ABSTRACT
The current study examined the effects of oxytocin administration on the response to infant crying in individuals with secure or insecure attachment representations as assessed with the Adult Attachment Interview. We measured feelings of irritation and the use of excessive force as indicated by grip strength using a handgrip dynamometer during exposure to infant crying in 42 women without children who were administered intranasal oxytocin or a placebo. In addition, amygdala responses to infant crying and control sounds were measured with functional magnetic resonance imaging (fMRI). The effects of oxytocin on reactivity to crying were moderated by attachment security. Oxytocin decreased the use of excessive handgrip force and amygdala reactivity in response to crying in individuals with insecure attachment representations. Our findings indicate that insecure individuals, who show emotional, behavioral, and neural hyperreactivity to crying, benefit the most from intranasal oxytocin.
Introduction
Infant crying is one of the most salient attachment signals in infancy. It communicates the needs of the child, elicits parental caregiving, and enhances infant survival by conveying information about the health condition of the child (Zeifman, Citation2001). For example, infants who are sick or in pain cry at higher fundamental frequencies than infants who cry for other reasons (Soltis, Citation2004). Infant crying evokes strong emotions in parents, ranging from feelings of empathy to anger, fear, or aversion. For some parents crying is an aversive stimulus (Leerkes, Parade, & Gudmundson, Citation2011) and negative feelings prevent them from responding in a sensitive way to their infant (Leerkes, Citation2010). Excessive infant crying can even trigger child abuse and neglect (Soltis, Citation2004). In the Netherlands, six months after birth nearly 6% of the parents report that they have shaken, smothered, or slapped their infant in order to stop the crying (Reijneveld, Van der Wal, Brugman, Sing, & Verloove-Vanhorick, Citation2004). It is therefore important to examine the mechanisms that are involved in reactions of adults to infant crying and to investigate why some parents respond sensitively to their crying infant, whereas other parents lack the empathic ability to abstain from abusive responses to their crying infant. The current study is the first to examine the combined influence of two important factors affecting responding to crying: the neuropeptide oxytocin and adult state of mind with respect to attachment.
Oxytocin
Previous studies have shown that the neuropeptide oxytocin is involved in explaining individual differences in responding to crying (Riem et al., Citation2011; Riem, Pieper, Out, Bakermans-Kranenburg, & van IJzendoorn, Citation2010).Oxytocin is crucial for mother–infant bonding, the initiation of maternal care, and sensitive responding to infant signals (Galbally, Lewis, van IJzendoorn, & Permezel, Citation2011; Insel, Citation2010). Higher maternal oxytocin levels during pregnancy and the postpartum period are predictive of increased maternal bonding behaviors, such as positive affect, motherese vocalizations, and affectionate touch (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, Citation2010; Feldman, Weller, Zagoory-Sharon, & Levine, Citation2007). Intranasal oxytocin administration experiments have shown that oxytocin stimulates infant-directed gestures in fathers, sensitive play, and reduces hostility and impatience (Naber, van IJzendoorn, Deschamps, Van Engeland, & Bakermans-Kranenburg, Citation2010; Weisman et al., Citation2013). A recent study examined oxytocin effects in depressed mothers and found that oxytocin increased protective responses in the presence of a socially intrusive stranger (Mah, Bakermans-Kranenburg, van IJzendoorn, & Smith, Citation2015). This finding is in line with animal and human models suggesting that oxytocin drives a “tend and defend” response (Carter, Citation1998; De Dreu et al., Citation2010).
Other studies have provided more insight into the neural effects of oxytocin with functional magnetic resonance imaging (fMRI) (for reviews see Rilling, Citation2013; Swain et al., Citation2014) . In a previous fMRI study, we found that intranasally administered oxytocin increases responses to infant crying in neural regions involved in empathy and emotion understanding, the insula and the inferior frontal gyrus (IFG), and at the same time reduces amygdala reactivity to crying (Riem et al., Citation2011). Reduced activation in the amygdala, a neural region involved in fear processing, has been suggested to be one of the mechanisms underlying the stress-reducing and anxiolytic effects of oxytocin (Domes et al., Citation2007; Kirsch et al., Citation2005). Thus, our previous finding might indicate that oxytocin promotes sensitive responding to infant crying by increasing empathic reactions to infants and reducing anxious feelings.
The role of family background and context in shaping oxytocin effects
Oxytocin is involved in a range of social behaviors other than parenting, and has been shown to stimulate trust, empathy, emotion understanding, and in-group altruism (for reviews see Heinrichs, Von Dawans, & Domes, Citation2009; MacDonald & MacDonald, Citation2010) . However, the prosocial effects of oxytocin appear to be dependent on social context (Bakermans-Kranenburg & van IJzendoorn, Citation2013; Bartz, Zaki, Bolger, & Ochsner, Citation2011). For example, intranasal oxytocin enhances in-group trust, but at the same time promotes defensive aggression toward individuals perceived as out-group members (De Dreu et al., Citation2010). Furthermore, trust-enhancing effects of oxytocin are only found when partners are known or believed to be reliable (Declerck, Boone, & Kiyonari, Citation2010; Mikolajczak et al., Citation2010). In a previous study, we examined effects of intranasal oxytocin on neural responses to crying that was indicated as coming from a sick infant and crying as coming from a bored infant (Riem, Voorthuis, Bakermans-Kranenburg, & van IJzendoorn, Citation2014). Although the crying sounds were the same in the two contexts, oxytocin increased insula and IFG responding to crying of a sick infant, but decreased activation in these brain regions during exposure to crying of an infant that was labeled as bored. This might indicate that oxytocin increases the salience of the context of crying, thereby affecting the perception of crying and facilitating flexible responses to the infant’s crying depending on context or meaning.
In addition to contextual factors, individual differences are also known to shape the effects of oxytocin on social behavior. A recent study indicates that effects of oxytocin on ingroup-favoritism are dependent on cognitive style (Ma, Liu, Rand, Heatherton, & Han, Citation2015). Oxytocin increased ingroup-favoritism among participants who were experimentally primed to be intuitive or those who preferred intuition in daily life, whereas it decreased ingroup-favoritism in participants primed to rely on reflective thinking or those who preferred reflective decision-making in daily life. Other studies have shown that the effects of oxytocin on social behavior are only present in individuals with positive child rearing experiences, and absent in individuals with experiences of harsh parenting. For example, in a previous study we found that oxytocin decreases the use of excessive handgrip force measured with a handgrip dynamometer during exposure to infant crying, but only in individuals who experienced sensitive parenting (Bakermans-Kranenburg, van IJzendoorn, Riem, Tops, & Alink, Citation2012). Excessive handgrip force has been interpreted as an analogue of a harsh response and reactive force to negative infant stimuli (Bugental, Lewis, Lin, Lyon, & Kopeikin, Citation1999) and has been observed in parents at risk for child abuse (Crouch, Skowronski, Milner, & Harris, Citation2008). In another study, we found that neural effects of oxytocin during rest were moderated by childhood experiences (Riem et al., Citation2013). During rest, oxytocin increased functional connectivity in complex brain networks involved in self-referential thinking and affectionate touch, but only in individuals who experienced low levels of maternal love withdrawal, a disciplinary strategy that involved withholding love and affection when a child misbehaves or fails at a task.
Interestingly, several studies show that the effects of oxytocin are a function of the baseline socio-emotional abilities of the individual. For example, Bartz et al. (Citation2010) showed that oxytocin increased empathic accuracy especially in less socially proficient individuals. Similarly, positive oxytocin effects on emotion recognition have been found in individuals with high alexithymia, but not in individuals with low alexithymia (Luminet, Grynberg, Ruzette, & Mikolajczak, Citation2011). In addition, a recent study showed that oxytocin normalizes the attentional bias to emotional faces in socially anxious individuals to levels seen in healthy individuals, suggesting stronger effects of oxytocin for individuals who could gain most with respect to socio-emotional functioning (Clark-Elford et al., Citation2014). Considering these findings, an interesting question is whether oxytocin also normalizes the perception of and responding to infant crying in individuals with an insecure attachment representation, that is, individuals who may be hyperreactive to infant crying.
Oxytocin and adult attachment
Since early childhood experiences and adult attachment representation are only modestly related and appear to be distinct constructs (Roisman et al., Citation2007; Schoenmaker et al., Citation2015; Waters, Hamilton, & Weinfield, Citation2000; Weinfield, Sroufe, & Egeland, Citation2000), it is still unclear whether adult attachment also moderates the effects of oxytocin on social behavior. Few studies on the effects of intranasal oxytocin in individuals with different attachment representations have been conducted. In a study using the Adult Attachment Projective Pictures System (AAP), Buchheim et al. (Citation2009) showed that intranasal administration of oxytocin increased secure responses to the AAP. Bartz et al. (Citation2010) tested the effects of oxytocin on recollections of maternal care and closeness as related to self-reported attachment style. They found that less anxiously attached individuals remembered their mother as more caring and close after oxytocin administration but more anxiously attached individuals remembered their mother as less caring and close in the oxytocin condition compared to a placebo condition. In another study, De Dreu (Citation2012) examined effects of intranasal oxytocin on betrayal aversion, cooperation, and trust in individuals with different self-reported attachment styles. Oxytocin reduced betrayal aversion and increased trust especially in individuals showing high attachment avoidance and less in those low in attachment avoidance. However, projective tests and self-report questionnaires on attachment have been shown to have little empirical or conceptual overlap with the Adult Attachment Interview (AAI), the gold standard for the measurement of adult attachment in parents or caregivers (George, Kaplan, & Main, Citation1985; Hesse, Citation2008; Main & Goldwyn, Citation1984).
The AAI is an hour-long semi-structured interview in which participants are asked to describe their childhood attachment experiences with their parents and how they think they were affected by these experiences (Hesse, Citation2008; Main & Goldwyn, Citation1984). Coding of the AAI yield three major attachment classifications: Secure-Autonomous, Insecure-Dismissing, and Insecure-Preoccupied (see Methods for a description of the classifications). Research with the AAI has shown that adult attachment influences parental caregiving and responding to infant crying. Secure parents respond adequately to their crying infants and, as a consequence, more often have infants who are securely attached (Ainsworth, Blehar, Waters, & Wall, Citation1978). Insecure parents, on the other hand, tend to make negative, internal attributions to the nature of the crying (e.g., the child is spoiled or has a difficult temperament) and are less accurate at identifying infant emotions (Leerkes & Siepak, Citation2006). This negative perception of the cry makes it more difficult to respond in a sensitive way (Dykas & Cassidy, Citation2011).
In a previous study, we found that women with insecure attachment representations showed heightened amygdala activation when exposed to infant crying compared to women with secure attachment representations (Riem, Bakermans-Kranenburg, van IJzendoorn, Out, & Rombouts, Citation2012). In addition, insecure women experienced more irritation during infant crying and used more excessive handgrip force measured with the handgrip dynamometer than women with a secure representation. Amygdala hyperreactivity might explain why insecure individuals experience more aversive and anxious feelings during exposure to infant crying and why they respond inadequately to infant signals. Oxytocin might reduce hyperreactivity to crying in insecure individuals because it has dampening effects on the amygdala. However, little research has been conducted on the relation between adult attachment, as measured with the AAI, and oxytocin. Pierrehumbert, Torrisi, Ansermet, Borghini, and Halfon (Citation2012) examined oxytocin responses before, during, and after an experimental psychosocial challenge (the Trier Social Stress Test) in individuals with different adult attachment representations. Although no attachment-related differences in oxytocin responses to stress were found, the overall levels of oxytocin differed between the attachment classifications, with significantly higher oxytocin levels in secure adults compared to insecure adults. Strathearn, Fonagy, Amico, and Montague (Citation2009) investigated brain responses to infant cues and oxytocin levels in mothers varying in adult attachment representations measured with the AAI, coded with the Crittenden system (Crittenden, Citation2004). Oxytocin responses to infant contact were found to be higher in secure mothers compared to mothers with dismissing attachment representations. Moreover, oxytocin levels after infant contact correlated with neural responses to own infant faces, indicating that oxytocin levels might be involved in explaining attachment-related differences in responding to crying.
As mentioned before, research has shown that oxytocin dampens amygdala activation, but it is unknown whether this effect is independent of adult attachment representation. The current study is the first to investigate the effects of intranasal administration of oxytocin in individuals with different attachment representations, measured with the AAI. Similar to our previous study with partly the same sample, we examine amygdala activation, feelings of irritation, and the use of excessive force during exposure to infant crying. Whereas our previous study only included participants from the placebo group, the current study focuses on the influence of oxytocin (compared to placebo) on reactivity to crying in women with different attachment representations. We expected that the effects of oxytocin on emotional, behavioral, and neural reactivity to crying would be moderated by adult attachment representations and that especially insecure individuals would benefit from intranasal oxytocin because they show hyperreactivity to infant crying.
Method
Participants
Participants were selected from a larger study investigating caregiving responses and physiological reactivity to infant crying (Out, Pieper, Bakermans-Kranenburg, & van IJzendoorn, Citation2010). The original sample consisted of 50 male and 134 female adult twin pairs. A group of 45 right-handed women, 22 from MZ twin pairs and 23 from DZ twin pairs, were selected to participate in an fMRI study investigating the influence of oxytocin administration on neural responses to infant signals (see Riem et al., Citation2011). Participants were screened for hearing impairments, MRI contraindications, pregnancy, psychiatric or neurological disorders, alcohol and drug use, and did not have children of their own. At the time of fMRI data acquisition the mean age of the participants was 29.00 years (SD = 7.40, range 22–49). Sixty-nine percent of the participants used oral contraceptives. One participant was excluded from the fMRI analyses due to excessive head movement during fMRI scanning (peak displacement 10 mm). Another participant was excluded from the handgrip force analyses because she failed to perform the dynamometer task. For two other participants neuroimaging and handgrip data were not acquired due to technical problems, resulting in a total sample of 42 participants, 21 who were administered oxytocin and 21 who were administered a placebo. Permission for this study was obtained from the Medical Ethics Committee of the Leiden University Medical Center and all participants gave informed consent.
Procedure
Participants were invited to the lab for two waves of data collection. In the first session, the AAI was administered in a quiet room. In the second session, oxytocin was administered, fMRI data acquisition was performed, and emotional and behavioral responses to infant crying were measured. For the second session, participants were invited preferably in the luteal phase of their menstrual cycle. One sibling from each twin pair was randomly assigned to the oxytocin condition, and the other sibling was assigned to the placebo condition. Participants who participated without twin sibling (n = 7) were also randomly assigned to the oxytocin or placebo condition. We tested the effect of sibling group (participating with a monozygotic twin sibling, participating with a dizygotic twin sibling, participating without a sibling) on brain activation. Sibling group was not significantly associated with change in brain activation, either at whole group level (placebo and oxytocin) or for the difference between the oxytocin and placebo condition (see Riem et al., Citation2011). Approximately 35 min before the start of the fMRI data acquisition, subjects took six puffs of nasal spray containing oxytocin (16 IU total) or six puffs of a placebo spray under supervision of the experimenter. Drug administration was double-blind. Afterwards, the fMRI procedure was explained and participants were instructed to comfortably position themselves on the scanner bed. Cushions were placed between the head coil and the participant in order to prevent head movement. Participants were instructed to attend to the sounds they would hear in the fMRI scanner. After fMRI scanning participants rated how much irritation they felt while listening to the crying sounds, and the handgrip-force task was administered.
Measures
Adult Attachment Interview
Ratings and classifications of adult attachment representations were derived from the AAI (Main, Kaplan, & Cassidy, Citation1985), which was conducted during a lab session (see Riem et al., Citation2012). The AAI is considered to be the gold standard for assessing attachment representations (Hesse, Citation2008). The AAI is an hour-long, semi-structured interview which assesses an individual’s current state of mind with respect to attachment. Participants are asked about their childhood attachment experiences with their parents and how they think they were affected by these experiences, as well as about the current relationship with their parents. It is the coherence of discourse rather than the content of the autobiographical account that determines their attachment classification (see Hesse, Citation2008, for a detailed description of the assessment). Coding of the AAI yields one of three main adult attachment classifications: Secure-Autonomous (F), Insecure-Dismissing (Ds), and Insecure-Preoccupied (E) . Adults with the F classification tend to value attachment relationships, to describe their attachment experiences (whether positive or negative) coherently, and to consider them important in the development of their personality. Adults with the Ds classification tend to idealize their childhood experiences without being able to provide concrete illustrations, or tend to minimize the importance of attachment in their own lives. Adults with the E classification tend to emphasize the impact, often negative, of their attachment experiences. They are still very much involved and preoccupied with these experiences. An additional classification, unresolved (U), is assigned when an interview shows signs of unresolved trauma or loss.
Interviews were audio-recorded, transcribed verbatim, and scored according to the standard AAI classification system (Main et al., 2008). The interviews were anonymously assigned and coded blindly by four raters who were trained to be reliable to the coding standards of the Berkeley laboratory of Mary Main and Erik Hesse. Scores for coherence of mind and unresolved trauma were are assigned using a nine-point rating scale (Hesse, Citation2008). Mean score for coherence of mind in the current sample was 4.76 (SD = 2.07). In the placebo group, seven participants were classified as secure, four participants as dismissing, four participants as preoccupied, and six participants as unresolved. Subjects were reclassified as either secure (autonomous) or insecure (dismissing, preoccupied, unresolved), resulting in a group of 14 insecure participants and a group of seven secure participants in the placebo group. In addition, participants were reclassified as unresolved or not unresolved, resulting in a group of six participants with an unresolved state of mind and 15 participants without an unresolved state of mind in the placebo group. In the oxytocin group, 14 participants were classified as secure, three participant as dismissing, four participants as preoccupied, and three participants as unresolved. Subjects were reclassified as either secure (autonomous) or insecure (dismissing, preoccupied, unresolved) and as unresolved or not unresolved, resulting in a group of 10 insecure participants and a group of 14 secure participants, and a group of three participants with an unresolved state of mind and 21 participants without an unresolved state of mind. For one participant it was not possible to assign a coherence of mind score because some AAI questions were missing due to problems with audio-recording. The missing value of this participant, who was classified as secure by two independent expert raters, was replaced by the mean coherence of mind score of individuals with a secure classification.
Harsh discipline
Participants completed an 18-item questionnaire on experiences of parental harsh discipline (see Bakermans-Kranenburg et al., Citation2012) that was based on the Parent–Child Conflict Tactics Scales (Straus, Hamby, Finkelhor, Moore, & Runyan, Citation1998). Items included experiences such as being spanked on the bottom, being kicked hard or hit with a fist, and threats to be sent away or kicked out of the house. Items were scored on a rating scale ranging from 1 = (almost) never to 5 = (almost) always. Participants received the harsh discipline questionnaire after the second lab session, completed it at home, and returned it by mail within two weeks of the experiment.
Emotional and behavioral responses to infant crying
After fMRI scanning the participants were asked to report whether they felt irritated while listening to the crying sounds on a 5-point Likert scale (1 = not irritated, 5 = irritated). In addition, an adult handgrip dynamometer was used as an indicator of the use of excessive force during listening to infant crying. The dynamometer (model TSD121 C) weighed 315 g and was 185 mm long, 42 mm wide, and 30 mm thick, with an isometric range from 0 to 100 kg. Squeeze intensities (in kg) were transferred directly from the dynamometer to the AcqKnowledge software program (version 3.8; Biopac Systems, 2004). Matlab (version 7.8.0, Mathworks, MA, USA) was used to identify peak intensities for each squeeze. Participants were asked to squeeze the handgrip dynamometer as hard as possible and then at 50% of their maximal handgrip strength. They performed as many trials as necessary for training, with their performance displayed on a monitor to check the 50% level of each second handgrip, until they were able to modulate the force of their second squeeze to half the strength of their first squeeze. Then the monitor was directed away from the participant in order to prevent them from receiving feedback regarding their performance during the remainder of the task.
The handgrip-force task was administered on a laptop using E-Prime software (version 2.0; Psychology Software Tools, Inc., PA, USA). During the task participants were seated in front of a computer screen wearing headphones (type König CMP). As a prompt, the words “squeeze maximally” were displayed briefly in the middle of the screen, after 2 s followed by the prompt “squeeze at half strength”, thus prompting the participants to perform a brief firm squeeze followed by a brief squeeze half the strength. After baseline squeezing (no sound), participants were requested to squeeze the handgrip dynamometer eight times at full and half strength, respectively, the first four times listening to infant laughter and then four times listening to infant crying. The infant laughter sound (duration = 2 min, average fundamental frequency = 215.96 Hz, constant volume) and the infant crying sound (duration = 2 min, average fundamental frequency = 360.06, constant volume) from Groh and Roisman (Citation2009) were used. The intervening time between full- and half-strength prompts was 2 s; the intervening time period between half-strength and the next full-strength prompt was 25 s. Similar to studies on the same and other samples (Bakermans-Kranenburg et al., Citation2012; Compier-de Block et al., Citation2015; Riem et al., Citation2012), grip strength modulation was calculated by dividing the half-strength squeeze intensity by the full-strength squeeze intensity, so that scores of over 0.50 indicated excessive force on the half-strength squeeze attempt. As a result of fatigue, the last trial yielded too many missing data. Therefore we decided to use the first three trials during infant crying, for which we added the numbers of trials with too much physical force (> 0.50).
Neural responses to infant crying
Blood oxygenation-level dependent responses to infant crying were measured with fMRI. Participants were instructed to attend to the sounds they would hear and they listened to the sounds through MRI compatible headphones. Cry sounds were derived from the spontaneous crying of a healthy two-day-old infant. A 10 s portion of the sustained period of crying was selected. The peak fundamental frequencies (Peak F0) of the entire cry were 515 ± 15 Hz. Two new 10 s cry sounds with overall Peak F0 of 714.5 Hz (700 Hz cry) and 895.8 Hz (900 Hz cry) were created by digitally increasing the pitch of the original cry (Dessureau, Kurowski, & Thompson, Citation1998; Out, Pieper, Bakermans-Kranenburg, Zeskind, & van IJzendoorn, Citation2010; Schuetze & Zeskind, Citation2001; Schuetze, Zeskind, & Das Eiden, Citation2003). We focused on neural responses to infant crying at different frequencies, because infant cries range from 500 Hz in normal, healthy infants to 900 Hz (and even higher) in infants in pain or with medical and neurological conditions (Soltis, Citation2004). We did not expect to find differences in brain activation between the frequency conditions, as there was no significant effect of frequency on neural responses to infant crying in a previous study on the same sample (Riem et al., Citation2011). Neutral auditory control stimuli were created identical to the original auditory stimuli in terms of duration, intensity, spectral content, and amplitude envelope but lacking an emotional meaning. Cry and control sounds were presented in eight cycles, each cycle consisting of six sounds (Cry 500 Hz, Cry 700 Hz, Cry 900 Hz, Control 500 Hz, Control 700 Hz, Control 900 Hz). The order of presentation of sounds within each cycle was random; the intertrial interval was 6 s. Cry sounds were collapsed across pitches to reduce the number of statistical tests.
FMRI data acquisition
Scanning was performed with a standard whole-head coil on a 3-T Philips Achieva MRI system (Philips Medical Systems, Best, the Netherlands) in the Leiden University Medical Center. Cushions were placed between the head coil and the participant in order to prevent head movement. First, a T1-weighted anatomical scan was acquired (flip angle = 8°, 140 slices, voxelsize .875 × .875 × 1.2 mm). For fMRI, a total of 360 T2*-weighted whole-brain EPIs were acquired (TR = 2.2 s; TE = 30 ms, flip angle = 80°, 38 transverse slices, voxelsize 2.75 × 2.75 × 2.75 mm (+10% interslice gap)). In accordance with Leiden University Medical Center policy, all anatomical scans were examined by a radiologist from the Radiology department. No anomalous findings were reported.
Data analysis
fMRI data analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB’s Software Library, www.FMRIb.ox.ac.uk/fsl; Smith et al., Citation2004). The following pre-statistics processing was applied: motion correction (Jenkinson, Bannister, Brady, & Smith, Citation2002), non-brain removal (Smith, Citation2002), spatial smoothing using a Gaussian kernel of full-width-at-half-maximum 5.0 mm, and high-pass temporal filtering (highpass filter cutoff = 50.0 s). Functional scans were registered to T1-weighted images, which were registered to standard space (Jenkinson et al., Citation2002; Jenkinson & Smith, Citation2001).
In native space, functional activation was examined using general linear model analysis. Each sound (Cry 500 Hz, 700 Hz, 900 Hz, and Control 500 Hz, 700 Hz, 900 Hz) was modeled separately as a square-wave function. Each predictor was then convolved with a double gamma hemodynamic response function and its temporal derivative was added to the model, giving 12 predictors. The contrast Cry combined 500, 700, 900 Hz > Control combined 500, 700, 900 Hz was assessed. A right amygdala region of interest was defined by identifying voxels within the anatomical confines of the amygdala (Harvard–Oxford subcortical atlas, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) that were significantly active in the placebo group for the contrast Cry versus Control (see Riem et al., Citation2011). The first-level contrast images and the corresponding variance images were transformed to standard space and submitted to second-level mixed-effects group region of interest analyses with the right amygdala to examine amygdala activation in the four subgroups: secure individuals in the placebo and oxytocin group and insecure individuals in the placebo and oxytocin group. Use of oral contraceptives and menstrual cycle (centered) were included as confound regressors in the model. The statistical images were thresholded using clusters determined by Z > 2.3 and a cluster corrected significance threshold of p < .05 (Worsley, Citation2001) . Mean Z values for the right amygdala were derived using Featquery (FMRIb.ox.ac.uk/fsl/feat5/featquery.html) and imported into SPSS.
Univariate analyses of variance were performed to examine the combined effects of AAI security and oxytocin administration on neural, emotional, and behavioral responding to crying. Age, menstrual cycle, and use of oral contraceptives were included as covariates in the analyses. In addition, hierarchical regression analyses were conducted to examine the combined effects on AAI coherence and oxytocin administration on neural, emotional, and behavioral responding to crying, with age, menstrual cycle, and use of oral contraceptives in the first step, condition (oxytocin versus placebo) and coherence in the second step, and the interaction between condition (centered) and coherence (centered) in the third step.
Results
Emotional and behavioral data
Irritation
A univariate analysis of variance was conducted to examine the effects of oxytocin administration and adult attachment representation on reported irritation during exposure to infant crying. Oxytocin administration did not significantly affect reported irritation (F(1,34) = 3.46, p = .07, partial ŋ2 = .09). However, there was a significant effect of AAI security (F(1,34) = 5.70, p = .02, partial ŋ2 = .14). Individuals with an insecure attachment representation reported higher levels of irritation (M = 3.98, SD = 0.20) compared to individuals with a secure representation (M = 3.28, SD = 0.21) (see Riem et al., Citation2012). There was no significant interaction between oxytocin administration and attachment security (F(1,34) = 1.66, p = .21, partial ŋ2 = .05). Since our previous study showed that harsh discipline moderated the effects of oxytocin on responding to crying, harsh discipline was included as an additional covariate in the analyses. There was no significant effect of harsh discipline (F(1,31) = 0.17, p = .69, partial ŋ2 = .01), nor a significant interaction between oxytocin and harsh discipline (F(1,31) = 0.29, p = .60, partial ŋ2 = .01). The interaction between oxytocin and attachment security did not remain significant when harsh discipline was included in the analysis (F(1,31) = 2.02, p = .17, partial ŋ2 = .06). The four-way attachment classification (F, Ds, E, U) did not have a significant effect on reported irritation (F(3,30) = 2.34, p = .09, partial ŋ2 = .09) and there was no significant interaction between four-way classification and oxytocin administration (F(3,30) = 2.00, p = .14, partial ŋ2 = .17). Neither was there a significant difference in reported irritation between individuals with an unresolved state of mind versus without an unresolved state of mind (F(1,34) = 0.94, p = .34, partial ŋ2 = .03) or a significant interaction between unresolved status and oxytocin administration (F(1,34) = 0.30, p = .59, partial ŋ2 = .01). Furthermore, the hierarchical regression analysis did not show a significant interaction between coherence of mind and oxytocin administration (ß = −.11, p = 0.51).
Handgrip force
A univariate analysis of variance was conducted to examine effects of oxytocin administration and adult attachment representation on excessive handgrip force during exposure to infant crying. The covariates age, menstrual cycle, and use of oral contraceptives were excluded from the analyses because they did not significantly affect handgrip force (ps > .29). There were no significant effects of oxytocin administration (F(1,38) = 2.60, p = .12, partial ŋ2 = .06) or AAI security (F(1,38) = 1.48, p = .23, partial ŋ2 = .04) on the use of excessive handgrip force. However, there was a marginally significant interaction between oxytocin administration and security (F(1,38) = 3.65, p = .06, partial ŋ2 = .09). Oxytocin reduced the use of excessive force but only for individuals with an insecure attachment representation (see ). Planned contrasts (insecure participants in the placebo group versus all other participants) showed that insecure individuals in the placebo group more often used excessive force compared to insecure individuals in the oxytocin group or secure individuals in the oxytocin or placebo group (t(38) = −6.13, p < .001). The four-way attachment classification did not have a significant effect on handgrip force (F(3,34) = 0.62, p = .61, partial ŋ2 = .05) and there was no significant interaction between four-way classification and oxytocin administration (F(3,34) = 1.39, p = .26, partial ŋ2 = .11). Furthermore, there was no significant difference in handgrip force between individuals with versus without an unresolved state of mind (F(1,38) = 0.03, p = .86, partial ŋ2 = .00) and no significant interaction between unresolved status and oxytocin administration (F(1,38) = 0.24, p = .63, partial ŋ2 = .01). Neither was there a significant interaction between coherence of mind and oxytocin administration in the hierarchical regression analysis (ß = .10, p = 0.52).
Figure 1. (A) Excessive handgrip force (M, SE) during exposure to infant crying for individuals with secure and insecure attachment representations in the placebo and oxytocin group. Oxytocin reduces the use of excessive force only for individuals with an insecure attachment representation. * p < .05. (B) (for illustrative purposes) Excessive handgrip force (M, SE) during exposure to infant crying for individuals with a secure (F, OT: n = 12, Pla: n = 7), dismissing (Ds, OT: n = 3, Pla: n = 4), preoccupied (E, OT: n = 3, Pla: n = 4), and unresolved (U, OT: n = 3, Pla: n = 6) classification in the placebo and oxytocin group. * p < .05.
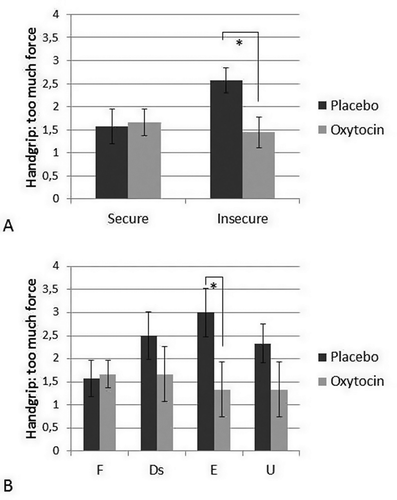
Since in a previous study on the same sample we found that oxytocin effects on handgrip force were more pronounced in individuals reporting little harsh discipline in their childhood, the correlation between experiences of harsh discipline and coherence of mind and the differences in experienced harsh discipline between secure and insecure individuals were examined. Secure and insecure individuals did not differ on experienced harsh discipline (t(42) = 0.77, p = .45) and the correlation between coherence and harsh discipline was not significant (r = −.12, p = .45), indicating that self-reported experiences of harsh discipline and adult attachment representation are unrelated. After including harsh discipline and the interaction between oxytocin and harsh discipline as a covariate in the analysis, the interaction between oxytocin administration and security remained significant (F(1,36) = 4.37, p = .04, partial ŋ2 = .11). Harsh discipline did not affect handgrip force (F(1,36) = 1.16, p = .29, partial ŋ2 = .03) and the interaction between harsh discipline and oxytocin was not significant either (F(1,36) = 3.28, p = .08, partial ŋ2 = .08).
Neuroimaging data
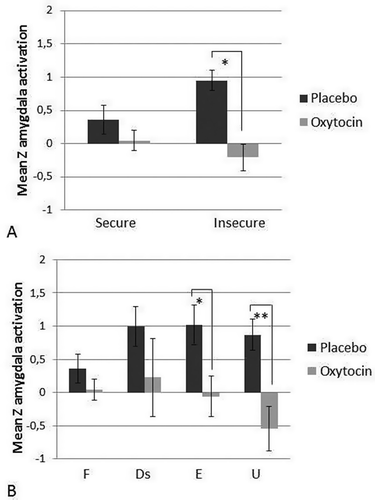
The contrast of infant crying (500 Hz, 700 Hz, 900 Hz) versus control sounds (500 Hz, 700 Hz, 900 Hz) revealed significant activation of the right amygdala in insecure and secure individuals in the placebo group (placebo secure: cluster size = 11 voxels, peak Z = 2.63, MNI coordinates x,y,z (mm) = 24, −8, −14, placebo insecure: cluster size = 96 voxels, peak Z = 3.99, MNI coordinates x,y,z (mm) = 20, −4, −26, see ). There was no significant amygdala activation in secure or insecure individuals in the oxytocin group. A univariate analysis of variance was performed to examine the effect of adult attachment representation and oxytocin administration on mean Z-values extracted from the right amygdala. Menstrual cycle and use of oral contraceptives were included as covariates in the model, but age was excluded because it was not significant (p = .54). There was no significant main effect of adult attachment security (F(1,36) = 0.86, p = .36, partial ŋ2 = .02). However, oxytocin administration significantly decreased amygdala activation (F(1,36) = 15.92, p < .001, partial ŋ2 = .31) (see Riem et al., Citation2011). Moreover, the interaction between attachment security and oxytocin administration was significant (F(1,36) = 5.12, p = .03, partial ŋ2 = .12). Oxytocin reduced amygdala activation only in individuals with an insecure attachment representation (see ). After including harsh discipline and the interaction between harsh discipline and oxytocin as an additional covariate in the analysis, the interaction between oxytocin administration and security was still significant (F(1,33) = 6.60, p = .02, partial ŋ2 = .17). Harsh discipline did not significantly affect amygdala activation (F(1,33) = 0.42, p = .52, partial ŋ2 = .01) and there was no significant interaction between oxytocin and harsh discipline (F(1,33) = 0.29, p = .60, partial ŋ2 = .01). Hierarchical regression analysis also showed a significant interaction between coherence of mind and oxytocin administration (ß = .30, p = 0.02). Oxytocin effects were more pronounced in individuals showing low coherence (median split, Oxytocin: M = −.15, SD = .73, Placebo: M = .89, SD = .50, Cohen’s d = −1.66) and less pronounced in individuals showing high coherence (Oxytocin: M = .12, SD = .64, Placebo: M = .41, SD = .69, Cohen’s d = −0.44) . However, the effect of four-way attachment classification on amygdala activation was not significant (F(3,32) = 0.89, p = .46, partial ŋ2 = .08) and there was no significant interaction between four-way attachment classification and oxytocin administration (F(3,32) = 1.86, p = .16, partial ŋ2 = .15). Furthermore, individuals with an unresolved state of mind did not have higher amygdala activation compared to individuals without an unresolved state of mind (F(1,36) = 0.88, p = .35, partial ŋ2 = .02) and the interaction between unresolved status and oxytocin administration was not significant (F(1,36) = 2.58, p = .12, partial ŋ2 = .07).
Figure 2. Right amygdala activation during infant crying (500, 700, 900 Hz) compared with control sounds (500, 700, 900 Hz) for individuals with secure and insecure attachment representations in the placebo condition. No significant amygdala activation was found in secure or insecure individuals in the oxytocin group.
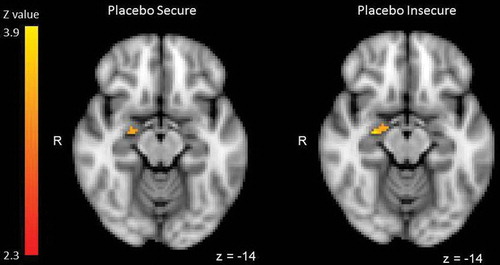
Figure 3. (A) Z-values (M, SE) of right amygdala activation during infant crying compared with control sounds for individuals with secure and insecure attachment representations in the placebo and oxytocin group. Oxytocin reduces amygdala activation, but this effect is most pronounced in individuals with an insecure attachment representation. * p < .05. (B) (for illustrative purposes) Z-values (M, SE) of right amygdala activation during infant crying compared with control sounds for individuals with a secure (F, OT: n = 13, Pla: n = 7), dismissing (Ds, OT: n = 1, Pla: n = 4), preoccupied (E, OT: n = 4, Pla: n = 4), and unresolved (U, OT: n = 3, Pla: n = 6) classification in the placebo and oxytocin group. * p < .05 ** p < .01.
Discussion
The current study is the first to investigate the effects of intranasal administration of oxytocin in individuals with different attachment representations, measured with the AAI. We examined oxytocin effects on amygdala activation, feelings of irritation, and the use of excessive force during exposure to infant crying in women with different attachment representations. We found that the effects of oxytocin on reactivity to crying were moderated by attachment security. Oxytocin decreased the use of excessive handgrip force and amygdala reactivity in response to crying in individuals with an insecure attachment representation. Oxytocin effects on feelings of irritation were not moderated by attachment security. Our findings indicate that insecure individuals benefit the most from intranasal oxytocin because in the placebo condition they show emotional, behavioral, and neural hyperreactivity to crying. This is consistent with previous studies showing that the influence of oxytocin is not the same for all people under all circumstances and that prosocial oxytocin effects are shaped by individual characteristics (Bakermans-Kranenburg & van IJzendoorn, Citation2013; Bartz et al., Citation2011).
In our previous study we found that individuals with an insecure attachment representation showed amygdala hyperreactivity during exposure to infant crying compared with individuals with a secure attachment representation (Riem et al., Citation2012). In addition, insecure individuals tended to experience more irritation during the perception of infant crying and they used more excessive force squeezing the handgrip. Since the amygdala is a brain region important for the processing of emotional arousal, fear, and aversion (LeDoux, Citation2000), amygdala hyperactivity might be involved in insecure individuals’ more aversive and angry feelings during exposure to infant crying. These negative emotions undermine sensitive child-oriented parental responses such as soothing the child and increase the risk of using harsh caregiving responses to stop the crying (Leerkes, Citation2010; Reijneveld et al., Citation2004). Oxytocin might lower negative emotions and the experience of stress during exposure to crying and this seems to be most beneficial for individuals with insecure attachment representations.
The finding that insecure individuals benefit the most from oxytocin might indicate that the effects of oxytocin are a function of the baseline reactivity to infant signals. Insecure individuals tend to process infant crying or other attachment-related information in a defensive and negatively biased manner (Dykas & Cassidy, Citation2011). The finding that insecure individuals are hyperreactive to crying may be the reason that it is especially this group who benefits from intranasal oxytocin. This is in line with De Dreu’s (Citation2012) study showing that oxytocin reduces betrayal aversion and increases trust in individuals with different attachment styles as measured with a self-report questionnaire, and it is also consistent with studies showing that oxytocin effects are a function of the baseline socio-emotional information processing of the individual (Bartz et al., Citation2010; Luminet et al., Citation2011). Oxytocin normalizes responding to infant crying in insecure individuals, showing amygdala hyperreactivity to crying, but appears to have a saturation point in that it has no effects in secure individuals showing low amygdala reactivity to crying. Thus, oxytocin may have a momentary “state-like” influence, changing neural and behavioral responses of individuals with insecure attachment representations temporarily into a secure direction. Such a security-enhancing influence of oxytocin was also observed by Buchheim et al. (Citation2009) using the AAP.
Our finding that oxytocin reduces hyperreactivity to crying in insecure individuals appears to be in contrast with studies that found lowered or absent positive effects of intranasal oxytocin in individuals with negative caregiving experiences (Bakermans-Kranenburg & van IJzendoorn, Citation2013). For example, oxytocin increased prosocial helping behavior toward an experimenter who was socially excluded during a virtual ball tossing game, but only in individuals reporting low levels of maternal love withdrawal (Riem, Bakermans-Kranenburg, Huffmeijer, & van IJzendoorn, Citation2013). Similarly, in a previous study with the same sample as the present study we found that oxytocin reduced the use of excessive force during exposure to infant crying in individuals who experienced little harsh discipline in their childhood (Bakermans-Kranenburg et al., Citation2012). Moreover, recent neuro-imaging studies found that early life stress modulates the effects of oxytocin on amygdala reactivity and connectivity during exposure to psychosocial stress (Fan et al., Citation2015; Grimm et al., Citation2014). The stress-reducing effects of oxytocin (Kirsch et al., Citation2005; Petrovic, Kalisch, Singer, & Dolan, Citation2008; Riem et al., Citation2011) were not found in individuals with experiences of early life stress (Fan et al., Citation2015; Grimm et al., Citation2014; Meinlschmidt & Heim, Citation2007), indicating that these individuals may have altered sensitivity to intranasal oxytocin.
One explanation for the inconsistence between the present findings and previous studies reporting lower effects of oxytocin in individuals with negative childhood experiences is that self-reported early parent–infant interactions do not determine adult attachment representations in a one-to-one manner (Schoenmaker et al., Citation2015; Waters et al., Citation2000). Experiences in subsequent social relationships appear to influence how individuals represent past and present attachment experiences. Indeed, in the present study experiences of harsh discipline and adult attachment security were unrelated. Previous research has shown that both adult attachment and childhood experiences are separate predictors of reactivity to infant distress. For example, abused mothers show increased physiological reactivity to infant crying (Casanova, Domanic, McCanne, & Milner, Citation1994). However, Leerkes and Siepak (Citation2006) showed that adult attachment security buffers the effects of negative childhood experiences on reactivity to infant distress. Mothers with experiences of emotional rejection were more likely to make negative attributions about a distressed infant and were more likely to be amused or neutral in response to infant distress than others, but only when they had an insecure attachment representation. Thus, parents with problematic childhood experiences who come to terms with their history because of positive experiences in subsequent relationships (labeled as “earned-secure” although the possibility of reliable assessment of earned security must be doubted, see Roisman, Haltigan, Haydon, & Booth‐LaForce, Citation2014 and Van IJzendoorn & Bakermans-Kranenburg, Citation2014) do not have a negative perception of infant distress and are able to respond in a sensitive way to infant crying (Egeland, Jacobvitz, & Sroufe, Citation1988; Leerkes & Siepak, Citation2006; Leon, Jacobvitz, & Hazen, Citation2004). In the present study, individuals with harsh caregiving experiences did not show amygdala hyperreactivity to crying, possibly because of the buffering effect of a secure representation in part of them. Therefore, the effects of oxytocin might be less pronounced for this group.
Some limitations should be noted. First, conclusions regarding the effects of oxytocin for insecure subclassifications could not be drawn because of the small sample size, which made it necessary to combine the insecure classifications in the analyses. There were only nine individuals classified as unresolved. Because a small sample may increase the risk for false positive results (David et al., Citation2013; Ioannidis, Munafo, Fusar-Poli, Nosek, & David, Citation2014), oxytocin effects for individuals with an unresolved status were not interpreted. Future studies with larger samples are needed for replication and to examine oxytocin effects in individuals with different insecure attachment representations or with an unresolved status. Second, we examined women without children to increase the comparability of our participants in terms of their experience with infant crying. As a consequence, our findings can not be generalized to mothers or parents in general. Previous research has shown that oxytocin increases parental sensitive responding to infant signals (Naber, Poslawsky, van IJzendoorn, Van Engeland, & Bakermans-Kranenburg, Citation2012; Naber et al., Citation2010). Future studies should examine whether this effect is indeed dependent on attachment representation and whether oxytocin effects on parental caregiving are most beneficial for insecure parents. Furthermore, we did not examine effects of adult attachment representations and oxytocin on other brain regions. In our previous study we found that the relation between attachment representation and emotional or behavioral responses to infant crying was not mediated by amygdala activation (Riem et al., Citation2012). This indicates that feelings of irritation and the use of excessive force in response to infant crying in insecure individuals can not be solely explained by a hyperactive amygdala and that other brain regions might be involved in attachment-related influences on the perception of infant crying. Future studies should examine the role of other brain regions as well as connectivity between regions in the differential effects of oxytocin in individuals with secure and insecure attachment representations. Furthermore, the findings of the present study can not be easily related to previous studies examining combined effects of oxytocin and adult attachment using the AAP and self-report questionnaires because these measures are unrelated to AAI classifications. Greater consistency in the way attachment is measured would improve our understanding of oxytocin effects in secure and insecure individuals. Lastly, in the current study, harsh parenting experiences were measured with a retrospective self-report questionnaire. Longitudinal studies or studies using certified child abuse data might shed more light on the differential effects of harsh caregiving experiences versus insecure adult attachment on neural reactivity to crying.
In conclusion, the current study is the first to examine the effects of oxytocin administration on the response to infant crying in individuals with different attachment representations, measured with the AAI. Although previous research has shown that adult attachment and oxytocin are two important factors influencing parental caregiving (Riem et al., Citation2011, Citation2012), it was as yet unknown whether oxytocin and adult attachment are two independent factors or whether they are interrelated. Somewhat in contrast to studies reporting lowered oxytocin effects in individuals with harsh caregiving experiences, we found that oxytocin is most beneficial for insecure individuals. Oxytocin decreased the use of excessive handgrip force and amygdala reactivity in response to crying, but only for individuals with an insecure attachment representation. Thus, oxytocin seems to have a security-enhancing influence on the perception of crying, changing neural and behavioral responses of individuals with insecure attachment representations into the direction of responses of secure individuals. This is consistent with our previous finding that oxytocin has a momentary positive influence on the salience of the context of infant crying (Riem et al., Citation2014), thereby making individuals more aware of the reason why an infant cries and facilitating flexible responses to the infant’s crying depending on meaning or context. In addition, our findings are in line with previous research showing that early caregiving experiences and adult attachment are unrelated (Roisman et al., Citation2007; Schoenmaker et al., Citation2015; Waters et al., Citation2000), and indicates that childhood experiences and adult attachment are two different moderating factors with differential influences on the prosocial effects of oxytocin.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ainsworth, M.D., Blehar, M., Waters, E., & Wall, S. (1978). Patterns of attachment. Hillsdale, NJ: Erlbaum.
- Bakermans-Kranenburg, M.J., & van IJzendoorn, M.H. (2013). Sniffing around oxytocin: Review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational Psychiatry, 3, e258. doi:10.1038/tp.2013.34
- Bakermans-Kranenburg, M.J., van IJzendoorn, M.H., Riem, M.M.E., Tops, M., & Alink, L.R. (2012). Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Social Cognitive and Affective Neuroscience, 7(8), 951–957. doi:10.1093/scan/nsr067
- Bartz, J.A., Zaki, J., Bolger, N., & Ochsner, K.N. (2011). Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences, 15(7), 301–309.
- Bartz, J.A., Zaki, J., Ochsner, K.N., Bolger, N., Kolevzon, A., Ludwig, N., & Lydon, J.E. (2010). Effects of oxytocin on recollections of maternal care and closeness. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21371–21375. doi:10.1073/pnas.1012669107
- Buchheim, A., Heinrichs, M., George, C., Pokorny, D., Koops, E., Henningsen, P., … Gündel, H. (2009). Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology, 34(9), 1417–1422. doi:10.1016/j.psyneuen.2009.04.002
- Bugental, D.B., Lewis, J.C., Lin, E., Lyon, J., & Kopeikin, H. (1999). In charge but not in control: The management of teaching relationships by adults with low perceived power. Developmental Psychology, 35(6), 1367–1378. doi:10.1037/0012-1649.35.6.1367
- Carter, C.S. (1998). Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology, 23(8), 779–818. doi:10.1016/S0306-4530(98)00055-9
- Casanova, G.M., Domanic, J., McCanne, T.R., & Milner, J.S. (1994). Physiological responses to child stimuli in mothers with and without a childhood history of physical abuse. Child Abuse & Neglect, 18(12), 995–1004. doi:10.1016/0145-2134(94)90124-4
- Clark-Elford, R., Nathan, P.J., Auyeung, B., Mogg, K., Bradley, B.P., Sule, A., … Baron-Cohen, S. (2014). Effects of oxytocin on attention to emotional faces in healthy volunteers and highly socially anxious males. International Journal of Neuropsychopharmacology, 18, pyu012.
- Compier-de Block, L.H.C.G., Alink, L.R.A., Reijman, S., Werner, C.D., Maras, A., Rijnberk, C., … Bakermans-Kranenburg, M.J. (2015). Handgrip force of maltreating mothers in reaction to infant signals. Child Abuse & Neglect, 40, 124–131. doi:10.1016/j.chiabu.2014.03.006
- Crittenden, P.M. (2004). Patterns of attachment in adulthood: A dynamic-maturational approach to analyzing. The Adult Attachment Interview. Unpublished manuscript.
- Crouch, J.L., Skowronski, J.J., Milner, J.S., & Harris, B. (2008). Parental responses to infant crying: The influence of child physical abuse risk and hostile priming. Child Abuse & Neglect, 32(7), 702–710. doi:10.1016/j.chiabu.2007.11.002
- David, S.P., Ware, J.J., Chu, I.M., Loftus, P.D., Fusar-Poli, P., Radua, J., … Kilner, J. (2013). Potential reporting bias in fMRI studies of the brain. PLoS One, 8(7), e70104. doi:10.1371/journal.pone.0070104
- De Dreu, C.K.W. (2012). Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology, 37(7), 871–880. doi:10.1016/j.psyneuen.2011.10.003
- De Dreu, C.K.W., Greer, L.L., Handgraaf, M.J., Shalvi, S., Van Kleef, G.A., Baas, M., … Feith, S.W.W. (2010). The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science, 328(5984), 1408–1411. doi:10.1126/science.1189047
- Declerck, C.H., Boone, C., & Kiyonari, T. (2010). Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Hormones and Behavior, 57(3), 368–374. doi:10.1016/j.yhbeh.2010.01.006
- Dessureau, B.K., Kurowski, C.O., & Thompson, N.S. (1998). A reassessment of the role of pitch and duration in adults’ responses to infant crying. Infant Behavior & Development, 21(2), 367–371. doi:10.1016/S0163-6383(98)90013-3
- Domes, G., Heinrichs, M., Gläscher, J., Büchel, C., Braus, D.F., & Herpertz, S.C. (2007). Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry, 62(10), 1187–1190. doi:10.1016/j.biopsych.2007.03.025
- Dykas, M.J., & Cassidy, J. (2011). Attachment and the processing of social information across the life span: Theory and evidence. Psychological Bulletin, 137(1), 19–46. doi:10.1037/a0021367
- Egeland, B., Jacobvitz, D., & Sroufe, A.L. (1988). Breaking the cycle of abuse. Child Development, 59, 1080–1088. doi:10.2307/1130274
- Fan, Y., Pestke, K., Feeser, M., Aust, S., Pruessner, J.C., Böker, H., … Grimm, S. (2015). Amygdala-hippocampal connectivity changes during acute psychosocial stress: Joint effect of early life stress and oxytocin. Neuropsychopharmacology, 40, 2736–2744. doi:10.1038/npp.2015.123
- Feldman, R., Gordon, I., Schneiderman, I., Weisman, O., & Zagoory-Sharon, O. (2010). Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology, 35(8), 1133–1141. doi:10.1016/j.psyneuen.2010.01.013
- Feldman, R., Weller, A., Zagoory-Sharon, O., & Levine, A. (2007). Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science, 18(11), 965–970. doi:10.1111/j.1467-9280.2007.02010.x
- Galbally, M., Lewis, A.J., van IJzendoorn, M.H., & Permezel, M. (2011). The role of oxytocin in mother-infant relations: A systematic review of human studies. Harvard Review of Psychiatry, 19(1), 1–14. doi:10.3109/10673229.2011.549771
- George, C., Kaplan, N., & Main, M. (1985). Adult attachment interview. Unpublished manuscript.
- Grimm, S., Pestke, K., Feeser, M., Aust, S., Weigand, A., Wang, J., … Bajbouj, M. (2014). Early life stress modulates oxytocin effects on limbic system during acute psychosocial stress. Social Cognitive and Affective Neuroscience, 9(11), 1828–1835. doi:10.1093/scan/nsu020
- Groh, A.M., & Roisman, G.I. (2009). Adults’ autonomic and subjective emotional responses to infant vocalizations: The role of secure base script knowledge. Developmental Psychology, 45(3), 889–893. doi:10.1037/a0014943
- Heinrichs, M., Von Dawans, B., & Domes, G. (2009). Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology, 30(4), 548–557. doi:10.1016/j.yfrne.2009.05.005
- Hesse, E. (2008). The Adult Attachment Interview: Protocol, method of analysis, and empirical studies. In J. Cassidy & P. R. Shaver (Eds.), Handbook of attachment: Theory, research, and clinical applications (2nd ed., pp. 552–598). New York, NY: Guilford.
- Insel, T.R. (2010). The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron, 65(6), 768–779. doi:10.1016/j.neuron.2010.03.005
- Ioannidis, J.P., Munafo, M.R., Fusar-Poli, P., Nosek, B.A., & David, S.P. (2014). Publication and other reporting biases in cognitive sciences: Detection, prevalence, and prevention. Trends in Cognitive Sciences, 18(5), 235–241. doi:10.1016/j.tics.2014.02.010
- Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825–841. doi:10.1006/nimg.2002.1132
- Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156. doi:10.1016/S1361-8415(01)00036-6
- Kirsch, P., Esslinger, C., Chen, Q., Mier, D., Lis, S., Siddhanti, S., … Meyer-Lindenberg, A. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience, 25(49), 11489–11493. doi:10.1523/JNEUROSCI.3984-05.2005
- LeDoux, J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. doi:10.1146/annurev.neuro.23.1.155
- Leerkes, E.M. (2010). Predictors of maternal sensitivity to infant distress. Parenting: Science and Practice, 10(3), 219–239. doi:10.1080/15295190903290840
- Leerkes, E.M., Parade, S.H., & Gudmundson, J.A. (2011). Mothers’ emotional reactions to crying pose risk for subsequent attachment insecurity. Journal of Family Psychology, 25(5), 635–643. doi:10.1037/a0023654
- Leerkes, E.M., & Siepak, K.J. (2006). Attachment linked predictors of women’s emotional and cognitive responses to infant distress. Attachment & Human Development, 8(1), 11–32. doi:10.1080/14616730600594450
- Leon, K., Jacobvitz, D.B., & Hazen, N.L. (2004). Maternal resolution of loss and abuse: Associations with adjustment to the transition to parenthood. Infant Mental Health Journal, 25, 130–148. doi:10.1002/(ISSN)1097-0355
- Luminet, O., Grynberg, D., Ruzette, N., & Mikolajczak, M. (2011). Personality-dependent effects of oxytocin: Greater social benefits for high alexithymia scorers. Biological Psychology, 87(3), 401–406. doi:10.1016/j.biopsycho.2011.05.005
- Ma, Y., Liu, Y., Rand, D.G., Heatherton, T.F., & Han, S. (2015). Opposing oxytocin effects on inter-group cooperative behavior in intuitive and reflective minds. Neuropsychopharmacology, 40, 2379–2387.
- MacDonald, K., & MacDonald, T.M. (2010). The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry, 18(1), 1–21. doi:10.3109/10673220903523615
- Mah, B.L., Bakermans-Kranenburg, M.J., van IJzendoorn, M.H., & Smith, R. (2015). Oxytocin promotes protective behavior in depressed mothers: A pilot study with the enthusiastic stranger paradigm. Depression and Anxiety, 32(2), 76–81. doi:10.1002/da.2015.32.issue-2
- Main, M., & Goldwyn, R. (1984). Adult attachment scoring and classification system. Unpublished manuscript.
- Main, M., Kaplan, N., & Cassidy, J. (1985). Security in infancy, childhood and adulthood: A move to the level of representation. In I. Bretherton & E. Waters (Eds). Growing points in attachment theory and research. Monographs of the Society for Research in Child Development, 50(1/2), 66–106. doi:10.2307/3333827
- Meinlschmidt, G., & Heim, C. (2007). Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological Psychiatry, 61(9), 1109–1111. doi:10.1016/j.biopsych.2006.09.007
- Mikolajczak, M., Gross, J.J., Lane, A., Corneille, O., de Timary, P., & Luminet, O. (2010). Oxytocin makes people trusting, not gullible. Psychological Science, 21(8), 1072–1074. doi:10.1177/0956797610377343
- Naber, F., Poslawsky, I.E., van IJzendoorn, M.H., Van Engeland, H., & Bakermans-Kranenburg, M.J. (2012). Brief report: Oxytocin enhances paternal sensitivity to a child with autism: A double-blind within-subject experiment with intranasally administered oxytocin. Journal of Autism and Developmental Disorders, 43, 1–6.
- Naber, F., van IJzendoorn, M.H., Deschamps, P., Van Engeland, H., & Bakermans-Kranenburg, M.J. (2010). Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: A double-blind within-subject experiment. Psychoneuroendocrinology, 35(10), 1583–1586. doi:10.1016/j.psyneuen.2010.04.007
- Out, D., Pieper, S., Bakermans-Kranenburg, M.J., & van IJzendoorn, M.H. (2010). Physiological reactivity to infant crying: A behavioral genetic study. Genes Brain and Behavior, 9(8), 868–876. doi:10.1111/gbb.2010.9.issue-8
- Out, D., Pieper, S., Bakermans-Kranenburg, M.J., Zeskind, P.S., & van IJzendoorn, M.H. (2010). Intended sensitive and harsh caregiving responses to infant crying: The role of cry pitch and perceived urgency in an adult twin sample. Child Abuse & Neglect, 34(11), 863–873. doi:10.1016/j.chiabu.2010.05.003
- Petrovic, P., Kalisch, R., Singer, T., & Dolan, R.J. (2008). Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience, 28(26), 6607–6615. doi:10.1523/JNEUROSCI.4572-07.2008
- Pierrehumbert, B., Torrisi, R., Ansermet, F., Borghini, A., & Halfon, O. (2012). Adult attachment representations predict cortisol and oxytocin responses to stress. Attachment & Human Development, 14(5), 453–476. doi:10.1080/14616734.2012.706394
- Reijneveld, S.A., Van der Wal, M.F., Brugman, E., Sing, R.A., & Verloove-Vanhorick, S.P. (2004). Infant crying and abuse. Lancet, 364(9442), 1340–1342. doi:10.1016/S0140-6736(04)17191-2
- Riem, M.M., Bakermans-Kranenburg, M.J., Huffmeijer, R., & van IJzendoorn, M.H. (2013). Does intranasal oxytocin promote prosocial behavior to an excluded fellow player? A randomized-controlled trial with Cyberball. Psychoneuroendocrinology, 38(8), 1418–1425. doi:10.1016/j.psyneuen.2012.12.023
- Riem, M.M., Voorthuis, A., Bakermans-Kranenburg, M.J., & van IJzendoorn, M.H. (2014). Pity or peanuts? Oxytocin induces different neural responses to the same infant crying labeled as sick or bored. Developmenta Science, 17(2), 248–256.
- Riem, M.M.E., Bakermans-Kranenburg, M.J., Pieper, S., Tops, M., Boksem, M.A.S., Vermeiren, R.R.J.M., … Rombouts, S.A.R.B. (2011). Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biological Psychiatry, 70(3), 291–297. doi:10.1016/j.biopsych.2011.02.006
- Riem, M.M.E., Bakermans-Kranenburg, M.J., van IJzendoorn, M.H., Out, D., & Rombouts, S.A.R.B. (2012). Attachment in the brain: Adult attachment representations predict amygdala and behavioral responses to infant crying. Attachment & Human Development, 14(6), 533–551. doi:10.1080/14616734.2012.727252
- Riem, M.M.E., Pieper, S., Out, D., Bakermans-Kranenburg, M.J., & van IJzendoorn, M.H. (2010). Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Social Cognitive and Affective Neuroscience, 6, 294–300.
- Riem, M.M.E., van IJzendoorn, M.H., Tops, M., Boksem, M.A., Rombouts, S.A., & Bakermans-Kranenburg, M.J. (2013). Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. European Neuropsychopharmacology, 23(10), 1288–1295. doi:10.1016/j.euroneuro.2013.01.011
- Rilling, J.K. (2013). The neural and hormonal bases of human parentalcare. Neuropsychologia, 51(4), 731–747. doi:10.1016/j.neuropsychologia.2012.12.017
- Roisman, G.I., Haltigan, J.D., Haydon, K.C., & Booth‐LaForce, C. (2014). VI. Earned-Security in retrospect: Depressive symptoms, family stress, and maternal and paternal sensitivity from early childhood to mid-adolescence. Monographs of the Society for Research in Child Development, 79(3), 85–107. doi:10.1111/mono.12115
- Roisman, G.I., Holland, A., Fortuna, K., Fraley, R.C., Clausell, E., & Clarke, A. (2007). The adult attachment interview and self-reports of attachment style: An empirical rapprochement. Journal of Personality and Social Psychology, 92(4), 678–697. doi:10.1037/0022-3514.92.4.678
- Schoenmaker, C., Juffer, F., van IJzendoorn, M.H., Linting, M., Van Der Voort, A., & Bakermans-Kranenburg, M.J. (2015). From maternal sensitivity in infancy to adult attachment representations: A longitudinal adoption study with secure base scripts. Attachment & Human Development, 17, 241–256.
- Schuetze, P., & Zeskind, P.S. (2001). Relations between women’s depressive symptoms and perceptions of infant distress signals varying in pitch. Infancy, 2(4), 483–499. doi:10.1207/S15327078IN0204_06
- Schuetze, P., Zeskind, P.S., & Das Eiden, R. (2003). The perceptions of infant distress signals varying in pitch by cocaine-using mothers. Infancy, 4(1), 65–83. doi:10.1207/S15327078IN0401_4
- Smith, S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. doi:10.1002/(ISSN)1097-0193
- Smith, S.M., Jenkinson, M., Woolrich, M.W., Beckmann, C.F., Behrens, T.E.J., Johansen-Berg, H., … Matthews, P.M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23(Suppl 1), S208–S219. doi:10.1016/j.neuroimage.2004.07.051
- Soltis, J. (2004). The signal functions of early infant crying. Behavioral and Brain Sciences, 27(4), 443–490.
- Strathearn, L., Fonagy, P., Amico, J., & Montague, P.R. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34(13), 2655–2666. doi:10.1038/npp.2009.103
- Straus, M.A., Hamby, S.L., Finkelhor, D., Moore, D.W., & Runyan, D. (1998). Identification of child maltreatment with the parent-child conflict tactics scales: Development and psychometric data for a national sample of American parents (vol 22, pg 249, 1998). Child Abuse & Neglect, 22(4), 249–270. doi:10.1016/S0145-2134(97)00174-9
- Swain, J.E., Kim, P., Spicer, J., Ho, S.S., Dayton, C.J., Elmadih, A., & Abel, K.M. (2014). Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research, 1580, 78–101. doi:10.1016/j.brainres.2014.03.007
- Van IJzendoorn, M.H., & Bakermans-Kranenburg, M.J. (2014). Confined quest for continuity: The categorical versus continuous nature of attachment. Monographs of the Society for Research in Child Development, 79(3), 157–167.
- Waters, E., Hamilton, C.E., & Weinfield, N.S. (2000). The stability of attachment security from infancy to adolescence and early adulthood: General introduction. Child Development, 71(3), 678–683. doi:10.1111/cdev.2000.71.issue-3
- Weinfield, N.S., Sroufe, L.A., & Egeland, B. (2000). Attachment from infancy to early adulthood in a high-risk sample: Continuity, discontinuity, and their correlates. Child Development, 71(3), 695–702. doi:10.1111/cdev.2000.71.issue-3
- Weisman, O., Delaherche, E., Rondeau, M., Chetouani, M., Cohen, D., & Feldman, R. (2013). Oxytocin shapes parental motion during father–infant interaction. Biology Letters, 9(6), 20130828. doi:10.1098/rsbl.2013.0828
- Worsley, K.J. (2001). Statistical analysis of activation images. In P. Jezzard, P. M. Matthews, & S. M. Smith (Eds.), Functional MRI: An introduction to methods (pp. 251–270). New York, NY: Oxford University Press.
- Zeifman, D.M. (2001). An ethological analysis of human infant crying: Answering Tinbergen’s four questions. Developmental Psychobiology, 39(4), 265–285. doi:10.1002/(ISSN)1098-2302
