ABSTRACT
Stable isotope studies have revolutionised our understanding of food webs, subsistence, mobility and their change through time. In the Andes, dietary stable isotopic studies have often focused on timing the adoption of maize as a staple food and identifying camelid pastoralism in selected valleys of the Pacific coast. Few studies have focused on highland societies and understanding pastoralist communities in particular, but the underlying assumption has been that camelid herders had essentially narrow and specialised diets. To evaluate this assertion, we analyzed dietary carbon and nitrogen stable isotopes of 27 human and 23 faunal specimens from nine archaeological sites located in the Central Altiplano of Bolivia supported by 27 radiocarbon dates. Our results suggest important diversity characterised both human and animal isotopic ecology between 1300 BCE and 1200 CE. The collected data also demonstrates that maize was not regularly consumed, suggesting interregional food exchange between this region of the Altiplano and its neighboring lowland inter-Andean valleys likely postdated 1100 CE. Consistent with reliance on cultivated crops such as chenopods and tubers and wild fauna including rodents, birds and fish, early camelid herders in the Andean Central Altiplano relied on a generalised form of pastoralism.
Introduction
Stable isotope analysis has revolutionised our understanding of subsistence transformation through time by facilitating qualitative and quantitative reconstructions of the diets and environments of ancient peoples (Lee-Thorp Citation2008; Schoeninger and Moore Citation1992; Ventresca Miller and Makarewicz Citation2017). Because the biochemical composition of vertebrate skeletal tissues is directly related to their diet and local ecology, stable isotope analysis can substantially increase our understanding of food webs, localised environmental change, socio-economic behaviour and mobility through space and over time (Janzen, Balasse, and Ambrose Citation2020; Otaola, Ugan, and Gil Citation2018; Pilaar Birch Citation2013; Zavodny et al. Citation2014). In the Andes, a number of archaeological dietary stable isotopic studies have focused on timing the agricultural reliance on maize (Finucane, Agurto, and Isbell Citation2006; Gil et al. Citation2014; Hastorf Citation1990; Hastorf and Johannessen Citation1993; Killian Galván, Samec, and Panarello Citation2016; Somerville et al. Citation2015; Tung and Knudson Citation2018), characterising the mobility strategies between the coast and interior valleys (Knudson, Aufderheide, and Buikstra Citation2007; Santana-Sagredo et al. Citation2019; Santana-Sagredo et al. Citation2021; Torres-Rouff, Pestle, and Gallardo Citation2012), and identifying camelid pastoralism in selected valleys of the Pacific coast (Dufour et al. Citation2014; Gayo et al. Citation2020; Szpak et al. Citation2016; Szpak and Valenzuela Citation2020). As these and other studies have allowed a greater appreciation of human subsistence diversity in relation to environmental and socioeconomic variability, various questions that could be addressed with these methods emerged (Dufour et al. Citation2020; Samec et al. Citation2020; Szpak et al. Citation2020; Takigami et al. Citation2020). For instance, how was animal husbandry as initially practiced by highland communities complemented by other subsistence activities, and how did it change over time?
Here we use stable isotope analysis to assess the nature of economic resource utilisation, subsistence and mobility by early pastoralist communities in the Bolivian Central Altiplano, a large high-elevation grassland, initially occupied by mobile foragers specialised in hunting wild camelids and eventually replaced by domestic camelid herders (Capriles Citation2014; Capriles Citation2017). The transition between foraging and herding occurred approximately 3500 years ago when an archaeological cultural complex known as Wankarani emerged and dispersed across the Central Altiplano (Bermann and Estévez Castillo Citation1995; Capriles Citation2011; Fox Citation2010; McAndrews Citation2005). Although increasing archaeological research focused on Wankarani sites has allowed a better understanding of its economic and settlement patterns, how this early pastoralist society led to successful adaptations to the harsh conditions of the Andean highlands, including its degree of reliance on wild and cultivated resources, remains uncertain.
Andean Pastoralism and Interregional Complementarity
Pastoralism is an economic subsistence system that is fundamentally, but not exclusively, based on the management, production and consumption of herding animals (Capriles and Tripcevich Citation2016; Chang and Koster Citation1986). The incorporation of domesticated animals marks a distinctive transition in human–animal relations and pastoralist societies vary substantially in terms of animals involved, degree of mobility and sociopolitical organisation (Marshall and Hildebrand Citation2002). Most pastoralist societies explicitly choose to differentiate themselves from farming and foraging communities because their primary subsistence activities are focused on feeding and safeguarding their herds in relation to environmental variability (Khazanov Citation1984). Nevertheless, the actual reliance, consumption and dependence of pastoralists on their domesticated herds varies cross-culturally (Cribb Citation1991). On one hand, specialised pastoralist societies focus on producing primary or secondary products and services such as meat, dairy, fat, wool, transportation and dung used for fuel and fertiliser, and therefore, often engage in symbiotic relationships with agricultural communities for exchanging complementary subsistence staple foods and products. On the other hand, generalist pastoralists often supplement animal husbandry with various other subsistence activities including crop cultivation, opportunistic hunting and gathering and even trade. Although most pastoralist societies fit somewhere in between these two extremes, the specialisation gradient serves as a helpful analytical framework for evaluating the degree of socioeconomic dependence that specific societies developed in relation to their animal herds as well as their eventual transformations over time (Marshall Citation1990).
In the Andes, ethnohistoric documentation suggests a form of specialised pastoralism developed as part of vertical complementarity, a socio-ecological system through which certain polities accessed resources from different ecological zones by means of population outposts. For instance, the Lupaqa settled in the Northern Altiplano around Lake Titicaca, defined themselves as pastoralists, and managed some of the largest herds in the Andes as well as differentiated themselves from the Uru fishermen of the lake, who were marginalised precisely because of their reliance on fish, waterfowl and other wild foods (Murra Citation1968). The Lupaqa also had permanent colonies in the inter-Andean valleys to the east and west of the lake to guarantee reliable access to essential goods not grown in the highlands, such as maize, chili peppers and coca (Murra Citation2002). Similarly, various other highland groups specialised in camelid pastoralism accessed non-highland goods through direct exchange facilitated by trains of llama caravans (Browman Citation1990). These societies were largely transformed after the Spanish conquest, but highly specialised Andean pastoralist communities persisted over time (Browman Citation1987). For instance, the herders of Paratia, a community situated west of Lake Titicaca and above the cultivation zone, had to either exchange or sell their camelid-derived products to supplement their subsistence (Flores-Ochoa Citation1979). Nevertheless, it remains largely unknown how these socio-ecological systems developed and transformed over time in the deeper archaeological past.
Located in the Bolivian Andes, the Central Altiplano extends between 14° and 22° south latitude, 66° and 71° west longitude and 3600 and 4200 m above sea level and is demarcated by a series of mountain ranges, highland plains and wetlands that drain in the Desaguadero River and Lake Poopó (Bermann and Estévez Castillo Citation1995; Capriles, Calla Maldonado, and Albarracin-Jordan Citation2011; Fox Citation2007). The climate of the Central Altiplano is semi-arid and marked by a strong contrast between the dry (May–October) and wet (November–April) seasons, which varied considerably in the course of the Holocene (Rigsby et al. Citation2005). Currently, the local vegetation is dominated by tussock grasslands and scrubby shrublands in addition to aquatic plants in marshes and bodies of water (Cuenca Sempertegui et al. Citation2005).
Archaeological research suggests that during the Archaic Period (8000–1500 BCE) under prevailing arid conditions, the highlands were occupied by dispersed bands of hunter-gatherers (Capriles et al. Citation2018; Osorio et al. Citation2017). The Wankarani archaeological cultural complex emerged in the Central Altiplano during the subsequent Early Formative Period (1500–500 BCE) featuring the establishment of mounded settlements containing overlapping residential areas with abundant domestic refuse including fragmented ceramics, bone and stone tools (Bermann and Estévez Castillo Citation1995; Ponce Sanginés Citation1970). The Wankarani are often cited as an example of a specialised herding society but evidence for limited engagement with chenopod and tuber dryland cultivation has been reported (Langlie et al. Citation2011; McAndrews Citation2005). During the Late Formative Period (500 BCE–500 CE), the dispersed settlements of the Wankarani persisted and might have contributed to the pastoralist foundation of the Tiwanaku state, which expanded into the Central Altiplano between 500 and 1100 CE (Beaule Citation2002; Bermann and Estévez Castillo Citation1993; Browman Citation1997; Janusek Citation2008). Following the disintegration of the Tiwanaku state, during the Late Intermediate Period or LIP (1100–1450 CE), the Central Altiplano was occupied by decentralised lineage-based pastoralist communities coalesced into larger segmentary polities, which were subsequently incorporated by the Inca through both conquest and diplomatic alliances (Catacora et al. Citation2002; Cruz et al. Citation2017; Michel López Citation2008).
Ethnohistoric research suggests that during the XVI century Spanish conquest, the Central Altiplano was populated by large Aymara speaking pastoralist polities subjugated by the Inca such as the Carangas, Soras and Quillacas (Gyarmati and Condarco Castellón Citation2014; Medinacelli Citation2010; Pärssinen Citation2005). Facilitated by llama caravans, these specialised herding communities exchanged staple dietary highland goods such as dry meat and salt for lowland foods such as maize and chili peppers, with farming populations settled in lowland valleys towards the east and west of the Altiplano (Lima Tórrez Citation2014; Núñez and Dillehay Citation1995; Saintenoy, González Estefane, and Uribe Rodríguez Citation2019). Although ethnohistoric accounts mention the establishment of highland colonies in these valleys (Durston and Hidalgo Citation1997), it remains uncertain when these patterns of social and resource complementarity emerged.
Stable Isotope Analyses and Subsistence Reconstruction
To improve our current understanding of the development of early camelid pastoralist communities in the Andean highlands we used dietary stable isotope analyses. Variation in stable carbon isotope ratios (δ13C) can be used to distinguish between the different photosynthetic pathways of plants. Most terrestrial plants follow the Calvin–Benson, or C3, pathway and have δ13C values of around −23‰. Plants that use the Hatch–Slack photosynthetic pathway, also called C4 plants, have less negative δ13C values than C3 plants, averaging around −13‰, and plants following the Crassulacean Acid Metabolism (CAM) photosynthetic pathway have intermediate δ13C values, between −27 and −13‰ (Farquhar, Ehleringer, and Hubick Citation1989; Kohn Citation2010; O’Leary Citation1988). The Andean landscape has plants from all three of these categories; whereas C3 plants predominate in the highland Andes, C4 plants are present with increasing frequency as altitude decreases, and stable isotope values can indicate which resources were used by these pastoralist communities over time (Berryman Citation2010; Thornton et al. Citation2011; Yacobaccio, Morales, and Samec Citation2009). By studying carbon isotope values from camelids and other fauna we can characterise their diets, and in turn the carbon isotope values of humans relate to the plants people consumed as well as the diets of the animals they consumed.
Archaeobotanical data from the Altiplano focuses on the plant species recovered from human settlements and may not represent the diversity of taxa consumed by pastoral peoples if they had highly specialised/narrow diets, frequently moved across different ecosystems and/or utilised more wild species than agriculturally focused communities. For example, remains from chenopods and tubers (C3 Andean highland domesticates) dominate archaeobotanical assemblages from the Formative Periods within the Lake Titicaca Basin (Bruno Citation2014), with maize (an introduced C4 plant) appearing in notable quantities only beginning in the Late Formative and particularly increasing during the Tiwanaku period (Logan, Hastorf, and Pearsall Citation2012). Within the Andean context, maize has been given exceptional attention due to its sociopolitical uses in multiple, large-scale polities (Wari, Tiwanaku, Chimu, Inka). Importantly, maize cannot be grown in most areas of the Altiplano environment, but it was actively exchanged into the Tiwanaku center during its height (Hastorf et al. Citation2006). The presence of enriched carbon isotope values for camelids and pastoral peoples from the Tiwanaku period could indicate the incorporation of maize into their diets but may also point to the use of other wild C4 or CAM plant species. However, the presence of C4 or CAM signatures in human samples from the Formative Periods may indicate the importance of wild plant species in pastoral diets.
To contextualise our faunal and human isotopic data, we rely on baseline data collected from other Bolivian highland studies. For instance, a small number of modern plant samples from the Lake Poopó region (elevation 3686 m) provides some guidance for expected carbon values for plants grown in the Central Altiplano. Three Chenopodium quinoa samples have a mean δ13C of −25.6‰ and a standard deviation (SD) of 1.1‰, that clearly separate from four Zea mays samples (mean δ13C = −12.3‰, SD = 0.5‰), along the expected C3/C4 isotopic groupings (Miller et al. Citation2021). Similarly, a set of modern and a few archaeological plant samples from the southern Lake Titicaca basin (Chiripa, Bolivia; elevation 3850 m) suggests highland crops follow the expected photosynthetic pathway structure of modern C3 plants and have low carbon values, such as tubers (Solanum tuberosum and Oxalis tuberosa; mean δ13C = −26.7‰), chenopods (Chenopodium spp.; mean δ13C = −23.9‰) and beans (Fabaceae; mean δ13C = −26.9‰) (Miller et al. Citation2021). Amaranth species have been reported to utilise either C3 or C4 pathways (Cadwallader et al. Citation2012) and two Lake Titicaca regional samples exhibited values typical of C3 plants (modern Amaranthus δ13C = −28.3‰; archaeological Amaranthus δ13C = −22.8‰) (Miller Citation2005; Miller et al. Citation2021). The modern Cactaceae species sampled, which are expected to use the CAM pathway, all show quite high δ13C values (Cactaceae mean δ13C = −12.7‰), and the C4 plant maize, Zea mays, follows with relatively higher carbon isotope values (mean δ13C = −11.3‰). These values are for modern plants and therefore their carbon values are ∼1.5‰ lower (more negative) than ancient plants would have been due to the Suess effect (modern burning of fossil fuels altering atmospheric carbon) (Keeling Citation1979).
Additionally, carbon stable isotope values can help distinguish between terrestrial and aquatic habitats: marine and some freshwater fish, including those from the Lake Titicaca basin, can be enriched in the heavier carbon isotope and can have isotopic values similar to C4 plants (Miller, Capriles, and Hastorf Citation2010). Consequently, we expect that if Altiplano herders practiced a specialised form of pastoralism with narrow diets based on C3 plants and C3-plant consuming camelids, their δ13C values should be characterised as slightly less negative than the camelid average. Generalised forms of pastoralism characterised by a wider dietary range including the consumption of freshwater fish might involve higher δ13C values. Furthermore, interregional exchange and C4 and/or CAM plant consumption should be identifiable by relatively high δ13C values. Distinguishing specific resource use can be a challenge if the resources overlap isotopically, which could be the case for many Altiplano freshwater aquatic foods and plants such as maize (Miller, Capriles, and Hastorf Citation2010), making the incorporation of other data sets such as archaeobotanical and faunal data particularly important, as well as the potential for emerging methodologies to improve our resolution of ancient diets in areas with the possibility of mixed resource use (Miller et al. Citation2021).
Skeletal tissue isotopic values reflect various dietary components. Collagen, the proteinaceous organic portion of bone and teeth, preferentially incorporates dietary protein contributions whereas apatite carbonate, derived from the inorganic hydroxyapatite matrix of bone and teeth, reflects total dietary sources (Ambrose and Norr Citation1993; Tieszen and Fagre Citation1993). An offset of approximately +5‰ in δ13C between the organic collagen fraction of bones and teeth and the average consumed diet has been observed (Sullivan and Krueger Citation1981). The carbon isotopic signature of omnivores reflects both the plant proteins as well as the proteins from any animals consumed, and as a result plants consumed by primary consumers (herbivores) are reflected in the signature of higher trophic level consumers, such as humans. The inorganic fraction of bones and teeth incorporate the carbon from all dietary sources and therefore reflect the range of foods consumed over the period that tissue was created, and this fraction also shows a dietary offset, ranging from +9 to +12‰ (Krueger and Sullivan Citation1984; Lee-Thorp, Sealy, and van der Merwe Citation1989). A combination of bone collagen and apatite data could help to further determine if a few or multiple food sources were present in studied samples (Froehle, Kellner, and Schoeninger Citation2012).
The nitrogen isotope values (δ15N) of consumers reflect their position in a food web because δ15N values increase by +3–4‰ per trophic level but can also vary in relation to latitude, altitude, climate and phenotypic variability (Schoeninger, DeNiro, and Tauber Citation1983; Schoeninger and DeNiro Citation1984). Except for nitrogen-fixing flora, most plants have δ15N ranges of +2–6‰ (DeNiro and Epstein Citation1981; Schoeninger and DeNiro Citation1984). Nevertheless, empirical research suggests that δ15N values can also be enriched as a consequence of specific physiological responses to aridity and other microenvironmental conditions (Szpak et al. Citation2013). Furthermore, the use of fertilisers, such as sea bird guano but also dung, can enrich the nitrogen content of plants, as documented in the north coast of Peru and the Atacama Desert (Santana-Sagredo et al. Citation2021; Szpak et al. Citation2012).
Modern plant samples collected from the Lake Poopó region (Miller et al. Citation2021) were fertilised with llama dung and therefore δ15N values are potentially altered due to fertiliser effects, so results should be interpreted with caution in their applicability to past conditions. The three Chenopodium quinoa samples have an average δ15N = +8.1‰, but the standard deviation is 2.3‰, while the four maize samples have an average δ15N = +8.6‰ and a standard deviation of 1.2‰, demonstrating the large variation in nitrogen isotope values possible within the same species, grown in the same area, under the same conditions (Miller et al. Citation2021). Isotopic analysis of modern plants from Chiripa in the Lake Titicaca basin further demonstrate a wide range of nitrogen isotope values across terrestrial and aquatic plants, with δ15N values ranging from −5.0 to +15.2‰, while most modern domesticated crops have δ15N values around +7‰ (Miller Citation2005; Miller et al. Citation2021). Specifically, tubers had an average δ15N of +7.7‰ (Solanum tuberosum and Oxalis tuberosa), chenopods (Chenopodium spp.) average δ15N = +7.1‰ and maize (Zea mays) average δ15N = +7.0‰, while beans, which fix atmospheric nitrogen, have an average δ15N of +0.4‰ (Fabaceae) (Miller et al. Citation2021). Based on this information, we expect that specialised altiplano pastoralists had δ15N values reflecting a predominantly terrestrial animal diet. More specifically, if camelid meat was the primary food source, human carbon isotopic values should be a trophic level above the camelid δ15N average value. Depending on additional resources consumed, generalised forms of pastoralism could involve proportionally lower or higher δ15N values. Finally, significant dietary variations including the consumption of agricultural products cultivated with manure as well as the potential consumption of imported marine resources should be reflected in high δ15N values.
Materials and Methods
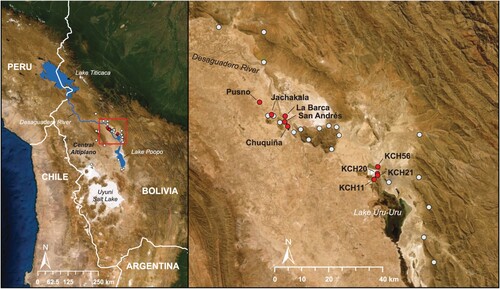
Archaeological excavations conducted at nine different sites located in the Wankarani hinterland of the Bolivian Central Altiplano provided the human and faunal remains analyzed for this study (). Human remains originate from seven sites and faunal remains from four sites. These sites correspond to Archaic, Early Formative, Late Formative, Tiwanaku and Late Intermediate periods. KCH20 is an open-air Archaic Period residential camp where a hearth feature containing stone tools and fragmented remains of wild fauna, but no human remains, were recovered and dated to 7000 BCE (Capriles et al. Citation2018). San Andrés, Chuquiña, La Barca and Pusno are Early Formative Wankarani mound sites containing overlapping circular residential structures built with stone foundations, mud-brick walls, food remains, and occasionally, human burials (Bermann and Estévez Castillo Citation1995; Fox Citation2007). KCH11, KCH21 and KCH56 are Wankarani residential bases occupied during the Late Formative Period and contain domestic structures, interior and exterior storage features, and abundant plant and animal remains, including a preponderance of domesticated camelids but also fish, aquatic birds and mid-sized rodents (Capriles Citation2011; Capriles Citation2017). Pusno, KCH11 and KCH21 also contained human burials, including some that postdated their residential occupations. Finally, Jachakala is a large, dispersed settlement that articulated interregional exchange during the Tiwanaku Period as suggested by an abundance of basalt hoes from this region that made their way to the Titicaca basin (Beaule Citation2002; Bermann and Estévez Castillo Citation1993).
Figure 1. Study area including the Central Altiplano, Wankarani early pastoralist sites (white circles) and archaeological sites that were sampled for this study (red circles).
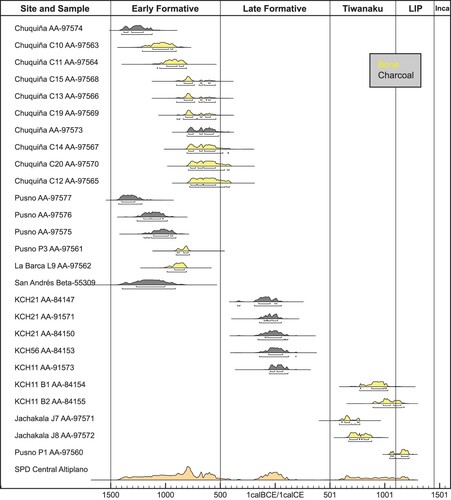
Skeletal samples from humans were selected from available burial contexts and faunal remains derive from sites where detailed zooarchaeological analyses were conducted. Basic bioarchaeological analyses of the human remains were carried out to estimate their sex and age at death (Buikstra and Ubelaker Citation1994). Most selected human samples were adults (n = 20), but a few juveniles (n = 4) are also present, and almost all human samples are bone, with only two individuals having a tooth sampled. Direct accelerated mass spectrometry (AMS) radiocarbon dating of most human specimens was conducted to precisely place these individuals in time and additional radiocarbon dates on charcoal from contexts near the undated human remains are also included. Radiocarbon dates were calibrated using the SHCal20 calibration curve (Hogg et al. Citation2020) in Oxcal 4.4.2 () (Bronk Ramsey Citation2020).
Figure 2. Calibrated radiocarbon dates of samples and contexts associated with this study including sum probability density (SPD) of the Central Altiplano using Oxcal 4.4.2 (Bronk Ramsey Citation2020) and SHCal20 (Hogg et al. Citation2020).
Bone and tooth samples were cleaned and prepared for isotopic analysis following published protocols for both collagen and apatite fractions (Koch, Tuross, and Fogel Citation1997; Sealy et al. Citation2014; Yoder and Bartelink Citation2010). Isotope values are described as parts per mil (‰) and presented in delta (δ) notation in relation to the standards of Vienna-Pee Dee Belemnite (VPDB) for δ13C and atmospheric nitrogen (AIR) for δ15N. The primary focus of this work is on δ13C and δ15N from bone collagen, but we also discuss δ13C results from a sub-set of bone apatite samples.
Isotopic samples were analyzed at two lab facilities. At Duke University’s Environmental Stable Isotope Laboratory (DEVIL), bone collagen samples were processed for δ13C and δ15N using a Carlo Erba Elemental Analyzer with zero-blank autosampler connected to a Conflo III interface, coupled with a Thermo Finnigan Delta Plus XL continuous flow mass spectrometer. Samples were analyzed with international reference materials including IAEA N-1, USGS 25, USGS 26 for nitrogen and NBS-22, Sucrose ANU, and PEFI foil for carbon as well as internal standards Costech acetanilide, Duke urea, Duke sucrose and USGS 26. External precision (relative to reference materials) is approximately ±0.2‰ at one standard deviation while internal precision (relative to internal standards) is generally better. At University of California, Berkeley’s Center for Stable Isotope Biogeochemistry (CSIB) bone collagen samples were analyzed for δ13C and δ15N using a Vario Isotope Cube Elemental Analyzer coupled with an IsoPrime IRMS. Samples were analyzed with NIST standards (SRM 1547 peach leaves, SRM 1577c bovine liver), IAEA-CH6 (sucrose) and internal project standards. Long term precision for δ13C is ±0.1‰ and δ15N is ±0.15‰. Bone apatite carbonate samples were analyzed for δ13C and δ18O at UC Berkeley’s Laboratory for Environmental and Sedimentary Isotope Geochemistry (LESIG) with NBS19, two lab standards, internal standards and LESIG reported long-term external precision of ±0.04‰. Collagen preservation was assessed by standard measures of percent (%) yield, %C, %N and atomic C:N ratios (DeNiro, Schoeninger, and Hastorf Citation1985; van Klinken Citation1999).
To describe food web and ecological relationships we applied single and bivariate statistics, grouping data taxonomically, temporally and spatially following standard representation of dietary stable isotopic data. Furthermore, for human and faunal samples with both organic and inorganic δ13C values we evaluate dietary data patterns in relation to controlled-feeding study dietary groups using data published by Froehle, Kellner, and Schoeninger (Citation2010). Finally, we compare our results with existing dietary stable isotopic studies from neighboring regions.
Results
Radiocarbon Dating and Chronology
Our chronology is based on 15 AMS radiocarbon dates directly conducted on human bone from the sites of Chuquiña, Pusno, Jachakala, La Barca and KCH11. In addition, we rely on 11 additional dates of charcoal from contexts associated with other analyzed human and faunal remains from Chuquiña, Pusno, San Andrés, KCH11, KCH21 and KCH56, to provide strong chronological control for our study sample (). In addition, a direct AMS date on a wild guinea pig bone from KCH20, dating to approximately 7000 BCE, strengthens the chronology of the samples included in this study. Radiocarbon dates in relation to the regional cultural chronology are used to present and discuss the results of the dietary stable isotope research.
Table 1. Radiocarbon dates of samples analyzed in this study including calibrated results using Oxcal 4.4.2 (Bronk Ramsey Citation2020) and SHCal20 (Hogg et al. Citation2020).
Dietary Stable Isotopes
Our collagen carbon (δ13Ccoll) and nitrogen (δ15Ncoll) stable isotope dataset corresponds to 23 faunal () and 27 human specimens () summarised in . Faunal remains derive from Archaic (n = 4), Late Formative (n = 16) and Tiwanaku (n = 3) period contexts. All faunal samples had good C:N ratios, ranging between 3.0 and 3.6, within the accepted range for biogenic collagen (DeNiro, Schoeninger, and Hastorf Citation1985; DeNiro & Schoeninger Citation1983). Human remains correspond to Early Formative (n = 17), Late Formative (n = 5 samples from 2 individuals), Tiwanaku (n = 4) and Late Intermediate (n = 1) Period contexts. All analyzed human samples had good C:N ratios, between 3.0 and 3.3 (Schwarcz and Nahal Citation2021), but sample P2 from Pusno, produced poor yield and its exceptionally low %C and %N content suggest degradation, and it was removed from subsequent discussion. In addition, we reported 28 paired apatite carbon (δ13Cap), 20 of which also included oxygen (δ18Oap) stable isotopes for most faunal and a few human specimens. The δ18Oap values, related to variable sources of water, are reported but not discussed in this text (Knudson Citation2009).
Table 2. Carbon and nitrogen stable isotope results of faunal remains.
Table 3. Carbon and nitrogen stable isotope results of human samples.
Table 4. Summarised results of stable isotope analysis by taxa and in the case of humans by temporal period including maximum (Max) and minimum (Min), mean and standard deviation (SD).
Animal Resources
Our faunal sample consists of 6 camelids, 4 tuco-tuco gophers, 3 guinea pigs, 5 birds and 5 freshwater fish (; and ). The six camelid remains were identified as domesticated llamas (Lama glama) based on osteometry and contextual evidence (Capriles Citation2011). The camelid δ13Ccoll values range from −19.6‰ to −15.2‰, with an average of −17‰ and a SD of 1.4‰, indicating most diets were predominantly C3-plant-based, with some individuals consuming small amounts of CAM, C4 plants and/or freshwater resources. The camelid δ15Ncoll values range from 8.1 to 11.1‰ with an average of 9.9‰ (SD = 1.1‰), which indicates the plant resources they generally consumed varied between 4 and 8‰ δ15N, which are consistent with both terrestrial and aquatic Andean environments (Juengst et al. Citation2021; Miller, Capriles, and Hastorf Citation2010; Pestle et al. Citation2017).
Figure 3. Bivariate plot showing carbon and nitrogen stable isotopes of bone collagen categorised by taxa and human samples by period.
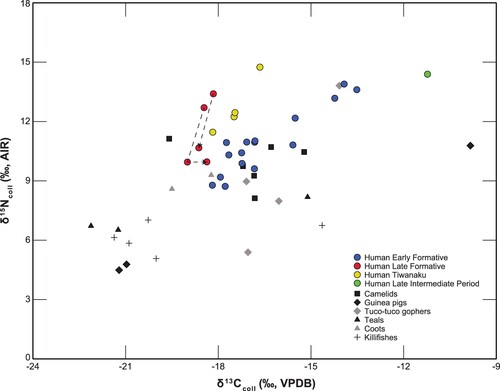
Two mid-sized rodent taxa were sampled for this study: cuy/guinea pigs (Cavia spp.) and tuco-tuco gophers (Ctenomys opimus). Although together these rodents have the largest range of stable isotope data for both carbon and nitrogen, indicating diverse dietary preferences, there are some concrete interspecific and intraspecific patterns. The two most similar Cavia samples, average −21.1‰ for carbon, and 4.5‰ for nitrogen, and most likely represent wild cuy (Cavia tschudii). These specimens were recovered from a feature containing dozens of wild guinea pig bones at KCH20. In contrast, the third guinea pig is from a Tiwanaku period context and had some of the highest δ13C (−9.8‰) and δ15N (10.8‰) values reported in the assemblage. It is possible that its enriched isotopic composition derives from the consumption of various sources including maize or other C4/CAM plants, and possibly gnawing of animal bones, as suggested by the fact that approximately 2.9% of all the camelid remains from the zooarchaeological study included rodent gnawing marks (Capriles Citation2011). Therefore, this specimen is either a commensal wild cuy or a domesticated guinea pig (Cavia porcellus).
The four tuco-tuco samples also show diversity, with an average δ13Ccoll value of −16‰ (SD = 1.4‰). Their nitrogen values range from 5‰ to almost 14‰. If we exclude the extremely high nitrogen value, the other three samples have an average δ15Ncoll value of 7.5‰ (SD = 1.9‰). This variability and range indicate the ability of tuco-tucos to successfully subsist on different diets that include various food sources (Takenaka et al. Citation2020). These values might also derive from commensal behaviour, as these animals were probably snared from the vicinity of the sites to which they were probably attracted as potential sources for food.
Two different bird taxa were sampled, teals (Anatidae) and coots (Rallidae). The teals are represented by three samples, two have very similar carbon isotope values (−22.1‰ and −21.2‰) and also very similar nitrogen values (6.5‰ and 6.7‰), while the third has a very different δ13C value of −15.1‰ and a δ15N value of 8.2‰. The significant difference in isotopic values (and therefore dietary differences) between these birds, suggests that at least two species may be represented. Given that there are over half a dozen different teal species that inhabit highland wetlands of the Central Altiplano including a few migrant speciates, this is not surprising. The two coots have very similar δ13C values, which average −18.9‰ and very similar δ15N values that average to 8.9‰. The coots generally have higher nitrogen values than the teals suggesting they are regularly consuming resources from a higher trophic level.
All fish specimens were identified as killifishes (Orestias agassii) and while most of them cluster around a δ13Ccoll value of −21‰, one sample had a very high value (−14.6‰), perhaps suggesting a dietary shift with age and/or size of the individual, but most likely, a distinct species. The δ15Ncoll values range from 5.1‰ to 7.0‰ and the average nitrogen value for these fish is 6.2‰, which is comparable with the trophic position of the genus in Lake Titicaca (Miller, Capriles, and Hastorf Citation2010; Miller et al. Citation2021). Although Orestias agassii is the only species currently represented in Lake Uru-Uru, zooarchaeological analysis from the study sites identified considerable variation within the size and morphology of killifish specimens (Capriles Citation2011).
Humans
The Early Formative sample of human remains consists of 17 individuals (n = 14 adults, n = 3 juveniles) from four Wankarani mound sites (; and ). Excluding individual P2 from Pusno, the 16 Early Formative humans have a mean δ13Ccoll of −16.5‰ (SD = 1.6‰) and a mean δ15Ncoll of 10.9‰ (SD = 1.6‰) (). Although the Early Formative includes our largest temporal sample, no clear effects were observed for sex or age. Nevertheless, there is also a good deal of diversity in the isotopic values recorded within sites. For example, Chuquiña, the site with the largest number of human burials (n = 11), includes δ13Ccoll values that range from −17.9‰ to −13.9‰ and δ15Ncoll values that range from 8.7‰ to 13.9‰. Many of the Early Formative individuals’ δ13C values cluster around −17‰, suggesting their diets were based almost exclusively on C3 plants and C3 consuming herbivores, which is consistent with most of the fauna reported above. A few individuals have values around −14‰, which indicates their diets had significant contributions from CAM or C4 plants and/or animal proteins such as some birds and fish. Individuals with high δ15Ncoll values likely consumed more meat in their diets or more animals that were higher on the food chain (such as birds and fish) compared to the individuals on the lower end of the nitrogen data range and whom may have had diets based on plants and herbivores.
The Late Formative period is represented by a small sample with only two adult individuals from the KCH21 site. However, these two people were sampled for multiple tissues (n = 5 samples total) to provide dietary data from different periods during their lifetimes. Individual L1003 was an adult female and we sampled both the crown of her first permanent molar to capture her diet during infancy including breastfeeding and weaning, and then the same tooth was sampled at the root tip to study her later childhood diet. The tooth dentin crown sample has a δ13Ccoll value of −18.5‰ and a δ15Ncoll value of 12.7‰, followed by her tooth dentin root sample having a carbon value of −19‰ and a nitrogen value of 10‰. The 2.7‰ drop in δ15N between the crown and root samples is consistent with the weaning process as an infant has breastmilk removed from the diet (Beaumont et al. Citation2013; Eerkens et al. Citation2018). A rib bone sample from the same individual, L1003, has a δ13Ccoll value of −18.4‰ and a δ15Ncoll value of 10‰, suggesting that her diet may have been relatively consistent between later childhood and her adult years. The crown from a permanent first molar from the L503 individual had a δ13Ccoll value of −18.2‰ and a δ15Ncoll value of 13.4‰, while its tooth root had a δ13Ccoll value of −18.6‰ and a δ15Ncoll value of 10.7‰. The decline in nitrogen across L503’s tooth samples is consistent with the expected change of an infant being weaned off milk. Both individuals’ isotopic values from later childhood/adulthood are very similar and are consistent with diets dominated by C3 plants and animals feeding mostly on C3 plants.
The four people sampled from the Tiwanaku period were all adults (3 males, 1 female) and show similar carbon values to the Late Formative individuals but differ in their nitrogen values. The Tiwanaku period individuals average δ13Ccoll is −17.5‰ (SD = 0.6‰), and average δ15Ncoll is 12.7‰ (SD = 1.4‰). Again, these isotopic values suggest diets dominated by C3 plants such as tubers and chenopods, and C3-consuming animals such as camelids, with possible addition of some small rodents, birds and fish (given the higher nitrogen isotope values observed for these Tiwanaku-period people). Interestingly, the carbon isotope values do not indicate significant consumption of maize (C4 plant). Finally, a Late Intermediate Period individual from Pusno (P1) included the highest δ13Ccoll (−11.2‰) and one of the highest δ15Ncoll (+14.4‰) values in the sample. Chronologically, this is the most recent sample in our study and its high isotopic values suggest that this individual consumed a higher proportion of CAM or C4 plants such as maize, and potentially other resources including higher proportions of animal protein, which might signal increased interregional mobility.
Combining Collagen and Apatite Datasets
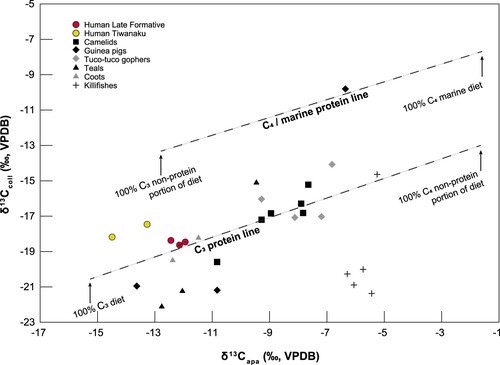
We sampled the inorganic δ13Cap from all 23 faunal specimens as well as from four of the Late Formative and Tiwanaku human individuals (with one, L1003, sampled twice) to further our dietary reconstructions (). Adding regression lines to the resulting dataset shows how animal and human diets are dynamically composed of multiple food sources of varying isotopic ranges (Froehle, Kellner, and Schoeninger Citation2010; Kellner and Schoeninger Citation2007). Our data mostly falls around the lower regression line, indicating that although some C4 or CAM plants could have occasionally been consumed, C3 resources dominated protein and non-protein sources for many of these species. Humans are also closer to the C3 protein line and are clustered to the left of that line, indicating diets that were almost exclusively C3 foods both in terms of protein sources and their overall diet (carbohydrates, fats and proteins). Interestingly, the camelids are further to the right on the C3 protein regression line, indicating that their diets had some ‘CAM' or 'C4’ contributions, but this could be also explained by feeding on aquatic plants (Miller, Capriles, and Hastorf Citation2010; Miller et al. Citation2021). The one outlying sample that lies just above the C4/marine protein line is the (likely domesticated) guinea pig.
Figure 4. Bivariate plot showing carbon isotopic results on bone collagen and bone apatite in relation to the predicted food consumption lines (following Froehle, Kellner, and Schoeninger Citation2010; Kellner and Schoeninger Citation2007).
Discussion
This study provides the first broad assessment of diet based on both human and animal stable isotope analyses for the Andean Central Altiplano. Regarding camelid herds, the specimens included in our study have nitrogen and carbon isotope values consistent with C3-plant based diets from the highlands albeit potentially with some amount of C4 and/or aquatic plants (Thornton et al. Citation2011). Camelids with a wide range of δ13C values have been recorded from a number of Andean sites and are largely attributed to herd management and animal feeding strategies (Berryman Citation2010; Finucane, Agurto, and Isbell Citation2006; López et al. Citation2017; Samec et al. Citation2020). In contrast, camelids herded in coastal sites have even higher nitrogen and carbon stable isotope values (Dufour et al. Citation2020; Szpak et al. Citation2020; Szpak and Valenzuela Citation2020). If the camelids from this study were used as pack animals or were herded over areas with large altitudinal differences, they may have consumed more C4 and/or aquatic plants and thus had more enriched δ13C and δ15N values (Finucane, Agurto, and Isbell Citation2006; Miller, Capriles, and Hastorf Citation2010; Szpak and Valenzuela Citation2020). Nevertheless, the enriched carbon values found in some of the camelid samples studied here may reflect the consumption of various highland resources including CAM succulents and highland aquatic resources (Cadwallader et al. Citation2012; Miller, Capriles, and Hastorf Citation2010; Miller et al. Citation2021).
Regarding human subsistence, based on previous archaeological and isotopic research in the ancient Andes, we hypothesised that if the early Altiplano pastoralist communities specialised in managing and consuming domesticated camelids, their diet breadth should be relatively narrow and show relatively low δ13C and δ15N values. Generalised pastoralists on the other hand, would have relied on a wider dietary breadth including agricultural and other faunal resources that should have resulted in wider isotopic ranges. Our data largely confirmed that the Central Altiplano early pastoralists had δ13C values reflecting a primarily C3 dependent diet, but with important variability within these choices and likely adaptability over time (). Consistent with zooarchaeological and paleoethnobotanical analyses that suggested Central Altiplano early camelid pastoralists utilised a wide range of dietary resources, isotopic results suggest consumption of C3 herbivores such as domesticated llamas, but also C3 plants including chenopods (such as quinoa, kañawa) and tubers (such as potatoes and ocas) as well as wild resources. For instance, about a third of the Early Formative people’s diets reflect the consumption of birds, fish and other C4/CAM/freshwater consumers. Furthermore, a few of the human δ13C values, like those from San Andrés (e.g. Sa5: −13.5‰, Sa4: −14.2‰) and a few from Chuquiña (e.g. C20: −13.9‰), suggest significant consumption of 13C enriched foods, likely as a result of consuming freshwater fish. Certainly, future research should further explore the nature of the observed variability and its potential association with specific intra-regional patterns of mobility, resource availability and the potential effects of climate change in shaping dietary change over time.
Figure 5. Carbon and nitrogen stable isotopes of human remains in relation to time and paleoenvironmental data derived from diatom analysis from Lake Titicaca (Bruno et al. Citation2021).
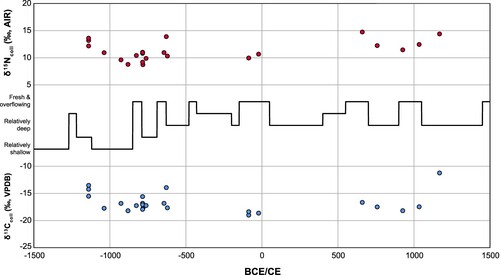
Concerning the question about the emergence of interregional complementary exchange identified in the ethnohistoric sources, our data do not provide strong evidence for the regular consumption of maize or any C4 plants until the end of Tiwanaku. Previous work has also failed to identify consistent evidence of maize in Wankarani settlements and with the exception of a few shell ornaments, archaeofaunal evidence for interregional trade is limited (Capriles Citation2017). These results further verify paleoethnobotanical research that underscores the importance of locally grown foods over time and the rarity of exotic cultigens, including maize, before the Late Intermediate Period (Bonzani Citation2021; Langlie and Capriles Citation2021). Nevertheless, the domesticated guinea pig from KCH21, likely fed with maize, is intriguing and might have been an exotic import. More research might verify if individuals of higher status could have accessed and consumed more maize, perhaps in the form of chicha beer, which at the time, was fundamentally a ritual beverage (Berryman Citation2010; Hastorf et al. Citation2006). In contrast, the human sample from Pusno (P1) dated to the very beginning of the Late Intermediate Period, suggests that interregional staple food exchange, including an emphasis on maize, might have begun quite early after the disintegration of Tiwanaku, which is consistent with growth of caravan networks and nodes in oases and valleys towards the interior of the Atacama Desert (Capriles et al. Citation2021; Santana-Sagredo et al. Citation2019; Torres-Rouff et al. Citation2015). Indeed, the consumption of maize and other lowland foods such as chili peppers and squash likely became feasible only after the establishment of strong networks of interregional exchange no earlier than the Late Intermediate Period (Korstanje Citation2015; Langlie and Capriles Citation2021; Santana-Sagredo et al. Citation2021b).
Conclusions
In this study, we reported dietary stable isotopic data from samples of human and animal archaeological skeletal remains from the Central Altiplano of Bolivia. Direct AMS radiocarbon dates verify the samples originate from a temporal range spanning between 1300 BCE and 1200 CE. The relatively broad dietary breadth documented in the human carbon and nitrogen stable isotopes is consistent with previously reported zooarchaeological and paleoethnobotanical data from the region indicating that from early on these groups practiced a generalised form of pastoralism with a strong reliance on camelid meat but also on other wild and domesticated plant and animal foods (Capriles Citation2014; Langlie and Capriles Citation2021). These results contrast with the ethnohistorically documented system of vertical complementarity as strong evidence for the consumption of maize is limited to the most recent human sample reported here. Therefore, maize does not seem to have been a significant component of the diet until the LIP confirming interregional exchange for staple goods developed later in time in this region. For the Central Altiplano peoples most dietary protein was derived from herbivore animals such as domesticated llamas, and C3 cultivated crops such as chenopods and tubers but also the consumption of wild resources including various amounts of freshwater fish, birds and plants. Over time, there is a persistence in this wide range of dietary sources and some temporal fluctuations potentially associated with resource intensification possibly connected to regional mobility patterns, population size and even climate change.
Our data suggest the early Central Altiplano pastoralists relied on a form of generalised pastoralism that mostly involved local plant cultivation and procurement of regionally available wild resources. Nevertheless, unlike the Carangas, Soras, Quillacas, and other ethnohistorically and ethnographically documented herding communities, they did not practice interregional complementarity. Beginning during the Early Formative Period, Wankarani communities structured residential mobility patterns tied to locally available resources. The integration of the central highlands and their local-scale subsistence systems into a larger political economy during the Tiwanaku Period does not seem to have involved strong processes of dietary change, but more research and larger sample sizes are needed to verify this proposition. Settlement patterns, site layouts and artefact analysis from Central Altiplano sites suggest that although the impact of the state was not dramatic, there were some significant differences between the Formative and Tiwanaku periods (Capriles Citation2017). As an intermediate region between the Titicaca basin, the Pacific western valleys and the tropical eastern valleys, the Central Altiplano herding communities could have benefited from increased traffic and regional interaction (Albarracin-Jordan Citation2007; Bonzani Citation2021; Browman Citation1997; Hastorf et al. Citation2006; Núñez and Dillehay Citation1995). Settlements such as Jachakala and KCH11, might have worked as network nodes for llama caravans moving to and from Tiwanaku and other consumption loci such as Cochabamba, Pica or the Azapa valley. Some of the larger sites might have served as gathering places for fairs, where exchanging prestige goods, consumption rituals (possibly involving maize chicha) and incipient political integration occurred. Nevertheless, the dietary stable isotopes along with other lines of archaeological evidence indicate that several of the resources consumed in earlier time periods continued to be utilised with subtle changes in the regional economic subsistence.
Acknowledgements
We thank Juan Albarracin-Jordan, Marc Bermann, Paul Brooks, Maria Bruno, Sergio Calla Maldonado, William Castellón, Todd Dawson, Alejandra Domic, Robert Drennan, John Janusek, Christine Hastorf, Stefania Mambelli, Fiona Marshall and Wenbo Yang for their support in conducting this research. Fieldwork in Oruro was authorised by the Unidad Nacional de Arqueología dependent of the Bolivian Ministerio de Culturas y Turismo, and facilitated by the Inti Raymi Mining Company, Universidad Mayor de San Andrés, Universidad Técnica de Oruro, and the people of the communities of Chuquiña, La Joya, Iroco, Cochiraya and Chuzequeri.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
José M. Capriles
José M. Capriles earned his PhD in Anthropology from Washington University in St. Louis in 2011 and is presently Assistant Professor of Anthropology at The Pennsylvania State University. He specialises in environmental archaeology, human ecology and zooarchaeology and has conducted extensive field and lab research in Bolivia and Chile.
Melanie J. Miller
Melanie J. Miller earned her PhD in Anthropology from the University of California, Berkeley, in 2016 and is currently a Postdoctoral Fellow in the Department of Anatomy at the University of Otago in New Zealand. Her research specialises in human biology and analytical chemistry, studying ancient human health and nutrition.
Jake R. Fox
Jake R. Fox earned his PhD in Anthropology from the University of Pittsburgh in 2007 and currently is Professor of Anthropology at Radford University. His research interests include subsistence economies in early village communities and foragers, lithic technology and cultural ecology.
David L. Browman
David L. Browman earned his PhD in Anthropology from Harvard University in 1970 and is presently Professor Emeritus at Washington University in St. Louis where he conducted research related to the origins of pastoralism, complex societies and historical archaeology particularly in the Andes for nearly 50 years.
Cassady Yoder Urista
Cassady Yoder Urista earned her PhD in Anthropology from Texas A&M University in 2006 and is currently Professor of Anthropology at Radford University. She specialises in biological anthropology and bone chemistry analysis and has conducted research on paleonutrition, paleopathology and skeletal biology in Medieval Denmark, Andean South America and the US.
References
- Albarracin-Jordan, J.. 2007. La Formación del Estado Prehispánico en los Andes: Origen y Desarrollo de la Sociedad Segmentaria Indígena. La Paz: Fundación Bartolomé de las Casas.
- Ambrose, S. H., and L. Norr. 1993. “Experimental Evidence for the Relationship of the Carbon Isotope Ratios of Whole Diet and Dietary Protein to Those of Bone Collagen and Carbonate.” In Prehistoric Human Bone, edited by J. B. Lambert and G. Grupe, 1–37. Berlin: Springer.
- Beaule, C. D. 2002. Late Intermediate Period Political Economy and Household Organization at Jachakala, Bolivia, xxiii + 334. Pittsburgh: Department of Anthropology, University of Pittsburgh.
- Beaumont, J., A. Gledhill, J. Lee-Thorp, and J. Montgomery. 2013. “Childhood Diet: A Closer Examination of the Evidence from Dental Tissues Using Stable Isotope Analysis of Incremental Human Dentine.” Archaeometry 55: 277–295.
- Bermann, M., and J. Estévez Castillo. 1993. “Jachakala: A New Archaeological Complex of the Department of Oruro, Bolivia.” Annals of the Carnegie Museum 62: 311–340.
- Bermann, M., and J. Estévez Castillo. 1995. “Domestic Artifact Assemblages and Ritual Activities in the Bolivian Formative.” Journal of Field Archaeology 22: 389–398.
- Berryman, C. A. 2010. Food, Feasts, and the Construction of Identity and Power in Ancient Tiwanaku: A Bioarchaeological Perspective. Nashville: Department of Anthropology, Vanderbilt University.
- Bonzani, R. M. 2021. “Ancient Paria, Bolivia: Macrobotanical Remains Recovered from an Administrative Site on the Royal Inca Highway.” In Andean Foodways: Pre-Columbian, Colonial, and Contemporary Food and Culture, edited by J. E. Staller, 137–186. Cham: Springer.
- Bronk Ramsey, C. 2020. OxCal Program, Version 4.4, Oxford Radiocarbon Accelerator Unit. Oxford: University of Oxford.
- Browman, D. L. 1987. “Agro-pastoral Risk Management in the Central Andes.” Research in Economic Anthropology 8: 171–200.
- Browman, D. L. 1990. “Camelid Pastoralism in the Andes: Llama Caravan Fleteros, and Their Importance in Production and Distribution.” In Nomads in a Changing World, edited by P. C. Salzman and J. G. Galaty, 395–438. Naples: Instituto Universitario Orientale.
- Browman, D. L. 1997. “Political Institutional Factors Contributing to the Integration of the Tiwanaku State.” In Emergence and Change in Early Urban Societies, edited by L. Manzanilla, 229–243. New York: Plenum Press.
- Bruno, M. C. 2014. “Beyond Raised Fields: Exploring Farming Practices and Processes of Agricultural Change in the Ancient Lake Titicaca Basin of the Andes.” American Anthropologist 116: 130–145.
- Bruno, M. C., J. M. Capriles, C. A. Hastorf, S. C. Fritz, D. M. Weide, A. I Domic, and P. A. Baker. 2021. “The Rise and Fall of Wiñaymarka: Rethinking Cultural and Environmental Interactions in the Southern Basin of Lake Titicaca.” Human Ecology 49 (2): 131–145.
- Buikstra, J. E., and D. H. Ubelaker. 1994. “Standards for Data Collection from Human Skeletal Remains”, Arkansas Archaeological Survey Research Series, Fayetteville.
- Cadwallader, L., D. G. Beresford-Jones, O. Q. Whaley, and T. C. O’Connell. 2012. “The Signs of Maize? A Reconsideration of What δ13C Values Say About Palaeodiet in the Andean Region.” Human Ecology 40: 487–509.
- Capriles, J. M. 2011. The Economic Organization of Early Camelid Pastoralism in the Andean Highlands of Bolivia. St. Louis: Department of Anthropology, Washington University in St. Louis.
- Capriles, J. M. 2014. “Mobile Communities and Pastoralist Landscapes During the Formative Period in the Central Altiplano of Bolivia.” Latin American Antiquity 25: 3–26.
- Capriles, J. M. 2017. Arqueología del Pastoralismo Temprano de Camélidos en el Altiplano Central de Bolivia. La Paz: Instituto Francés de Estudios Andinos (IFEA), Plural Editores.
- Capriles, J. M., J. Albarracin-Jordan, D. W. Bird, S. T. Goldstein, G. M. Jarpa, S. Calla Maldonado, and C. M. Santoro. 2018. “Mobility, Subsistence, and Technological Strategies of Early Holocene Hunter-Gatherers in the Bolivian Altiplano.” Quaternary International 473: 190–205.
- Capriles, J. M., S. Calla Maldonado, and J. Albarracin-Jordan. 2011. “Tecnología Lítica y Estrategias de Subsistencia durante los Períodos Arcaico y Formativo en el Altiplano Central, Bolivia.” Chungara Revista de Antropología Chilena 43: 455–468.
- Capriles, J. M, C. M. Santoro, R. J. George, E. Flores Bedregal, D. J. Kennett, L. Kistler, and F. Rothhammer. 2021. “Pre-Columbian Transregional Captive Rearing of Amazonian Parrots in the Atacama Desert.” Proceedings of the National Academy of Sciences 118 (15): e2020020118.
- Capriles, J. M., and N. Tripcevich. 2016. The Archaeology of Andean Pastoralism. Albuquerque: University of New Mexico Press.
- Catacora, H., M. Clavijo, S. Fernández, P. Lima, F. Michel, and M. Michel. 2002. “Una Aproximación Histórico-Espacial a la Relación Hombre-Medio Ambiente en la Cuenca del Poopó: El Caso de Quillacas.” In Diagnóstico de Recursos Naturales y Culturales de los Lagos Poopó y Uru Uru, Oruro – Bolivia, edited by O. Rocha Olivo, 133–166. La Paz: Convención de Humedales – Ramsar.
- Chang, C., and H. A. Koster. 1986. “Beyond Bones: Toward an Archaeology of Pastoralism.” Advances in Archaeology Method and Theory 9: 97–148.
- Cribb, R. 1991. Nomads in Archaeology. Cambridge: Cambridge University Press.
- Cruz, P., T. Winkel, M.-P. Ledru, C. Bernard, N. Egan, D. Swingedouw, and R. Joffre. 2017. “Rain-fed Agriculture Thrived Despite Climate Degradation in the pre-Hispanic Arid Andes.” Science Advances 3: e1701740.
- Cuenca Sempertegui, A., E. Garnica Bahoz, E. López Canelas, and I. Marca Cáceres. 2005. Más Allá de las Pajas y Espinas: Biodiversidad en el Municipio de Oruro (Comunidades Cochiraya – Iroco – Chuzekery). Oruro: Centro de Ecología y Pueblos Andinos (CEPA), Latinas Editores.
- DeNiro, M. J., and S. Epstein. 1981. “Influence of Diet on the Distribution of Nitrogen Isotopes in Animals.” Geochimica et Cosmochimica Acta 45: 341–351.
- DeNiro, Michael J., and Margaret J. Schoeniger. 1983. “Stable Carbon and Nitrogen Isotope Ratios of Bone Collagen: Variations within Individuals, between Sexes, and within Populations Raised on Monotonous Diets.” Journal of Archaeological Science 10 (3): 199–203.
- DeNiro, M. J., M. J. Schoeninger, and C. A. Hastorf. 1985. “Effect of Heating on the Stable Carbon and Nitrogen Isotope Ratios of Bone Collagen.” Journal of Archaeological Science 12: 1–7.
- Dufour, E., N. Goepfert, M. Le Neün, G. Prieto, and J. W. Verano. 2020. “Life History and Origin of the Camelids Provisioning a Mass Killing Sacrifice During the Chimú Period: Insight from Stable Isotopes.” Environmental Archaeology 25: 310–324.
- Dufour, E., N. Goepfert, B. G. Léon, C. Chauchat, R. F. Jordan, and S. V. Sánchez. 2014. “Pastoralism in Northern Peru During pre-Hispanic Times: Insights from the Mochica Period (100–800 AD) Based on Stable Isotopic Analysis of Domestic Camelids.” Plos One 9: e87559.
- Durston, A., and J. Hidalgo. 1997. “La Presencia Andina en los Valles de Arica, Siglos XVI-XVIII: Casos de Regeneración Colonial de Estructuras Archipielágicas.” Chungara Revista de Antropología Chilena 29: 249–274.
- Eerkens, J. W., A. de Voogt, T. L. Dupras, V. Francigny, and A. M. Greenwald. 2018. “Early Childhood Diets on the Nile: δ13C and δ15N in Serial Samples of Permanent First Molars in an Elite Meroitic Population from Sai Island, Sudan.” International Journal of Osteoarchaeology 28: 552–562.
- Farquhar, G. D., J. R. Ehleringer, and K. T. Hubick. 1989. “Carbon Isotope Discrimination and Photosynthesis.” Annual Review of Plant Biology 40: 503–537.
- Finucane, B., P. M. Agurto, and W. H. Isbell. 2006. “Human and Animal Diet at Conchopata, Peru: Stable Isotope Evidence for Maize Agriculture and Animal Management Practices During the Middle Horizon.” Journal of Archaeological Science 33: 1766–1776.
- Flores-Ochoa, J. 1979. Pastoralists of the Andes. The Alpaca Herders of Paratía. Philadelphia: Institute for the Study of Human Issues.
- Fox, J. R. 2007. Time and Process in an Early Village Settlement System on the Bolivian Southern Altiplano, xviii + 268. Pittsburgh: Department of Anthropology, University of Pittsburgh.
- Fox, J. R. 2010. “A Persistent Early Village Settlement System on the Bolivian Southern Altiplano.” In Becoming Villagers: Comparing Early Village Societies, edited by M. S. Bandy and J. R. Fox, 184–204. Tucson: University of Arizona Press.
- Froehle, A. W., C. M. Kellner, and M. J. Schoeninger. 2010. “FOCUS: Effect of Diet and Protein Source on Carbon Stable Isotope Ratios in Collagen: Follow up to Warinner and Tuross (2009).” Journal of Archaeological Science 37: 2662–2670.
- Froehle, A. W., C. M. Kellner, and M. J. Schoeninger. 2012. “Multivariate Carbon and Nitrogen Stable Isotope Model for the Reconstruction of Prehistoric Human Diet.” American Journal of Physical Anthropology 147: 352–369.
- Gayo, E. M., T. Martens, H. Stuart-Williams, J. Fenner, C. M. Santoro, C. Carter, and J. Cameron. 2020. “Procurement of Camelid Fiber in the Hyperarid Atacama Desert Coast: Insights from Stable Isotopes.” Quaternary International 548: 71–83.
- Gil, A. F., R. Villalba, A. Ugan, V. Cortegoso, G. Neme, C. T. Michieli, P. Novellino, and V. Durán. 2014. “Isotopic Evidence on Human Bone for Declining Maize Consumption During the Little Ice Age in Central Western Argentina.” Journal of Archaeological Science 49: 213–227.
- Gyarmati, J., and C. Condarco Castellón. 2014. Paria la Viexa: Pre-Hispanic Settlement Patterns in the Paria Basin, Bolivia, and Its Inka Provincial Center. Budapest: Museum of Ethnography.
- Hastorf, C. A. 1990. “The Effect of the Inka State on Sausa Agricultural Production and Crop Consumption.” American Antiquity 55: 262–290.
- Hastorf, C. A., and S. Johannessen. 1993. “Pre-Hispanic Political Change and the Role of Maize in the Central Andes of Peru.” American Anthropologist 95: 115–138.
- Hastorf, C. A., W. T. Whitehead, M. C. Bruno, and M. Wright. 2006. “The Movements of Maize Into the Middle Horizon Tiwanaku, Bolivia.” In Histories of Maize: Multidisciplinary Approaches to the Prehistory, Linguistics, Biogeography, Domestication, and Evolution of Maize, edited by J. Staller, R. Tykot, and B. Benz, 429–448. San Diego: Academic Press.
- Hogg, A. G., T. J. Heaton, Q. Hua, J. G. Palmer, C. S. M. Turney, J. Southon, A. Bayliss, et al. 2020. “SHCal20 Southern Hemisphere Calibration, 0–55,000 Years cal BP.” Radiocarbon 62: 759–778.
- Janusek, J. W. 2008. Ancient Tiwanaku. Cambridge: Cambridge University Press.
- Janzen, A., M. Balasse, and S. H. Ambrose. 2020. “Early Pastoral Mobility and Seasonality in Kenya Assessed Through Stable Isotope Analysis.” Journal of Archaeological Science 117: 105099.
- Juengst, S. L., D. L. Hutchinson, K. M. Chávez, S. J. Chávez, S. R. Chávez, J. Krigbaum, T. Schober, and L. Norr. 2021. “The Resiliency of Diet on the Copacabana Peninsula, Bolivia.” Journal of Anthropological Archaeology 61: 101260.
- Keeling, C. D. 1979. “The Suess Effect: 13Carbon−14Carbon Interrelations.” Environment International 2: 229–300.
- Kellner, C. M., and M. J. Schoeninger. 2007. “A Simple Carbon Isotope Model for Reconstructing Prehistoric Human Diet.” American Journal of Physical Anthropology 133: 1112–1127.
- Khazanov, A. M. 1984. Nomads and the Outside World. Madison: University of Wisconsin Press.
- Killian Galván, V. A., C. T. Samec, and H. O. Panarello. 2016. “When Maize is not the First Choice: Advances in Paleodietary Studies in the Archaeological Site Río Doncellas (Jujuy, Argentina).” Anthropological Review 79: 265–279.
- Knudson, K. J. 2009. “Oxygen Isotope Analysis in a Land of Environmental Extremes: The Complexities of Isotopic Work in the Andes.” International Journal of Osteoarchaeology 19: 171–191.
- Knudson, K. J., A. E. Aufderheide, and J. E. Buikstra. 2007. “Seasonality and Paleodiet in the Chiribaya Polity of Southern Peru.” Journal of Archaeological Science 34: 451–462.
- Koch, P. L., N. Tuross, and M. L. Fogel. 1997. “The Effects of Sample Treatment and Diagenesis on the Isotopic Integrity of Carbonate in Biogenic Hydroxylapatite.” Journal of Archaeological Science 24: 417–429.
- Kohn, M. J. 2010. “Carbon Isotope Compositions of Terrestrial C3 Plants as Indicators of (Paleo)Ecology and (Paleo)Climate.” Proceedings of the National Academy of Sciences 107: 19691–19695.
- Korstanje, M. A. 2015. “Andenes en los Andes: Paisajes Agrícolas Tardíos Sin Maíz.” In Racionalidades Campesinas en los Andes del Sur. Reflexiones en Torno al Cultivo de la Quinoa y otros Vegetales Andinos, edited by P. Cruz, R. Joffre and T. Winkel, 21–57. Jujuy: Editorial Universidad Nacional de Jujuy.
- Krueger, H. W., and C. H. Sullivan. 1984. “Models for Carbon Isotope Fractionation Between Diet and Bone, Stable Isotopes in Nutrition.” American Chemical Society Symposium Series 258: 205–220.
- Langlie, B. S., and J. M. Capriles. 2021. “Paleoethnobotanical Evidence Points to Agricultural Mutualism among Early Camelid Pastoralists of the Andean Central Altiplano.” Archaeological and Anthropological Sciences 13: 107.
- Langlie, B. S., C. A. Hastorf, M. C. Bruno, M. Bermann, R. M. Bonzani, and W. C. Condarco. 2011. “Diversity in Andean Chenopodium Domestication: Describing a new Morphological Type from La Barca, Bolivia 1300-1250 B.C.” Journal of Ethnobiology 31: 72–88.
- Lee-Thorp, J. A. 2008. “On Isotopes and old Bones.” Archaeometry 50: 925–950.
- Lee-Thorp, J. A., J. C. Sealy, and N. J. van der Merwe. 1989. “Stable Carbon Isotope Ratio Differences Between Bone Collagen and Bone Apatite, and Their Relationship to Diet.” Journal of Archaeological Science 16: 585–599.
- Lima Tórrez, M. P. 2014. “La Presencia Inka y su Relación con las Poblaciones Locales en la Región Occidental de Bolivia: Los Casos de Carangas y Quillacas, Oruro.” In Ocupación Inka y Dinámicas Regionales en los Andes (Siglos XV-XVII), edited by C. Rivera Casanovas, 45–66. Lima: Instituto Francés de Estudios Andinos (IFEA), Plural Editores.
- Logan, A., C. Hastorf, and D. Pearsall. 2012. ““Let’s Drink Together”: Early Ceremonial use of Maize in the Titicaca Basin.” Latin American Antiquity 23: 235–258.
- López, P., I. Cartajena, R. Loyola, L. Núñez, and C. Carrasco. 2017. “The use of Hunting and Herding Spaces: Stable Isotope Analysis of Late Archaic and Early Formative Camelids in the Tulan Transect (Puna de Atacama, Chile).” International Journal of Osteoarchaeology 27: 1059–1069.
- Marshall, F. 1990. “Origins of Specialized Pastoral Production in East Africa.” American Anthropologist 92: 873–894.
- Marshall, F., and E. Hildebrand. 2002. “Cattle Before Crops: The Beginnings of Food Production in Africa.” Journal of World Prehistory 16: 99–143.
- McAndrews, T. 2005. Wankarani Settlement Systems in Evolutionary Perspective: A Study in Early Village-Based Society and Long-Term Cultural Evolution in the South-Central Andean Altiplano. Pittsburgh: University of Pittsburgh, Plural Editores.
- Medinacelli, X. 2010. Sariri. Los Llameros y la Construcción de la Sociedad Colonial. La Paz: Instituto Francés de Estudios Andinos (IFEA), Plural Editores.
- Michel López, M. 2008. Patrones de Asentamiento Precolombino del Altiplano Boliviano: Lugares Centrales de la Región de Quillacas, Departamento de Oruro, Bolivia. Uppsala: Department of Archaeology and Ancient History, Uppsala University.
- Miller, M. J. 2005. What’s in That Pot?: Using Stable Isotope Analysis to Understand Cuisines of the Taraco Peninsula, Bolivia, 1500 BC-AD 1000. Berkeley: Department of Anthropology, University of California.
- Miller, M. J., J. M. Capriles, and C. A. Hastorf. 2010. “The Fish of Lake Titicaca: Implications for Archaeology and Changing Ecology Through Stable Isotope Analysis.” Journal of Archaeological Science 37: 317–327.
- Miller, M. J., I. Kendall, J. M. Capriles, M. C. Bruno, R. P. Evershed, and C. A. Hastorf. 2021. “Quinoa, Potatoes, and Llamas Fueled Emergent Social Complexity in the Lake Titicaca Basin of the Andes.” Proceedings of the National Academy of Sciences. Accepted.
- Murra, J. V. 1968. “An Aymara Kingdom in 1567.” Ethnohistory (Columbus, Ohio) 15: 115–151.
- Murra, J. V. 2002. El Mundo Andino: Población, Medio Ambiente y Economía, Instituto de Estudios Peruanos. Lima: Pontíficia Universidad Católica del Perú.
- Núñez, L., and T. D. Dillehay. 1995. Movilidad giratoria, armonía social y desarrollo en los Andes meridionales: Patrones de tráfico e interacción económica. Antofagasta: Universidad Católica del Norte.
- O’Leary, M. H. 1988. “Carbon Isotopes in Photosynthesis.” BioScience 38: 328–336.
- Osorio, D., J. M. Capriles, P. C. Ugalde, K. A. Herrera, M. Sepúlveda, E. M. Gayo, C. Latorre, D. Jackson, R. De Pol-Holz, and C. M. Santoro. 2017. “Hunter-gatherer Mobility Strategies in the High Andes of Northern Chile During the Late Pleistocene-Early Holocene Transition (ca. 11,500–9500 cal B.P.).” Journal of Field Archaeology 42: 228–240.
- Otaola, C., A. Ugan, and A. F. Gil. 2018. “Environmental Diversity and Stable Isotope Variation in Faunas: Implications for Human Diet Reconstruction in Argentine mid-Latitude Deserts.” Journal of Archaeological Science: Reports 20: 57–71.
- Pärssinen, M. 2005. Caquiaviri y la Provincia Pacasa: Desde el Alto – Formativo Hasta la Conquista Española, 1–1533. La Paz: Producciones CIMA.
- Pestle, W. J., C. Torres-Rouff, M. Hubbe, and E. K. Smith. 2017. “Eating out or Dining in: Modeling Diverse Dietary Strategies in the Middle Period, San Pedro de Atacama, Chile.” Archaeological and Anthropological Sciences 9: 1363–1377.
- Pilaar Birch, S. E. 2013. “Stable Isotopes in Zooarchaeology: An Introduction.” Archaeological and Anthropological Sciences 5: 81–83.
- Ponce Sanginés, C. 1970. Las culturas Wankarani y Chiripa y su relación con Tiwanaku. La Paz: Academia Nacional de Ciencias de Bolivia.
- Rigsby, C. A., J. P. Bradbury, P. A. Baker, S. M. Rollins, and M. R. Warren. 2005. “Late Quaternary Palaeolakes, Rivers, and Wetlands on the Bolivian Altiplano and Their Palaeoclimatic Implications.” Journal of Quaternary Science 20: 671–691.
- Saintenoy, T., F. González Estefane, and M. Uribe Rodríguez. 2019. “Desde la perspectiva de la isla: el ordenamiento territorial incaico en la transecta andina Arica-Carangas (18 S).” Latin American Antiquity 30: 393–414.
- Samec, C. T., M. Pirola, H. D. Yacobaccio, and H. O. Panarello. 2020. “Assessing Prehispanic Herding Strategies Through Stable Isotope Analysis: A Case Study from the Dry Puna of Argentina.” Environmental Archaeology 25: 353–364.
- Santana-Sagredo, F., J. Lee-Thorp, R. Schulting, and M. Uribe. 2019. “Mobility in the Atacama Desert, Northern Chile, in the Late Intermediate Period (AD 900–1450): A re-Evaluation Using Stable Isotope Analysis.” Quaternary International 533: 66–77.
- Santana-Sagredo, F., R. J. Schulting, P. Méndez-Quiros, A. Vidal-Elgueta, M. Uribe, R. Loyola, A. Maturana-Fernández, et al. 2021. “‘White Gold’ Guano Fertilizer Drove Agricultural Intensification in the Atacama Desert from AD 1000.” Nature Plants 7: 152–158.
- Schoeninger, M. J., and M. J. DeNiro. 1984. “Nitrogen and Carbon Isotopic Composition of Bone Collagen from Marine and Terrestrial Animals.” Geochimica et Cosmochimica Acta 48: 625–639.
- Schoeninger, M., M. DeNiro, and H. Tauber. 1983. “Stable Nitrogen Isotope Ratios of Bone Collagen Reflect Marine and Terrestrial Components of Prehistoric Human Diet.” Science 220: 1381–1383.
- Schoeninger, M. J., and K. Moore. 1992. “Bone Stable Isotope Studies in Archaeology.” Journal of World Prehistory 6: 247–296.
- Schwarcz, H. P., and H. Nahal. 2021. “Theoretical and Observed C/N Ratios in Human Bone Collagen.” Journal of Archaeological Science 131: 105396.
- Sealy, J., M. Johnson, M. Richards, and O. Nehlich. 2014. “Comparison of Two Methods of Extracting Bone Collagen for Stable Carbon and Nitrogen Isotope Analysis: Comparing Whole Bone Demineralization with Gelatinization and Ultrafiltration.” Journal of Archaeological Science 47: 64–69.
- Somerville, A. D., P. S. Goldstein, S. I. Baitzel, K. L. Bruwelheide, A. C. Dahlstedt, L. Yzurdiaga, S. Raubenheimer, K. J. Knudson, and M. J. Schoeninger. 2015. “Diet and Gender in the Tiwanaku Colonies: Stable Isotope Analysis of Human Bone Collagen and Apatite from Moquegua, Peru.” American Journal of Physical Anthropology 158: 408–422.
- Sullivan, C. H., and H. W. Krueger. 1981. “Carbon Isotope Analysis of Separate Chemical Phases in Modern and Fossil Bone.” Nature 292: 333–335.
- Szpak, P., D. Chicoine, J.-F. Millaire, C. D. White, R. Parry, and F. J. Longstaffe. 2016. “Early Horizon Camelid Management Practices in the Nepeña Valley, North-Central Coast of Peru.” Environmental Archaeology 21: 230–245.
- Szpak, P., J.-F. Millaire, C. Chapdelaine, C. D. White, and F. J. Longstaffe. 2020. “An Integrated Isotopic Study of Early Intermediate Period Camelid Husbandry in the Santa Valley, Perú.” Environmental Archaeology 25: 279–295.
- Szpak, P., J.-F. Millaire, C. D. White, and F. J. Longstaffe. 2012. “Influence of Seabird Guano and Camelid Dung Fertilization on the Nitrogen Isotopic Composition of Field-Grown Maize (Zea mays).” Journal of Archaeological Science 39: 3721–3740.
- Szpak, P., and D. Valenzuela. 2020. “Camelid Husbandry in the Atacama Desert? A Stable Isotope Study of Camelid Bone Collagen and Textiles from the Lluta and Camarones Valleys, Northern Chile.” Plos One 15: e0228332.
- Szpak, P., C. D. White, F. J. Longstaffe, J.-F. Millaire, and V. F. Vásquez Sánchez. 2013. “Carbon and Nitrogen Isotopic Survey of Northern Peruvian Plants: Baselines for Paleodietary and Paleoecological Studies.” Plos One 8: e53763.
- Takenaka, R., M. J. Miller, M. N. Tammone, T. E. Dawson, and E. A. Lacey. 2020. “Stable Isotopes Reveal Differential Patterns of Holocene Environmental Change among Tuco-Tucos (Rodentia: Ctenomyidae, Ctenomys) from Patagonia.” Palaeogeography, Palaeoclimatology, Palaeoecology 540: 109522.
- Takigami, M., K. Uzawa, Y. Seki, D. M. Chocano, and M. Yoneda. 2020. “Isotopic Evidence for Camelid Husbandry During the Formative Period at the Pacopampa Site, Peru.” Environmental Archaeology 25: 262–278.
- Thornton, E. K., S. D. Defrance, J. Krigbaum, and P. R. Williams. 2011. “Isotopic Evidence for Middle Horizon to 16th Century Camelid Herding in the Osmore Valley, Peru.” International Journal of Osteoarchaeology 21: 544–567.
- Tieszen, L. L., and T. Fagre. 1993. “Carbon Isotopic Variability in Modern and Archaeological Maize.” Journal of Archaeological Science 20: 25–25.
- Torres-Rouff, C., K. J. Knudson, W. J. Pestle, and E. M. Stovel. 2015. “Tiwanaku Influence and Social Inequality: A Bioarchaeological, Biogeochemical, and Contextual Analysis of the Larache Cemetery, San Pedro de Atacama, Northern Chile.” American Journal of Physical Anthropology 158: 592–606.
- Torres-Rouff, C., W. J. Pestle, and F. Gallardo. 2012. “Eating Fish in the Driest Desert in the World: Osteological and Biogeochemical Analyses of Human Skeletal Remains from the San Salvador Cemetery, North Chile.” Latin American Antiquity 23: 51–69.
- Tung, T. A., and K. J. Knudson. 2018. “Stable Isotope Analysis of a pre-Hispanic Andean Community: Reconstructing pre-Wari and Wari Era Diets in the Hinterland of the Wari Empire, Peru.” American Journal of Physical Anthropology 165: 149–172.
- van Klinken, G. J. 1999. “Bone Collagen Quality Indicators for Palaeodietary and Radiocarbon Measurements.” Journal of Archaeological Science 26: 687–695.
- Ventresca Miller, A. R., and C. A. Makarewicz. 2017. “Isotopic Approaches to Pastoralism in Prehistory: Diet, Mobility, and Isotopic Reference Sets.” In Isotopic Investigations of Pastoralism in Prehistory, edited by A. R. Ventresca Miller and C. A. Makarewicz, 1–14. London: Routledge.
- Yacobaccio, H. D., M. R. Morales, and C. T. Samec. 2009. “Towards an Isotopic Ecology of Herbivory in the Puna Ecosystem: New Results and Patterns on Lama glama.” International Journal of Osteoarchaeology 19: 144–155.
- Yoder, C., and E. J. Bartelink. 2010. “Effects of Different Sample Preparation Methods on Stable Carbon and Oxygen Isotope Values of Bone Apatite: A Comparison of Two Treatment Protocols.” Archaeometry 52: 115–130.
- Zavodny, E., S. B. McClure, B. J. Culleton, E. Podrug, and D. J. Kennett. 2014. “Neolithic Animal Management Practices and Stable Isotope Studies in the Adriatic.” Environmental Archaeology 19: 184–195.
