collection much of the fish was not identified beyond the family level. Most identifications were based on the five paired elements; dentaries, maxillae, premaxillae, articulars and quadrates, plus special taxa-specific elements, such as pharyngeal grinders (Leach Citation1997). Fish vertebrae were few within the three units (NISP = 83) and therefore we deemed the lack of vertebrae identification as a minor limitation.
We calculated the MNI of land crabs (Gecarcinidae and Ucididae) by counting mandibles and pairing elements, the majority of which were chelipeds. The lack of a reference collection however prohibited identifications to a lower taxonomic order. The assemblage is likely comprised of species from the land crab (Gecarcidinae) family, blue land crab (Cardisoma guanhumi), black mountain crab (Gecarcinus ruricola), and red land crab (G. lateralis) or belong to mangrove adapted swamp ghost crab (Ucides cordatus), which are all commonly trapped in the same modern crabberies in the north-western Dominican Republic (Herrera et al. Citation2011; Wing Citation1995).
We applied different methods of quantification for vertebrates and invertebrates. For vertebrates, the number of identified specimens (NISP) was calculated, and samples were weighed. Elements of the same taxa were paired according to size to calculate the MNI. For marine invertebrates we only calculated the MNIs as counting the NISP of fragmented material was unfeasible. The MNI, NISPs and biomass of each taxon, or soft tissue weight in the case of invertebrates, were calculated for the site. All marine invertebrates were sorted and identified by students under supervision during the excavation seasons using a reference collection also housed at the NEXUS1492 field unit.
There are inherent inconsistencies with calculating biomass from fragmented archaeological material; however, we applied this metric to provide another quantification beyond NISP and MNI, which are subject to their own biases (Domínguez-Rodrigo Citation2012). Biomass was calculated using published average weights of terrestrial vertebrate taxa, with the exception of Isolobodon portoricensis (n = 230), I. montanus (n = 1), Brotomys sp. (n = 37), and guinea pig (Cavia porcellus) (n = 1), for which average body masses were directly calculated by measuring lower tooth row length using allometric regression values as outlined by Hopkins (Citation2008) for rodents of <5 kg in weight. The anterior centrum width of complete fish vertebrae (n = 50) was measured to provided relative estimations of body mass range. Fish body mass, and soft tissue weights for marine invertebrates were calculated from centrum width and shell weights respectively, using the allometric formula Y = aXb (Y = live weight, X = shell weight/centrum width, a = Y-intercept, b = slope) following regression values outlined for fish vertebrae by Wing (Citation2001) and Colten and Worthington (Citation2014), and for gastropods, general bivalves and oysters by Reitz and Wing (Citation2008, 68). We used published live weights of all identified species when enough shell was not present to accurately calculate soft tissue weight, as in the case of queen conch (Aliger gigas). An analogous average body mass for land crab of 144.9 g was derived from weight ranges of Cardisoma guanhami taken over a six month study by Olalekan (Citation2015). Land crab meat yields were deduced from fishery studies conducted on live blue swimming crabs (Portunus pelagicus), which average 29.4% of total body weight (Pathak et al. Citation2019). To calculate the soft tissue weigh of Queen conch (Aliger gigas) we applied an average live weight of 2.3 kg of which approximately 7–8% is meat (Davis Citation2005).
We recorded bone discolourations to provide an indication of the temperature to which the bone was exposed, in order to suggest modes of cooking (Ellingham et al. Citation2015). Bone colour was recorded using a Munsell soil colour chart.
Results
Faunal Distribution Across the Excavated Area of El Flaco
The faunal remains of the excavated site included a wide range of taxonomic classes (; ). The sorted faunal material from the entire excavated area totalled 14,798 bones, 8,589 of which were identifiable to taxonomic class, and 18,249 land crab remains. These were distributed in mainly three areas, with the majority in the western artificial mound (Units 70–74), and southern area (including Unit 69) that show mixed usage. Land crab, the most numerous of all faunal remains (75.19% of NISP), are mainly clustered in the western, southern, and eastern refuse areas. Terrestrial gastropod (Pleurodonte sp. and Obeliscus sp.) shells appear to be most dense in the eastern part of the site. The high density of land snail in this eastern section may also be an indication of a greater time of exposure of archaeological deposits to open air (Gutiérrez Zugasti Citation2011; Newsom and Wing Citation2004; Wing, Ray, and Kozuch Citation2002), which may also have negatively impacted the preservation of animal bone. No further work regarding the taxonomic identification of sorted faunal material, or other qualitative analysis, was conducted on material from the entire excavated area of the site.
Figure 3. Assortment of faunal remains recovered from El Flaco: (a) Isolobodon portoricensis mandibles; (b) Aves: left – Hispaniolan woocock (Scolopax brachycarpus) tarsometatarsus; right – white-winged warbler (Xenoligea montana) humerus; (c) Hispaniolan boa (Chilabothrus striatus) maxilla and dentary; (d) anguid/galliwasp (Celestus sp.) dentary/mandible; (e) Nesophontes paramicrus mandible; (f) Dominican giant anole (Anolis baleatus) frontal; (g) Brotomys sp. mandibles; (h) Hispaniolan slider (Trachemys s. vicina) carapace fragment; (h) parrotfish (Scarinae) lower pharyngeal grinder; (j) land crab (Gecarcinidae) chelipeds. (photo G. Shev).

Table 1. Identified vertebrates and invertebrates from excavation Units 69, 71 and 72 from El Flaco. Includes NISP, MNI and biomass/soft tissue weight of all fauna calculated using allometric formulae and applying regression values from listed publications.
All the following results relate only to findings from the three case study excavation units: Unit 69, 71 and 72, which contain 36.3% (NISP = 3114) of the identifiable bones, and 51.7% of all land crab remains from the entire excavated area.
Last-Occurrence Dates
Direct radiocarbon dating of an individual I. portoricensis from Unit 72, layer 4, indicates a new last occurrence date of 570 ± 30 BP, updated from 710 ± 50 BP (Mcfarlane et al. Citation2000). Nesophontes paramicrus, a species of West Indies shrew, has a new relative last occurrence date of between 445 and 550 ± 30 BP, based on radiocarbon dating of archaeological material in the same contexts in the upper three layers of Unit 69. The previous last occurrence date was 680 ± 50 BP (cal. AD 1295) (Macphee, Flemming, and Lunde Citation1999). An extinct lizard, Leiocephalus anonymous, present in Unit 72 layer 2, also has a relative last occurrence date of 600 ± 30 BP; however, it is suspected to have survived into the sixteenth century (Pregill Citation1984).
Terrestrial Vertebrates
Eight mammal taxa are represented including two domestic species, dog and guinea pig, the latter represented by one mandible and being one of only four instances that this species has been recovered from pre-Columbian contexts in Hispaniola (LeFebvre and deFrance Citation2014). Thirty-one C. familiaris specimens (MNI = 3) were recovered. The majority of mammal remains were of the Echimyidae family of rodents, which includes the subfamily of hutias (Capromyinae). Hispaniolan hutia (Plagiodontia aedium) is represented by four mandibles (MNI = 3), whereas I. portoricensis dominates the terrestrial vertebrates (NISP = 438; MNI = 234). The second-most abundant terrestrial vertebrate is the edible rat (Brotomys sp.) (NISP = 242; MNI = 131), which may have been one of four highly esteemed elite foodstuffs known to Indigenous peoples as ‘mohuy’ (de Oviedo y Valdez Citation1851, 35). Seven Nesophontes paramicrus individuals are present (NISP = 15; MNI = 7), as well as one mandible of a fig-eating bat (Phyllops falcatus).
Herpetofauna formed a large part of the diet at the site with a clear preference for large endemic lizard species, which are still found locally. The largest reptile species is rhinoceros iguana (Cyclura cornuta) (NISP = 37; MNI = 6); however, the most frequently occurring reptiles are anguids (Celestus sp.) (NISP = 165; MNI = 67) and anoles (Anolis sp.) (NISP = 165; MNI = 57), followed by the now extinct Leiocephalus anonymous (NISP = 79; MNI = 41). Hispaniolan boa (Chilabothrus striatus) (NISP = 24; MNI = 12) is well represented as is freshwater Dominican slider (Trachemys stejnegeri viscina) (NISP = 134; MNI = 7).
Bird remains were mostly non-diagnostic (NISP = 20); however, seven species from five families were identified, three from the pigeon family, Columbidae (NISP = 6; MNI = 3). One species, the white-winged warbler (Xenoligea montana) (NISP = 1) is only found on Hispaniola (BirdLife International Citation2021). Hispaniolan woodcock (Scolopax brachycarpa) (NISP = 1) was also identified, a species which was one of many endemic animals that persisted throughout the late-Holocene but disappeared sometime after the arrival of Europeans in the region (Steadman and Takano Citation2013).
Marine Vertebrates
A total of 23 fish species encompassing 12 genera and 14 families were identified. Most species prefer inshore, estuarine, and reef habitats, with the exceptions being scalloped hammerhead (Sphyrna lewini) (NISP = 1) and crevalle jack (Caranx hippos) (NISP = 1) which are coastal pelagic species, though crevalle jack also frequents brackish waters (Cervigón et al. Citation1992) (). Possibly, the only true pelagic species is represented by two vertebrae of a fish from the Scombridae family (tuna, mackeral and bonitos). The most represented species is rock hind (Epinephelus adscensionis; NISP = 4; MNI = 3). Two families are represented by several species, Scarinae (# of taxa = 4) and Serranidae (# of taxa = 3), but no species dominates the assemblage. Most of the identified fish inhabit coastal inshore (31.25% of marine vertebrate MNI) and reef environments (50% of marine invertebrate MNI), suggesting that environments close to the shore were the primary target for fishing activities. Although freshwater fish could have feasibly been caught from the Yaque del Norte River or tributaries no freshwater species were identified. Nevertheless, the presence of freshwater turtle, Hispaniolan slider (Trachemys stejnegeri viscina), suggests that the site’s inhabitants occasionally utilised rivers and streams.
Figure 4. Fishing techniques and habitat preferences of identified fish taxa following criteria of Wing (Citation1972).
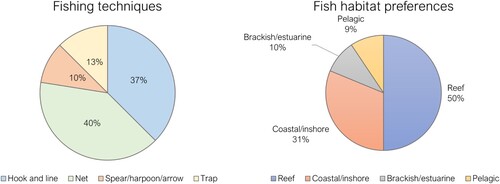
There was likely a range of fishing techniques employed (). Netting was probably a common practice, with 40% of all identified individual fish being susceptible to be captured using this method, due either to their size or their herbivorous diets, which inhibit the use of hook-and-lines. However, it must be noted that netting was likely incompatible with reef environments. Parrotfish (Scarinae) may have also been captured using traps, a method for which 13% of identified individual fish (MNI) are amenable for capture. Hook-and-line methods may have been employed for most predatory fish (37% of marine vertebrate MNI), whilst the use of spears, harpoons or arrows may have been employed for larger catches, such as requiem sharks (Carcharhinidae), rays (Myliobatiformes) and groupers (Serranidae). Fish weight ranged from 129.3g to 11689.6 g, (μ = 1545.5g, Mdn = 672.8g). Most fish (n = 31, 62%) weighed under 1 kg, whereas only 7 individuals exceeded 2 kg in live weight.
Invertebrates
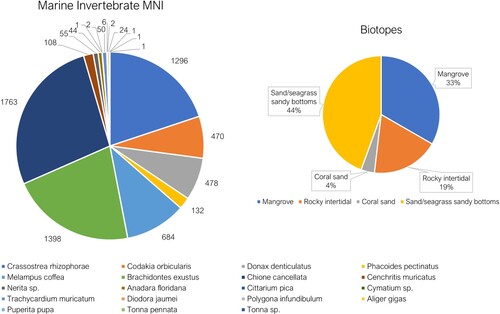
Terrestrial invertebrates, such as land crabs (Gecarcinidae and Ucididae; NISP = 9437; MNI = 3293), and snails (Pluerodonte spp.) (MNI = 5446), account for the majority of the site’s total MNI, although according to biomass calculations (12.3% of site total) land crabs were the second most important source of meat. The lack of an adequate reference collection for land crabs prevented distinguishing between species, but these taxa all inhabit open forested areas located behind coastal shores, often favouring mangrove areas (Hartnoll et al. Citation2006; Herrera et al. Citation2011). The significant presence of Pleurodonte sp. (MNI = 5446) may be an indication of the intensity of agriculture that was being practiced at the site.
The Indigenous people living at El Flaco targeted twenty coastal-dwelling species of marine gastropods and bivalves. Venus clam (Chione cancellata, MNI = 1763) dominates, accounting for 31.1% of marine invertebrate soft tissue weight, whilst another bivalve, Mangrove oyster (Crassostrea rhizophorae, MNI = 1296) is also well represented (23.5% of soft tissue weight). Small gastropods, Melampus coffea (MNI = 684) and Cenchritis muricatus (MNI = 108), were gathered, yet their small size likely meant that little nutrition was gained from these species, and they may have been gathered accidently because they inhabit nearshore environments exploited for more economically important molluscs. Queen conch (Aliger gigas) is represented by MNI = 24 and comprises 14% of the edible meat weight of marine invertebrates, yet throughout the Caribbean conch middens are often found close to the coast so this species may have been frequently butchered prior to transportation to the site (Newsom and Wing Citation2004; Posada et al. Citation2007). Conch shell was also manipulated and used as tools throughout the Caribbean region (Newsom and Wing Citation2004). The near absence of gastropods, such as West Indian whelk (Cittarium pica) (MNI = 1), an important food elsewhere in the Caribbean (Robertson Citation2003), suggests that rocky intertidal areas were rarely targeted. The taxonomic composition of the marine invertebrate assemblage supports a heavy exploitation of mangrove forests, sandy-bottom, and seagrass environments as determined from the high proportions of venus clam, mangrove oyster, and the presence of conch (Aliger gigas) ().
Evidence of Burning
Only 6.3% (NISP = 196) of identifiable vertebrate specimens showed clear signs of burning, with Anolis sp. demonstrating the highest frequency of all genera (10.3%, NISP = 17). This was followed in frequency of burning by Anguids (Celestus sp.) (7.9%, NISP = 13), and Echimyidae (hutias and edible rats) (7.5%, NISP = 154). Of burnt bones, 46.9% (NISP = 92) are of reddish-brown to dark brown hue (Munsell 7-5YR–7-5YR8/3) possibly indicating cooking temperatures of between 300°C and 400°C (Ellingham et al. Citation2015; Quatrehomme et al. Citation1998; Shipman, Foster, and Schoeninger Citation1984; Wahl Citation1981). Land crab remains demonstrate the highest rate of burning (10.4%, NISP = 987) of all animal remains.
Biomass
The calculation of biomass provides another method of examining the relative dietary importance of taxa beyond NISP and MNI. Terrestrial animals, including land crabs, account for a biomass of 1036.6 kg, or at 90.7% of the total site biomass, a figure disparate to the total site MNI that place this group at 40.5% of the total. Marine invertebrates account for 2.6% of the total biomass, whilst marine vertebrates account for 6.8%.
One terrestrial vertebrate dominates, Isolobodon portoricensis, accounting for 13.4% of the site biomass. When all Echimyidae postcrania (hutias and edible rats) are combined they account for 65.7% of the site biomass. Land crab, although the most numerous of terrestrial animal remains, were the second highest in biomass, accounting for only 12.3% of the total, therefore their overrepresentation in NISP and MNI does not indicate that these animals formed most of the diet. Additionally, taphonomic processes may have favoured the preservation of heavily calcified land crab chelipeds (Locatelli Citation2013) over more gracile vertebrate remains.
Diachronic Study of Units 71 and 72
In Unit 71 most identified vertebrates are found (NISP = 72) in layer 9, the majority of which are hutia, while land crab remains are minimal (NISP = 51) compared to later deposits, increasing to NISP = 266 in layer 4 (). This layer corresponds to a stratum consisting of ash and charcoal deposits, and a mix of silt and fine gravel. Radiocarbon dating () of a I. portoricensis mandible from this layer yielded a conventional 14C date of 670 ± 30 BP (cal. AD 1277–1322, 37.8% probability; cal. AD 1356–1392, 42.3% probability). The sole guinea pig mandible recovered from this unit comes from Layer 10. Vertebrates are relatively few in upper layers, while land crab increases up to layer 4 which consisted of slightly ashy earth deposits containing relatively less charcoal. Based on stratigraphic profiles this layer likely corresponds to Unit 72, layer 4, with radiocarbon dating of another hutia mandible giving a conventional date of 570 ± 30 BP.
Figure 6. Distribution of faunal material according to excavation layer within Unit 71; (a) the NISP of vertebrate classes; (b) crab (Gecarcindae and Ucididae) NISP; (c) MNI of marine invertebrate species.
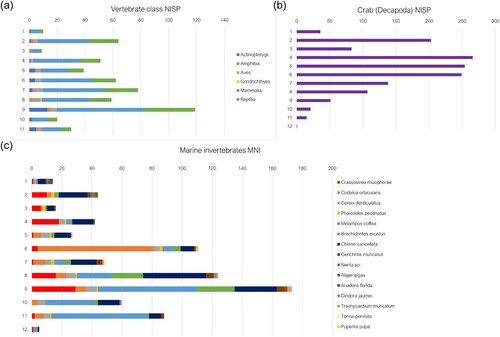
Table 2. Radiocarbon sample dates from Units 71 and 72 (n = 3).
Marine invertebrate MNIs decrease in the upper layers of Unit 71, with evidence of a relative decrease above layer 9, like that of vertebrates, but with perhaps a greater presence of queen conch (Aliger gigas) in upper layers. However, given the relatively short date range with overlapping calibrated dates, these differences may be associated with different activities being conducted in this area of the site and may not be representative of changes to overall subsistence strategies. Several features are present within Unit 71, including hearths at layers 8 and layer 3. Layers 4 and 9, with the most faunal remains, directly underly these hearths and therefore are likely linked to these features.
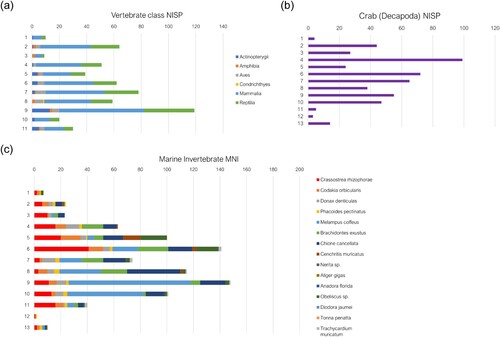
In Unit 72 the highest quantity of identified vertebrates is within layer 10 (NISP = 119), which corresponds stratigraphically to layer 9 in Unit 71 and therefore likely dating to 670 ± 30 BP (; ). Land crab is present in similar quantities in layer 10, corresponding to layer 9 in Unit 71. There is again an increase in land crab consumption culminating in layer 4, dating to 570 ± 30 BP. Layer 9 contains most marine invertebrates but is dominated by Melampus coffea, which has little nutritional yield. Layer 3, as in Unit 71, has relatively few faunal remains. An I. portoricensis mandible yielded a radiocarbon date of 600 ± 30 BP which is earlier than the sample from layer 4 but with considerable overlap in calibrated date ranges, indicating that these upper layers were likely contemporaneous. These layers correspond to silty deposits containing gravel, ash and charcoal. Unit 72, like Unit 71, appears to demonstrate a trend of decreasing reliance on marine invertebrates, with an increase in land crab in the upper layers.
Figure 7. Distribution of faunal material according to excavation layer within Unit 72; (a) the NISP of vertebrate classes; (b) crab (Gecarcindae and Ucididae) NISP; (c) MNI of marine invertebrate species.
Based on the available chronological data, the start date for this deposit is modelled at cal AD 1255 to 1361 (68.3% probability), with a median of cal AD 1278 (). The end date for this deposit is modelled at cal AD 1334 to 1439 (68.3% probability), with a median of cal AD 1401. As a result, these events can be securely established as belonging to a timeframe somewhere between cal AD 1255 and 1439. However, it is important to note that the inclusion of additional chronological data in the future may impact these boundaries. The raw calibrated distribution plots, Bayesian start and end models, and the code used to produce the data is available online (SI 2).
Figure 8. Modelled Bayesian start and end date ranges produced using the available conventional radiocarbon ages. All ranges are shown at 68.3% confidence and statistical medians are illustrated as crosses.
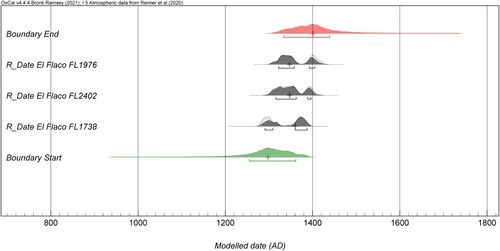
Generally, most marine molluscs and vertebrates were deposited sometime around cal AD 1278, while land crab usage increased progressively, peaking sometime in the late fourteenth century. A change in faunal exploitation is not clear as this mound was in use for a relatively short time compared to the occupational date range of the site.
Discussion
The faunal assemblage from Units 69, 71 and 72 at El Flaco suggests that the inhabitants mainly hunted or trapped locally available terrestrial animals and supplemented their diets with distantly acquired marine foods. Marine resources were sourced predominantly from mangrove forests, sandy-bottom inshore environments, and inshore reef systems. Although vertebrates and marine invertebrate taxa numbers fluctuate over time, their relative percentages remain similar in each layer and do not clearly suggest an overexploitation of any one taxa or environment. The presence of marine animals serves as evidence of persistent interaction with coastal areas; however, fishing and shellfish gathering were likely not important subsistence strategies for the inhabitants of this small, horticultural settlement. The presence of a smaller mangrove-adapted gastropod (Melampus coffea) that had little nutritional yield may reflect the non-optimal foraging of inexperienced members of the community, such as children (Keegan et al. Citation2019).
The only clear change in faunal exploitation observed is an increasing predation on land crab during the creation of the western artificial mound. These observations are contrary to the decrease in land crab exploitation during the Saladoid – post-Saladoid cultural transition in much of the Caribbean, which for example is seen throughout Puerto Rico and Guadeloupe (Grouard Citation2001; Wing Citation2001). Although, a similar anachronistic phenomenon of increasing land crab consumption was observed from sample columns at La Jácanas, Puerto Rico, dating to cal AD 1088 (DuChemin Citation2013). In the Dominican Republic, large modern land crab exploitation areas exist within mangrove and coastal areas of Montecristi province where these three species are found abundantly (Herrera et al. Citation2011). An increase of land crab consumption may reflect the broadening of exchange networks with coastal communities between AD 1280 and 1380, but there is no clear increase in the consumption of marine foods to support this. An expansion in land crab trapping may also relate to the increasingly moist conditions present in Hispaniola between cal AD 1250 and 1600 (Castilla-Beltrán et al. Citation2018; Lane et al. Citation2009), which may have bolstered populations of land crab species that are particularly susceptible to climatic fluctuations (McGaw et al. Citation2019; Olalekan Citation2015). However, given the relatively short duration of usage for the mounds comprising Units 71 and 72, further analysis of other contexts within the site will improve our understanding regarding changes in foodways or the exploitation of ecosystems.
An increase of land crab and a decrease in marine invertebrates does not mean that different environments were targeted, as all these marine resources may have feasibly been accessed from the same region. Montecristi province, along with containing abundant mangroves is home to the largest reef formation in the Dominican Republic, reaching 64.2 km in length (Geraldes Citation2003), so possibly all reef-dwelling fish identified may also have broadly come from this region. Southwest Puerto Plata province also contains mangrove and reef systems and can be considered as a continuation of similar biomes and therefore serves as another candidate.
Observations of bone discolouration due to heat indicated that a lot of the fauna were subject to cooking temperatures of between 300°C and 400°C, suggesting that roasting meat over an open flame may have been a popular technique of food preparation. Hutia, land crabs and some lizard species were either occasionally roasted over open flame or their remains were commonly disposed of in hearths and burnt after consumption. Most specimens did not demonstrate observable signs of burning, perhaps reflecting methods of cooking that did not reach temperatures leading to bone discolouration or that did not expose bone directly to flame, such as boiling in ‘pepper pots’ at temperatures below 200°C (Ellingham et al. Citation2015; Kalsbeek and Richter Citation2006).
The presence and relative quantities of some vertebrate species may reflect the impact of human niche construction activities and garden hunting strategies. Most meat came from terrestrial vertebrates, particularly hutia. This sharply contrasts with the coastal site of En Bas Saline (AD 1200–1530), northern Haiti, where only 24 I. portoricensis individuals are represented (NISP = 123) and rarely comprise more than 1% of the identified fauna within each sample, whereas marine taxa largely dominate the assemblage (LeFebvre Citation2015). I. portoricensis, the dominant vertebrate at El Flaco, was likely synanthropic according to its ubiquity in many archaeological sites. This is supported by a genomic study of human coprolites from Saladoid-Huecoid contexts at Sorcé, Vieques that identified zoonotic parasites likely transmitted from hutia to humans (Wiscovitch-Russo et al. Citation2020). It was likely a ground-dwelling species of hutia (Cooke and Crowley Citation2018; DuChemin Citation2013; Garner Citation2002; Newsom and Wing Citation2004), and therefore it may have benefited from the clearance of high-canopied flora. Las Casas (Citation1875, 384) also mentions how hutia were found in great number in the tall grasses of the Cibao Valley when Europeans first arrived, noting also the systematic burning of the landscape. Palaeoenvironmental studies from northern Hispaniola support this early historical mention of fire usage, confirming an increase in agricultural activity and evidence of more fire in the landscape around cal AD 1150 (Castilla-Beltrán et al. Citation2020, Citation2018). Slash-and-burn farming likely created mosaic environments and may have bolstered populations of hutia, while cultivated garden plots possibly attracted them to human settlements.
Isotopic investigation of human and animal bone collagen from El Flaco positively determined that some hutias had diets that led to isotopic values similar to that of humans (Shev, Laffoon, and Hofman Citation2021). These similarities are indicated by relatively high carbon isotope values falling into the range of humans and domesticated dogs from El Flaco, but being very different from other analysed faunal species that are not considered to have been managed by Indigenous peoples (Newsom and Wing Citation2004). Nonetheless, due to the high degree of variance of isotopic values within hutia populations it is unlikely this is associated with their systematic management. These data, although not discounting that some hutia may have been purposefully fed, or possibly tamed, more likely suggests that some hutias were attracted to and habitually scavenged from human garden plots (Shev, Laffoon, and Hofman Citation2021). Similar phenomena, such as the increase in agouti (Dasyprocta leporina) and overall reliance on terrestrial animals in Late Ceramic contexts at the site of Roseau in Basse Terre, Guadeloupe, has also been hypothesised as being the result of garden hunting practices (Bochaton et al. Citation2021). Based on our evidence we hesitate to suggest that some endemic animals were undergoing a process of nascent domestication. Instead we consider garden hunting as a viable explanation for the composition of the faunal assemblage, and of how some animals, such as I. portoricensis, demonstrate dietary similarities with humans while at the same time they were also an important source of food for Indigenous peoples.
The presence of other taxa in the three studied units at El Flaco may also be the result of garden hunting. Two insectivorous lizard genera, anoles (Anolis spp.) and anguids (Celestus spp.) may have been attracted to garden plots due to an abundance of terrestrial snails and other agricultural pests. Anguids are opportunistic predators but mostly target prey within their preferred habitat of leaf litter areas, often making use of human-made clearings. Some endemic species of Hispaniolan anoles demonstrably thrive better within anthropogenic landscapes (Incháustegui, Schwartz, and Henderson Citation1985; Powell, Ottenwalder, and Incháustegui Citation1999). It may be that the presence of these species in such relatively large quantities is the result of localised hunting or trapping within garden plots or mosaic plant communities close to the settlement. According to Linares (Citation1976), the presence of certain bird species may reflect an attraction to anthropogenic mosaic environments, like in the case of pigeons (Columbidae) which are represented in the El Flaco assemblage, although identified avian taxa were only few. Little is known about the behaviour of the second-most represented vertebrate at El Flaco, the edible rat (Brotomys sp.), though an isotopic study by Cooke and Crowley (Citation2018) suggests an arboreal lifestyle with a frugivorous diet of largely homogenous sources for Brotomys voratus. It is possible that their presence within the assemblage may also be the result of an attraction to fruit trees, which were cultivated by Indigenous peoples throughout the Caribbean (Newsom and Wing Citation2004). It is likely that the inhabitants of El Flaco spent little time hunting far from their settlement and were more reliant on easily catchable prey that were attracted to human horticulture.
The large quantity of marine invertebrates, as well as land crabs which are found prolifically nearby mangrove areas and forests bordering the coastline (Olalekan Citation2015), suggest that there was consistent transportation of animal resources from the coast to the hinterland. The likely region of origin for much of the mangrove-inhabiting fauna is northern Montecristi and southwest Puerto Plata, located approximately 25 km to the northwest. The Montecristi coastal region is known for its large mangrove forests, but it is thought that it has much less agricultural potential compared to the Cibao Valley partly due to its more arid climate (Herrera et al. Citation2011; Hoy and Fisher Citation1974). An exchange of resources between El Flaco and the coast to the northwest was therefore a good possibility. As supported by evidence from examinations of cultural material from the site (Falci et al. Citation2020; Hofman, Valcárcel Rojas, and Ulloa Hung Citation2020), this study confirms that El Flaco was likely one node within a larger network of interconnected settlements engaged in the trade of locally acquired or cultivated products, specific to their regions.
In comparing inland El Flaco to coastal En Bas Saline, distinctions can be made primarily with the ratios of marine to terrestrial fauna. This is likely a reflection of the main food production activities that were taking place at each site, with the latter placing more emphasis on the harvesting of sea-based resources. The assemblage at El Flaco, located in the agriculturally productive interior Cibao Valley, contains more locally acquired terrestrial animals than coastal sites. It is likely that most terrestrial species were easily hunted or trapped close to the site as they may have been attracted to human activities, as has been previously demonstrated with the isotopic evidence of hutia from the site sharing dietary similarities with the human inhabitants (Shev, Laffoon, and Hofman Citation2021). Although not discounting hunting, fishing and the gathering of shellfish as common activities, this study suggests that the primary labour focus at El Flaco was on horticulture, while coastal resources, particularly land crab and marine bivalves, were available via local exchange networks.
It is possible that agricultural produce from the hinterland was exchanged for marine resources obtained from coastal settlements, with kinship ties facilitating these networks of exchange. A recent ancient DNA study indicates population sizes in Hispaniola were much smaller than previously surmised, with low genetic differentiation and genetically closely related peoples buried relatively far apart (Fernandes et al. Citation2020). It may be those connections between groups inhabiting the interior and the coast were familial, with members of both groups part of extended kinship groups that may have helped facilitate the exchange of resources. El Flaco likely served as only one node within a system of mobility and exchange in which resources were moved between coastal communities bountiful in marine resources, and the agriculturally productive hinterland of the Cibao Valley.
Conclusion
Our study of the faunal remains from the precolumbian ‘hamlet’ of El Flaco has revealed a plethora of information regarding the subsistence strategies of the site’s inhabitants. Our data also suggest the Indigenous inhabitants of northern Hispaniola practiced active trade with peoples occupying different ecological zones, indicating the existence of complex regional interconnections prior to the European invasion of the island. We have provided updated last occurrence dates for some taxa that went extinct after the European invasion, such as Isolobodon portoricensis, Nesophontes paramicrus and Leiocephalus anonymous, examined the spatial distribution of fauna across the excavated area of the site, and utilised previously conducted palaeoenvironmental studies to improve our interpretations of human-animal relations in the region. Our findings suggest that terrestrial animals were primarily targeted and provided the greatest source of meat for the inhabitants of El Flaco. These animals, particularly endemic rodents such as hutias and edible rats, were likely attracted to horticultural plots either within or nearby the settlement. The practice of garden hunting meant that Indigenous peoples likely did not venture far, nor spent too much time trapping or hunting animals, meaning that the primary labour focus was likely on plant food production. Alongside the abundance of terrestrial fauna, significant quantities of land crab and shellfish were recovered, suggesting that networks of exchange facilitated the trade of resources from coastal areas beyond the Cordillera Septentrional into the hinterland of the Cibao Valley.
Previous isotopic work has established that some hutias were possibly scavenging horticultural produce from humans or were possibly tamed and purposefully fed by humans. To further elucidate these relationships, more isotopic research is needed, such as employing Bayesian dietary mixing models to establish the relative proportions of certain types of plants that were consumed. In addition, examining the isotope ecology of humans and hutia in early archaeological contexts that predate the supposed introduction of widespread agriculture during the Ceramic Age would be greatly beneficial to assess the differences between a wild diet and one influenced by human activities. Further studies of faunal remains from sites in Hispaniola, both located on the coast and in the interior of the island, will undoubtedly improve our understanding of regional differences in food procurement as well as establish the existence of exchange networks between settlements occupying different regions and environments. Our study, in considering previous work on palaeoenvironmental reconstructions and the isotope ecology of animals, highlights the need to acknowledge the impact Indigenous peoples had on their environments and resident animals. Doing so within a broader explanatory framework of human niche construction allows us to better contextualise and interpret faunal assemblages, and more holistically assess human-animal relations prior to the European invasion of the Americas.
Supplemental Appendices
Download MS Word (791.2 KB)Acknowledgements
The results of this research were generated under the NWO PhDs in the Humanities grant (PGW.18.0.015) ‘Human-animal entanglements on the eve of Columbus’ landfall: a study of indigenous animal husbandry practices in the island of Hispaniola’ and co-funded by the ERC-Synergy project, NEXUS1492 (grant number 319209), under which El Flaco was excavated. We would like to thank all the students and local team members who assisted with the sorting of the faunal assemblage during the field seasons of 2017–2019. We would also like to acknowledge our colleagues at the Faculty of Archaeology, Leiden University who provided comments and feedback on various drafts of this paper, notably Dr. Jason Laffoon and Dr. Andrzej Antczak. We are grateful for the important palaeobotanical and palaeoenvironmental work conducted by Alvaro Castilla-Beltrán and colleagues, Jaime Pagán Jimenéz and colleagues, and Henry Hooghiemstra and colleagues, from which our interpretations of the faunal data greatly benefited. We also would like to acknowledge the time spent reading this manuscript and the helpful comments given by the anonymous reviewers. Special thanks goes to Dr Carlos Suriel and the Museo Nacional de Historia Natural ‘Prof. Eugenio de Jesús Marcano’ for facilitating access to the faunal collection and providing logistical support, and to the Museo del Hombre Dominicano and the Ministerio de Cultura, República Dominicana for allowing access to sites and materials.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Gene T. Shev
Gene T. Shev is a PhD researcher at Leiden University. His research interests lie in the study of human-animal interactions at late precolumbian sites throughout the Greater Antilles, employing zooarchaeological approaches and isotope ratio analysis. His NWO-funded PhD research, entitled ‘Human-animal entanglements on the eve of Columbus’ landfall: a study of Indigenous animal husbandry practices in the island of Hispaniola’, has investigated the potential captive management of certain endemic faunal species found prolifically at Indigenous archaeological sites in the Greater Antilles. The information generated from this research allows us to reconceptualise our current perceptions of the relationships Indigenous peoples had with animals, and to provide empirical evidence of complex environmental management practices.
Zara Ali
Zara Ali has worked as a research assistant at both the Archaeology Centre, University of the West Indies, St. Augustine, where her research focus was on zooarchaeology, and at the Faculty of Archaeology, Leiden University, Leiden, where she studied Caribbean palaeoethnobotany. She has participated in field work and finds processing in both Trinidad and Tobago and the Dominican Republic at several precolumbian and historical era sites.
Juan N. Almonte Milan
Juan N. Almonte Milan is a Dominican herpetologist and expert in endemic microfauna from the Caribbean. He is the curator of the vertebrate fossil collection at the Museo de Historia Natural ‘Prof. Eugenio de Jesús Marcano’ in Santo Domingo.
Simone Casale
Simone Casale is a PhD researcher at Leiden University, Faculty of Archaeology. He performs geochemical and petrographic analysis on ceramic materials on different geographical areas and historical periods moving from precolumbian Americas to late Medieval European trades. His PhD research aims to target questions linking ceramic manufacturing practices, social complexity, and multiculturality among ancient Caribbean societies in the context of the CaribTRAILS project at KITLV.
Igor Djakovic
Igor Djakovic is an Australian-born PhD researcher at the Human Origins group of Leiden University, Faculty of Archaeology. He is currently part of the NWO-funded ‘Neandertal Legacy’ VICI project, aimed at identifying cultural interactions between incoming Homo sapiens and indigenous Neanderthal populations in Europe prior to their disappearance around 40 thousand years ago. His research primarily centres on lithic technological analysis and chronological modelling, with a regional focus on France and northern Spain during the Middle to Upper Palaeolithic transition. More broadly, his work aims towards the development of novel methodological tools for lithic analysis – namely through the adoption of three-dimensional and network-based approaches.
Corinne L. Hofman
Corinne L. Hofman is Professor of Caribbean Archaeology at the Faculty of Archaeology, Leiden University, The Netherlands, and Principal Investigator of the CaribTRAILS project at the Royal Netherlands Institute of Southeast Asian and Caribbean Studies (KITLV/KNAW). Hofman has conducted fieldwork throughout the Caribbean. Her research and publications are highly multi-disciplinary and major themes of interest centre around mobility and exchange, colonial encounters, inter-cultural dynamics, settlement archaeology, artefact analyses, and provenance studies. Hofman’s projects are designed to contribute to the historical awareness, valorisation of archaeological heritage, and knowledge exchange in the Caribbean. In 2013 she obtained an ERC-Synergy Grant for the NEXUS1492 project which led to results discussed in this article.
References
- Alfonso, Y. U., P. Charruau, L. R. Schettino, and S. M. Riveaux. 2013. “Diet and Sexual Dimorphism in the Curly Tail Lizard Leiocephalus macropus (Sauria: Tropiduridae) at Yacabo Abajo, Guantanamo Province, Cuba.” Caribbean Journal of Science 47 (2–3): 339–343.
- Arce-Peña, N. P., V. Arroyo-Rodríguez, M. San-José, D. Jiménez-González, I. Franch-Pardo, E. Andresen, and L. D. Ávila-Cabadilla. 2019. “Landscape Predictors of Rodent Dynamics in Fragmented Rainforests.” Biodiversity and Conservation 28 (3): 655–669.
- BirdLife International. 2021. "Species Factsheet: Xenoligea Montana" (On-Line). BirdLife International. Accessed 14 April 2021. http://www.birdlife.org.
- Bochaton, C., B. Ephrem, B. Bérard, D. Cochard, M. Gala, K. K. Richter, A. Le Lay, S. Renou, and A. Lenoble. 2021. “The Pre-Columbian Site of Roseau (Guadeloupe, F. W. I.): Intra-Site Chronological Variability of the Subsistence Strategies in a Late Ceramic Archeological Vertebrate Assemblage.” Archaeological and Anthropological Sciences 13 (1): 16.
- Boivin, N. L., M. A. Zeder, D. Q. Fuller, A. Crowther, G. Larson, J. M. Erlandson, T. Denham, and M. D. Petraglia. 2016. “Ecological Consequences of Human Niche Construction: Examining Long-Term Anthropogenic Shaping of Global Species Distributions.” Proceedings of the National Academy of Sciences 113 (23): 6388–6396.
- Carder, N., and J. G. Crock. 2012. “A Pre-Columbian Fisheries Baseline from the Caribbean.” Journal of Archaeological Science 39 (10): 3115–3124.
- Carder, N., E. J. Reitz, and J. G. Crock. 2007. “Fish Communities and Populations During the Post-Saladoid Period (AD 600/800–1500), Anguilla, Lesser Antilles.” Journal of Archaeological Science 34 (4): 588–599.
- Carey, W. M. 1975. “The Rock Iguana, Cyclura pinguis, on Anegada, British Virgin Islands, with Notes on Cyclura Ricordi and Cyclura Cornuta on Hispaniola.” Florida State Museum Biological Sciences 19 (4): 189–234.
- Carlson, L. A., and D. W. Steadman. 2009. “Examining Temporal Differences in Faunal Exploitation at Two Ceramic Age Sites in Puerto Rico.” The Journal of Island and Coastal Archaeology 4 (2): 207–222.
- Carmona, E. C., A. V. Ramírez, and A. Cano-Ortiz. 2010. “Contribution to the Biogeography of the Hispaniola (Dominican Republic, Haiti).” Acta Botanica Gallica 157 (4): 581–598.
- Castilla-Beltrán, A., H. Hooghiemstra, M. L. P. Hoogland, T. H. Donders, J. R. Pagán-Jiménez, C. N. H. McMichael, S. M. F. Rolefes, et al. 2020. “Ecological Responses to Land Use Change in the Face of European Colonization of Haytí Island.” Quaternary Science Reviews 241: 106407.
- Castilla-Beltrán, A., H. Hooghiemstra, M. L. P. Hoogland, J. Pagán-Jiménez, B. van Geel, M. H. Field, M. Prins, et al. 2018. “Columbus’ Footprint in Hispaniola: A Paleoenvironmental Record of Indigenous and Colonial Impacts on the Landscape of the Central Cibao Valley, Northern Dominican Republic.” Anthropocene 22: 66–80.
- Cervigón, F., R. Cipriani, L. W. Fischer, L. Garibaldi, M. Hendrickx, A. J. Lemus, R. Márquez, J. M. Poutiers, G. Robaina, and B. Rodriquez. 1992. Guia de campo de las espécies comerciales marinas y de aguas salobres de la costa septentrional de Sur América. Roma: FAO.
- Colten, R. H., and B. Worthington. 2014. “Faunal Remains from the Archaic and Archaic Ceramic Site of Vega del Palmar, Cuba.” Journal of Caribbean Archaeology 14: 23-49.
- Cooke, S. B., and B. E. Crowley. 2018. “Deciphering the Isotopic Niches of Now-Extinct Hispaniolan Rodents.” Journal of Vertebrate Paleontology 38 (5): e1510414.
- Crosby, A. W. 2003. The Columbian Exchange: Biological and Cultural Consequences of 1492. 30th anniversary ed. Westport, CN: Praeger.
- Davis, M. 2005. “Species Profile: Queen conch, Strombus gigas.” Southern Regional Aquaculture Centre 7203: 1–12.
- de Oviedo y Valdez, G. F. 1851. Historia general y natural de las Indias, islas y tierra-firme del mar océano. Primera parte., Real Academia de la Historia, Madrid. Accessed 7 April 2021. http://www.cervantesvirtual.com/obra-visor/historia-general-y-natural-de-las-indias-islas-y-tierrafirme-del-mar-oceano-primera-parte–0/html/014747fa-82b2-11df-acc7-002185ce6064_4.htm.
- Domínguez-Rodrigo, M. 2012. “Critical Review of the MNI (Minimum Number of Individuals) as a Zooarchaeological Unit of Quantification.” Archaeological and Anthropological Sciences 4 (1): 47–59.
- DuChemin, G. R. 2013. “Animal Use and Community in Pre-Columbian Puerto Rico: Zooarchaeology of the Río Portugués.” PhD diss., University of Florida, Gainesville.
- Dunning, Jr., J. B. 2007. CRC Handbook of Avian Body Masses. Boca Raton, FL: CRC Press.
- Ellingham, S. T. D., T. J. U. Thompson, M. Islam, and G. Taylor. 2015. “Estimating Temperature Exposure of Burnt Bone – a Methodological Review.” Science and Justice 55 (3): 181–188.
- Falci, C. G., D. Ngan-Tillard, C. L. Hofman, and A. Van Gijn. 2020. “The Biographies of Bodily Ornaments from Indigenous Settlements of the Dominican Republic (AD 800–1600).” Latin American Antiquity 31 (1): 180–201.
- Fernandes, D. M., K. A. Sirak, H. Ringbauer, J. Sedig, N. Rohland, O. Cheronet, M. Mah, et al. 2020. “A Genetic History of the Pre-Contact Caribbean.” bioRxiv, 2020.06.01.126730.
- Field, D. J., C. Lynner, C. Brown, and S. A. F. Darroch. 2013. “Skeletal Correlates for Body Mass Estimation in Modern and Fossil Flying Birds.” PLoS ONE 8: 11. Accessed 25 February 2021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3843728/.
- Flemming, C., and R. D. E. MacPhee. 1999. “Redetermination of Holotype of Isolobodon portoricensis (Rodentia, Capromyidae) : With Notes on Recent Mammalian Extinctions in Puerto Rico. American Museum novitates ; no. 3278.” Accessed 19 February 2021. http://digitallibrary.amnh.org/handle/2246/3012.
- Ford, B. 2013. Coastal Archaeology, The Oxford Handbook of Maritime Archaeology. Accessed 4 August 2021. https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780199336005.001.0001/oxfordhb-9780199336005-e-33.
- Garner, B. 2002. “On the Possible Prehistoric Domestication of the Caribbean hutia, Isolobodon portoricensis.” Journal of Undergraduate Research of the University of Florida 6 (9): 8.
- Geraldes, F. X. 2003. “Coral Reefs of the Dominican Republic.” In Latin American Coral Reefs, edited by Jorge Cortés, 77–110. Amsterdam: Elsevier.
- Giovas, C. M. 2013. “Foraging Variability in the Prehistoric Caribbean: Multiple Foraging Optima, Resource Use, and Anthropogenic Impacts on Carriacou, Grenada.” PhD diss., University of Washington, Seattle.
- Giovas, C. M. 2016. “Though She Be But Little: Resource Resilience, Amerindian Foraging, and Long-Term Adaptive Strategies in the Grenadines, West Indies.” The Journal of Island and Coastal Archaeology 11 (2): 238–263.
- Grouard, S. 2001. “Faunal Remains Associated with Late Saladoid and PostSaladoid Occupations at Anse a la Gourde, Guadeloupe, West Indies: Preliminary Results.” Archaeofauna 10: 71–98.
- Grouard, S. 2002. “Subsistance et mode de vie … ” Prehistoires Méditerranéennes 10–11: 26.
- Grouard, S. 2010. “Caribbean Archaeozoology.” In Current Advances in Latin-American Archaeozoology., edited by G. Mengoni Goñalons, J. Arroyo-Cabrales, O. J. Polaco, and F. J. Aguilar, 133–151. México: Instituto Nacional de Antropología e Historia. Consejo Nacional para la Ciencia y la Tecnología, International Council for Archaeozoology y Universidad de Buenos Aires.
- Grouard, S., S. Perdikaris, and K. Debue. 2013. “Dog Burials Associated with Human Burials in the West Indies During the Early Pre-Columbian Ceramic Age (500 BC–600 AD).” Anthropozoologica 48 (2): 447–465.
- Guerrero, J. G., and M. Veloz Maggiolo. 1988. Los inicios de la colonización en América: (la arqueología como historia). Universidad Central del Este, San Pedro de Macorís.
- Gutiérrez Zugasti, F. I. 2011. “Early Holocene Land Snail Exploitation in Northern Spain: the Case of La Fragua Cave.” Environmental Archaeology : The Journal of Human Palaeoecology 16 (1): 36–48.
- Hansford, J., J. M. Nuñez-Miño, R. P. Young, S. Brace, J. L. Brocca, and S. T. Turvey. 2012. “Taxonomy-testing and the “Goldilocks Hypothesis”: Morphometric Analysis of Species Diversity in Living and Extinct Hispaniolan hutias.” Systematics and Biodiversity 10 (4): 491–507.
- Hartnoll, Richard G, Mark SP Baine, Yolima Grandas, Jennifer James, and H. Atkin. 2006. “Population Biology of the Black Land Crab, Gecarcinus Ruricola, in the San Andres Archipelago, Western Caribbean.” Journal of Crustacean Biology 26 (3): 316–325.
- Hedges, S. B. 2007. “The Amphibians and Reptiles of Hispaniola and Their Conservation.” In Hispaniola: A Photographic Journeuy Through Island Biodiversity, edited by Eladio Fernández, 18–25. Cambridge, MA: Beknap Press.
- Hermanson, John W, and C. A. Woods. 2012. “Anatomical Specializations of Capromyid Rodents: Functional and Phylogenetic Considerations.” In Terrestrial Mammals of the West Indies: Contributions, edited by R. Borotto-Páez, Charles A. Woods, and Florence E. Sergile, 179–194. Gainesville: The Florida Museum of Natural History and Wacahoota Press.
- Herrera, A., L. Betancourt, M. Silva, P. Lamelas, and A. Melo. 2011. “Coastal Fisheries of the Dominican Republic.” In Coastal Fisheries of Latin America and the Caribbean. FAO Fisheries and Aquaculture Technical Paper, edited by S. Salas, R. Chuenpagdee, A. Charles, and J. C. Seijo, 175–217. Rome: FAO.
- Herrera Malatesta, E. 2018. “Una Isla, Dos Mundos: Estudio Arqueológico Sobre el Paisaje Indigena de Hayti y su Transformación al Paisaje Colonial de La Española (1200–1550).” PhD diss., Leiden University, Leiden.
- Herrera Malatesta, E., and C. L. Hofman. 2019. “Indigenous Landscape Transformation on Northern Haytí: An Archaeological and Environmental Database of the Montecristi Coast.” Journal of Open Archaeology Data 7: 1–5.
- Hofman, C. L., and M. L. P. Hoogland. 2015. “Investigaciones arqueológicas en los sitios El Flaco (Loma de Guayacanes) y La Luperona (UNIJICA).” Boletín del Museo del Hombre Dominicano 46 (42): 61–74.
- Hofman, C. L., J. U. Hung, E. H. Malatesta, J. S. Jean, T. Sonnemann, and M. Hoogland. 2018. “Indigenous Caribbean Perspectives: Archaeologies and Legacies of the First Colonised Region in the New World.” Antiquity 92 (361): 200–216.
- Hofman, C. L., and J. Ulloa Hung. 2019. “NEXUS 1492: Encuentros del Nuevo Mundo con un mundo en globalización.” Ciencia y sociedad 44 (4): 95–115.
- Hofman, C. L., R. Valcárcel Rojas, and J. Ulloa Hung. 2020. “Colonization, Transformations, and Indigenous Cultural Persistence in the Caribbean.” In The Global Spanish Empire: Five Hundred Years of Place Making and Pluralism, edited by C. D. Beaule and G. Douglas, 55–82. Tucson: The University of Arizona Press.
- Hooghiemstra, H., T. Olijhoek, M. Hoogland, M. Prins, B. van Geel, T. Donders, W. Gosling, and C. Hofman. 2018. “Columbus’ Environmental Impact in the New World : Land use Change in the Yaque River Valley, Dominican Republic.” The Holocene 28 (11): 1818–1835.
- Hopkins, S. S. B. 2008. “Reassessing the Mass of Exceptionally Large Rodents Using Toothrow Length and Area as Proxies for Body Mass.” Journal of Mammalogy 89 (1): 232–243.
- Hoy, D. R., and J. S. Fisher. 1974. “Primary Production and the Measurement of Agricultural Potential in the Caribbean.” Revista Geográfica 80: 71–87.
- Incháustegui, S. J., A. Schwartz, and R. Henderson. 1985. “Hispaniolan Giant Diploglossus (Sauria: Anguidae): Description of a New Species and Notes on the Ecology of D. warreni.” Amphibia-Reptilia 6 (2): 195–201.
- Jackson, J. T. 2010. Demography and Population Structure of a Rio Grande Endemic Emydid, The Big Bend Slider (Unpublished thesis). San Marcos: Texas State University.
- Kalsbeek, N., and J. Richter. 2006. “Preservation of Burned Bones: An Investigation of the Effects of Temperature and pH on Hardness.” Studies in Conservation 51 (2): 123–138.
- Keegan, W. F., L. A. Carlson, K. M. Delancy, and D. Hayes. 2019. “Child Labor in Saladoid St. Thomas, U.S.V.I. (300–500 CE).” Journal of Anthropological Archaeology 53: 222–228.
- Keegan, W. F., and C. L. Hofman. 2017. The Caribbean before Columbus, The Caribbean before Columbus, Oxford University Press. Accessed 17 March 2021. https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780190605247.001.0001/acprof-9780190605247.
- Kier, G., H. Kreft, T. M. Lee, W. Jetz, P. L. Ibisch, C. Nowicki, J. Mutke, and W. Barthlott. 2009. “A Global Assessment of Endemism and Species Richness Across Island and Mainland Regions.” Proceedings of the National Academy of Sciences 106 (23): 9322–9327.
- Kimura, B. K., M. J. LeFebvre, S. D. de France, H. I. Knodel, M. S. Turner, N. S. Fitzsimmons, S. M. Fitzpatrick, and C. J. Mulligan. 2016. “Origin of Pre-Columbian Guinea Pigs from Caribbean Archeological Sites Revealed Through Genetic Analysis.” Journal of Archaeological Science: Reports 5: 442–452.
- Lane, C. S., S. P. Horn, C. I. Mora, and K. H. Orvis. 2009. “Late-Holocene Paleoenvironmental Change at Mid-Elevation on the Caribbean Slope of the Cordillera Central, Dominican Republic: A Multi-Site, Multi-Proxy analysis.” Quaternary Science Reviews 28 (23): 2239–2260.
- Las Casas, B de. 1875. Historia de las Indias, Tomo I. Madrid: Imprenta de Miguel Ginesta.
- Leach, F. 1997. A Guide to the Identification of Fish Remains from New Zealand Archaeological Sites. New Zealand Journal of Archaeology Special Publication, New Zealand Journal of Archaeology, Wellington. Accessed 26 March 2021. https://www.academia.edu/1764885/Leach_B_F_1997_A_guide_to_the_identification_of_fish_remains_from_New_Zealand_archaeological_sites_New_Zealand_Journal_of_Archaeology_Special_Publication_129_pp.
- LeFebvre, M. J. 2007. “Zooarchaeological Analysis of Prehistoric Vertebrate Exploitation at the Grand Bay Site, Carriacou, West Indies.” Coral Reefs 26 (4): 931–944.
- LeFebvre, M. J. 2015. “Animals, Food, and Social Life Among the Pre-Columbian Taíno of En Bas Saline, Hispaniola.” PhD thesis, University of Florida.
- LeFebvre, M. J., and S. D. deFrance. 2014. “Guinea Pigs in the Pre-Columbian West Indies.” The Journal of Island and Coastal Archaeology 9 (1): 16–44.
- LeFebvre, M., and S. deFrance. 2018. “Animal Management and Domestication in the Realm of Ceramic Age Farming, edited by The Archaeology of Caribbean and Circum-Caribbean Farmers 6000 BC - AD 1500, edited by Basil A. Reid, 149–170.
- LeFebvre, M. J., S. D. de France, G. D. Kamenov, W. F. Keegan, and J. Krigbaum. 2019. “The Zooarchaeology and Isotopic Ecology of the Bahamian hutia (Geocapromys ingrahami): Evidence for pre-Columbian Anthropogenic Management.” PLOS ONE 14 (9): e0220284.
- LeFebvre, M. J., and C. M. Giovas. 2009. “The Zooarchaeology of Islands: Towards Synergy and Synthesis.” The Journal of Island and Coastal Archaeology 4 (2): 141–150.
- Lentini, A. 2002. Husbandry Manual: Puerto Rican Crested Toad (Peltophryne lemur). Toronto: Toronto Zoo.
- Linares, O. F. 1976. “‘Garden Hunting’ in the American Tropics.” Human Ecology 4 (4): 331–349.
- Locatelli, E. R. 2013. “Preservation Potential of Gecarcinid Land Crabs (Decapoda, Brachyura, Gecarcinidae) from San Salvador.” PALAIOS 28 (11/12): 867–874.
- Loiselle, B. A., and J. G. Blake. 1992. “Population Variation in a Tropical Bird Community.” BioScience 42 (11): 838–845.
- Macphee, R., C. Flemming, and D. Lunde. 1999. ““Last Occurrence” of the Antillean Insectivoran Nesophontes: New Radiometric Dates and Their Interpretation.” American Museum Novitates 3261: 1–20.
- Maiorana, K. 2006. "Eleutherodactylus coqui" (On-line), Animal Diversity Web. Accessed 20 April 2021. http://animaldiversity.ummz.umich.edu/site/accounts/information/Eleutherodactylus_coqui.html.
- Mcfarlane, D., A. Vale, K. Christenson, J. Lundberg, G. Atilles, and S. Lauritzen. 2000. “New Specimens of Late Quaternary Extinct Mammals from Caves in Sanchez Ramirez Province, Dominican Republic.” Caribbean Journal of Science 36: 163–166.
- McGaw, I. J., T. E. Van Leeuwen, R. H. Trehern, and A. E. Bates. 2019. “Changes in Precipitation may Alter Food Preference in an Ecosystem Engineer, the Black Land Crab, Gecarcinus ruricola.” PeerJ 7. Accessed 15 February 2021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6511230/.
- Meijía, M. 2006. “Flora de la Española: Conocimiento Actual y Estado de Conservación.” In Actas IX Congreso Latinoamericano de Botánica; El 18-25 de Junio, edited by Jardín Botánico Nacional Dr. Rafael Ma, 11–12. Santo Domingo: Moscoso.
- Mollat, H., B. M. Wagner, P. Cepek, and W. Weiss. 2004. Mapa Geológico de la República Dominicana 1:250.000. Hannover: Geologisches Jahrbuch.
- Morgan, G. S., R. D. E. Macphee, R. Woods, and S. T. Turvey. 2019. “Late Quaternary Fossil Mammals from the Cayman Islands, West Indies.” Bulletin of the American Museum of Natural History 2019 (428): 1.
- Newsom, L. A., and E. S. Wing. 2004. On Land and Sea: Native American Uses of Biological Resources in the West Indies. Tuscaloosa: The University of Alabama Press.
- Noviello, N., L. McRae, R. Freeman, and C. Clements. 2020. “Body Mass and Latitude Predict the Presence of Multiple Stressors in Global Vertebrate Populations.” bioRxiv, 2020.12.17.423192.
- Odling-Smee, F. J., K. N. Laland, and M. W. Feldman. 1996. “Niche Construction.” The American Naturalist 147 (4): 641–648.
- Olalekan, E. I. 2015. “Size and Growth of Cardiosoma armatum and Cardiosoma guanhumi as Ecological Parameters for Mangrove Ecosystem.” Journal of Marine Science: Research and Development 05 (02). Accessed 13 April 2021. https://www.omicsonline.org/open-access/size-and-growth-of-cardiosoma-armatum-and-cardiosoma-guanhumi-as-ecological-parameters-for-mangrove-ecosystem-2155-9910-1000164.php?aid=58062.
- Ortega, E. J. 1988. La Isabela y Arqueología en la Ruta de Colón. San Pedro de Macorís: Editorial Universidad Central del Este.
- Oswald, J. A., J. M. Allen, M. J. LeFebvre, B. J. Stucky, R. A. Folk, N. A. Albury, G. S. Morgan, R. P. Guralnick, and D. W. Steadman. 2020. “Ancient DNA and High-Resolution Chronometry Reveal a Long-Term Human Role in the Historical Diversity and Biogeography of the Bahamian hutia.” Scientific Reports 10 (1): 1373.
- Pagán-Jiménez, J. R., Z. Ali, C. G. Santiago-Marrero, and C. L. Hofman. 2020. “Plantscapes of Dwelling: Precolonial Household Mounds, Phytocultural Dynamics and the Ensuing Human Ecosystems at El Flaco and El Carril (cal. AD 990–1450), Northern Dominican Republic.” Review of Palaeobotany and Palynology 274: 104160.
- Pathak, N., J. Robinson, G. Jeyasekaran, R. Shalini, N. Neethiselvan, and P. Padmavathy. 2019. “Pre-processing Yields of Blue Swimming Crabs Collected from Different Crab Collection Points in South East Coast of India.” Journal of Experimental Zoology India 22 (1): 125–130.
- Posada, J. M., A. Stoner, K. Sealey, A. Antczak, D. Schapira, R. Torres, I. Montaño, M. Ray-Culp, and D. Aranda. 2007. “Regional Initiative for the Evaluation of Queen Conch (Strombus gigas) Exploitation Under an Historical Perspective.” Proceedings of the Gulf and Caribbean Fisheries Institute 59: 23–30.
- Powell, R. 2012. “Hispaniola and Navassa.” In Island List of West Indian Amphibians and Reptiles, edited by Robert Powell, and R. W. Henderson, 85–166. Gainesville: Florida Museum of Natural History.
- Powell, R., J. A. Ottenwalder, and S. J. Incháustegui. 1999. “The Hispaniolan Herpetofauna: Diversity, Endemism, and Historical Perspectives, with Comments on Navassa Island.” In Caribbean Amphibeans and Reptiles, edited by Brian I. Crother, 93–168. San Diego: Academic Press.
- Pregill, G. 1984. “An Extinct Species of Leicephalus from Haiti (Sauria, Iguanidae).” Proceedings of the Biological Society of Washington 97: 827–833.
- Quatrehomme, G., M. Bolla, M. Muller, J. P. Rocca, G. Grévin, P. Bailet, and A. Ollier. 1998. “Experimental Single Controlled Study of Burned Bones: Contribution of Scanning Electron Microscopy.” Journal of Forensic Sciences 43 (2): 16160J–161422.
- Ramírez-Barajas, P. J., and S. Calmé. 2015. “Subsistence Hunting and Conservation.” In Biodiversity and Conservation of the Yucatán Peninsula, edited by Gerald Alexander Islebe, Sophie Calmé, Jorge L. León-Cortés, and Birgit Schmook, 333–351. Cham: Springer International Publishing. Accessed 26 January 2021. http://link.springer.com/10.1007/978-3-319-06529-8_13.
- Reimer, P. J., W. E. N. Austin, E. Bard, A. Bayliss, P. G. Blackwell, C. B. Ramsey, M. Butzin, et al. 2020. “The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP).” Radiocarbon 62 (4): 725–757.
- Reitz, E. J., and E. S. Wing. 2008. Zooarchaeology. 2nd ed. Cambridge Manuals in Archaeology. Cambridge: Cambridge University Press.
- Robertson, R. 2003. “The Edible West Indian ‘Whelk’ Cittarium pica (Gastropoda: Trochidae): Natural History with New Observations.” Proceedings of the Academy of Natural Sciences of Philadelphia 153: 27–47.
- Schill, S. R., V. P. McNulty, F. J. Pollock, F. Lüthje, J. Li, D. Knapp, J. Kington, et al.. 2021. “Regional-Scale High-Resolution Benthic Habitat Data from PlanetScope Imagery for Conservation Decision-Making and Marine Planning.” Remote Sensing in prep.
- Scudder, S. J. 1991. “Early Arawak Subsistence Strategies on the South Coast of Jamaica.” Proceedings of the Thirteenth International Congress for Caribbean Archaeology. Report of the Archaeological-Anthropological Institute of the Netherlands Antilles 11: 307–325.
- Shev, G. T., J. E. Laffoon, and C. L. Hofman. 2021. “Human and Hutia (Isolobodon portoricensis) Interactions in Pre-Columbian Hispaniola: The Isotopic and Morphological Evidence.” Journal of Archaeological Science: Reports 37: 102913.
- Shine, R. 1992. “Relative Clutch Mass and Body Shape in Lizards and Snakes: Is Reproductive Investment Constrained or Optimized?” Evolution 46 (3): 828–833.
- Shipman, P., G. Foster, and M. Schoeninger. 1984. “Burnt Bones and Teeth: an Experimental Study of Color, Morphology, Crystal Structure and Shrinkage.” Journal of Archaeological Science 11 (4): 307–325.
- Sibly, R. M., C. C. Witt, N. A. Wright, C. Venditti, W. Jetz, and J. H. Brown. 2012. “Energetics, Lifestyle, and Reproduction in Birds.” Proceedings of the National Academy of Sciences 109 (27): 10937–10941.
- Siders, R. 2014. “Ameiva ameiva.” Animal Diversity Web. Accessed 20 April 20 2021. https://animaldiversity.org/accounts/Ameiva_ameiva/.
- Smith, B. D. 2001. “Low-Level Food Production.” Journal of Archaeological Research 9: 1–43.
- Smith, D. A. 2005. “Garden Game: Shifting Cultivation, Indigenous Hunting and Wildlife Ecology in Western Panama.” Human Ecology 33 (4): 505–537.
- Smith, B. D. 2007. “Niche Construction and the Behavioral Context of Plant and Animal Domestication.” Evolutionary Anthropology: Issues, News, and Reviews 16 (5): 188–199.
- Smith, B. D. 2011. “General Patterns of Niche Construction and the Management of ‘Wild’ Plant and Animal Resources by Small-Scale Pre-Industrial Societies.” Philosophical Transactions of the Royal Society B: Biological Sciences 366 (1566): 836–848.
- Spalding, M. 2010. World Atlas of Mangroves. Abingdon: Routledge.
- Spalding, M., M. Kainuma, and L. Collins. 2010. World Atlas of Mangroves (version 3.1). A Collaborative Project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. London: Earthscan. doi:10.34892/w2ew-m835
- Steadman, D. W., and S. Jones. 2006. “Long-Term Trends in Prehistoric Fishing and Hunting on Tobago, West Indies.” Latin American Antiquity 17 (3): 316–334.
- Steadman, D. W., and O. M. Takano. 2013. “A Late-Holocene Bird Community from Hispaniola: Refining the Chronology of Vertebrate Extinction in the West Indies.” The Holocene 23 (7): 936–944.
- Sugiyama, N., M. F. Martínez-Polanco, C. A. M. France, and R. G. Cooke. 2020. “Domesticated Landscapes of the Neotropics: Isotope Signatures of Human-Animal Relationships in Pre-Columbian Panama.” Journal of Anthropological Archaeology 59: 101195.
- Takano, O., and D. Steadman. 2015. “A New Species of Woodcock (Aves: Scolopacidae: Scolopax) from Hispaniola, West Indies.” Zootaxa 4032: 117.
- Tavares, V., and C. Mancina. 2008. “Phyllops falcatus (Chiroptera: Phyllostomidae).” Mammalian Species 811: 1–7.
- The Cornell Lab of Ornithology. 2019. "Common Gallinule identification" (On-line), All About Birds. Accessed 20 April 2021. https://www.allaboutbirds.org/guide/Common_Gallinule/id.
- Turvey, Samuel T., Rosalind J. Kennerley, Jose M. Nuñez-Miño, and Richard P. Young. 2017. “The Last Survivors: Current Status and Conservation of the Non-Volant Land Mammals of the Insular Caribbean.” Journal of Mammalogy 98 (4): 918–936.
- Turvey, S. T., J. R. Oliver, N. Storde, and P. Rye. 2007. “Late Holocene Extinction of Puerto Rican Native Land Mammals.” Biology Letters 3 (2): 193–196.
- Ulloa Hung, J. 2014. Arqueología en la Línea noroeste de La Española. Paisajes, Cerámicas e Interacciones, Santo Domingo: Instituto Tecnológico de Santo Domingo.
- Wahl, J. 1981. “Beobachtungen zur Verbrennung menschlicher Leichname. Über die Vergleichbarkeit moderner Krematorien mit prähistorischen Leichenbränden.” Archäologisches Korrespondenzblatt 11: 271–279. Abb.
- Whidden, H. P., and R. J. Asher. 2001. “The Origin of the Greater Antillean Insectivorans.” In Biogeography of the West Indies: Patterns and Perspectives, edited by Charles A. Woods and Florence E. Sergile, 237–252. Boca Raton, FL: CRC Press.
- Wiley, J. W. 2010. “Food Habits of the Endemic Ashy-Faced Owl (Tyto glaucops) and Recently Arrived Barn Owl (T. alba) in Hispaniola.” Journal of Raptor Research 44 (2): 87–100.
- Wilkins, L. 2001. “Impact of Hunting on Jamaican Hutia (Geocapromys brownii) Populations: Evidence from Zooarchaeology and Hunter Surveys.” In Biogeography of the West Indies: Patterns and Perspectives, edited by Charles A. Woods, and Florence E. Sergile, 529–546. Boca Raton, FL: CRC Press.
- Wing, E. S. 1972. “Identification and Interpretation of Faunal Remains.” In The White Marl Site in Jamaica: Report of the 1964 Robert R. Howard Excavation, edited by James Silverberg, 18–35. Milwaukee: Department of Anthropology, The University of Wisconsin-Milwaukee.
- Wing, E. S. 1995. “Land Crab Remains in Caribbean Sites.” In Proceedings of XVI International Congress for Caribbean Archaeology, Cultural Resource Solutions, pp. 105–113. St. Petersburg, FL. Accessed 17 March 2021. https://ufdc.ufl.edu/AA00061961/00590.
- Wing, E. S. 2001. “The Sustainability of Resources Used by Native Americans on Four Caribbean Islands.” International Journal of Osteoarchaeology 11 (1-2): 112–126.
- Wing, E. S. 2008a. “Pets and Camp Followers in the West Indies.” In Case Studies in Environmental Archaeology, Interdisciplinary Contributions to Archaeology, edited by Elizabeth J. Reitz, Sylvia J. Scudder, C. Margaret Scarry, Michael Jochim, and Roy S. Dickens, 405–425. New York: Springer New York. Accessed 26 January 2021, http://link.springer.com/10.1007/978-0-387-71303-8_21.
- Wing, E. S. 2008b. “Pets and Camp Followers in the West Indies.” In Case Studies in Environmental Archaeology. Interdisciplinary Contributions to Archaeology, edited by Elizabeth J. Reitz, Sylvia J. Scudder, and C. Margaret Scarry, 405–425. New York: Springer. Accessed 1 February 2021. https://doi.org/10.1007/978-0-387-71303-8_21.
- Wing, E. S. 2012. “Zooarchaeology of West Indian Land Mammals.” In Terrestrial Mammals of the West Indies: Contributions, edited by R. Borotto-Páez, Charles A. Woods, and Florence E. Sergile, 341–356. Gainesville: Florida Museum of Natural History and Wacahoota Press.
- Wing, E. S., C. E. Ray, and L. Kozuch. 2002. “Faunal Remains from the Tutu Archaeological Village Site, St. Thomas.” In The Tutu Archaeological Village Site: A Multidisciplinary Case Study in Human Adaptation, edited by E. Righter, 141–165. London: Routledge.
- Wing, E. S., and S. R. Wing. 2001. “Prehistoric Fisheries in the Caribbean.” Coral Reefs 20 (1): 1–8.
- Wiscovitch-Russo, R., J. Rivera-Perez, Y. M. Narganes-Storde, E. García-Roldán, L. Bunkley-Williams, R. Cano, and G. A. Toranzos. 2020. “Pre-Columbian Zoonotic Enteric Parasites: An Insight Into Puerto Rican Indigenous Culture Diets and Life Styles.” PLOS ONE 15 (1): e0227810.
- Woods, R., S. T. Turvey, S. Brace, C. V. McCabe, L. Dalén, E. J. Rayfield, M. J. F. Brown, and I. Barnes. 2020. “Rapid Size Change Associated with Intra-Island Evolutionary Radiation in Extinct Caribbean ‘Island-Shrews’.” BMC Evolutionary Biology 20 (1): 106.
- Zeder, M. A. 2015. “Core Questions in Domestication Research.” Proceedings of the National Academy of Sciences 112 (11): 3191–3198.
- Zootierliste. 2021. "Chilabothrus Striatus" (On-line), Zootierlisted. Accessed 18 April 2021. https://www.zootierliste.de/en/?klasse=3&ordnung=305&familie=30506&art=50906021.