Abstract
Therapeutic applications of genomic medicine are slowly finding their way into the healthcare framework of developing countries. The establishment of equitable innovation policies is a determining factor in how genomic-based therapeutic applications will evolve in these countries. In the biomedical field, the commercialization of research results has established itself as the dominant paradigm in the innovation system. However, many recent studies have demonstrated that this emphasis on commercialization and the protection of intellectual property has led to disappointing results. A growing number of stakeholders in this debate argue that it is now necessary to go beyond the commercialization of research and implement policies based on the research valorization paradigm, which supports the achievement of social as well as economic objectives. We thus propose a new set of more inclusive research performance indicators to help policymakers measure the impact of international genomics projects on developing countries.
Introduction
Recent scientific advancements in genomics have the potential in the near future to significantly impact the health of people living in developing countries. The innovation system in genomics is, however, facing several challenges, one of which is a stronger emphasis placed on the commercialization of research findings through intellectual property that sometimes has a negative effect on research and development (R&D) leading to new therapeutic applications. This tendency is all the more worrying in the initial research stage, generally funded by the public sector and carried out in universities. In this context, funding bodies and technology transfer offices use indicators for measuring research project performance that focus on commercialization. Some innovation experts are now arguing that we should go beyond commercialization of research funded by the public sector and establish policies for assessing research based on the research valorization paradigm. Valorization, sometimes also called mobilization of innovation, is broader than commercialization in that it favors the attainment of both social and economic objectives (Joly, Livingstone, and Dove Citation2012, 3). The choice of the appropriate innovation model is especially important with respect to access to new medical technologies in developing countries.
In this article, we will suggest a set of indicators that we have developed to measure the impact on developing countries of research done in the context of large-scale international projects in genomics. Our indicators, inspired by the valorization paradigm, are based on criteria that are much more diverse and inclusive than those suggested by commercialization. First we provide a summary of recent advances made in genomic medicine and then we discuss the need to develop new indicators with the objective of creating both social and economic value. The methodology used to select the indicators will be explained in the third section of this article.
Innovation in genomic medicine and developing countries
The potential benefits of genomic medicine
Science, technology and innovation have played an important role in the evolution of health care over the last century (UNMPSTTF Citation2004, 4). It has often been the case that advances in scientific research materialized following the development of new technologies such as instruments for observing and measuring. These tools can provide users with an advantage over those without access to similar instruments. Furthermore, they can advance knowledge in a specific area given their greater capacity. For example, Galileo’s famous discoveries in astronomy were largely due to his ability to build a superior telescope compared to what existed at the time (Seung Citation2012, 139–140). In the framework of genomic research, major research infrastructures and databases that can be accessed by researchers the world over are prerequisites to major discoveries (Knoppers Citation2013, 537).
Indeed, such genetic databases have already played a critical role in the discovery of some 3000 genes associated with Mendelian diseases (rare diseases) and 900 genetic variations associated with multifactorial diseases (chronic diseases) (Brown, Hay, and Ostrer Citation2008, 54; Green, Guyer and NHGRI Citation2011, 204–205). However, the number of functional genes in the human genome is estimated to be nearly 24,000, and each of these genes is associated in turn with different genetic variants, which may be linked to diseases with hereditary components (Brown, Hay, and Ostrer Citation2008, 103). Complete characterization of the genetic aspects of complex diseases thus requires identifying the whole spectrum of human genomic variations and their interactions through new research efforts. Moreover, databases on genetic variations in non-human species, such as insects, bacteria and viruses, are also very valuable in medical genomic research. For example, understanding the genetic variations involved in the transmission of infectious disease by insect vectors could possibly inform the development of strategies for preventing the transmission of infectious disease generally. Knowledge of the genetic variations among microbial pathogens could also be useful in the development of new vaccines (Green, Guyer and NHGRI Citation2011, 205).
This progress in human genomics has resulted in the creation of thousands of biotechnology firms (McCarty, McLeod, and Ginburg Citation2013, 1) as well as new medical technologies currently in use or with the potential to improve health care on a global level (Singer et al. Citation2005, 113–117). The progress made in genomic medicine has however taken place mainly in developed countries,Footnote1 while in developing countries,Footnote2 advances in genomics are perceived as out of reach. In this respect, the Global Health Forum reported that 90% of medical research still addresses the healthcare needs of only 10% of the world population (WHO, Citationn.d.). Several authors are worried that if developing countries delay appropriating the benefits offered by genomics, this will lead to a technological lag comparable to that associated with the information technology revolution in Western countries (Smith et al. Citation2004, 386). This technology gap is especially problematic since nearly 85% of the world population lives in developing countries, and they are afflicted with 92% of the global burden of disease (WHO, Citationn.d.). Moreover, the portion of the world population living in developing countries increases each year, as can be seen from a recent report by UNICEF (Citation2014, 13), which shows that nearly 25% of the world population will be African in 2050. Furthermore, developing countries used to be affected mostly by transmissible diseases, such as malaria, HIV/AIDS and tuberculosis, but we are now seeing growth in health problems caused by chronic diseases in these countries. A third of deaths in the world are now caused by cardiovascular diseases, and nearly 80% of these deaths occur in developing countries (Séguin et al. Citation2008, 487).
While technologies stemming from genomic research could, in the long term, improve equity in world health (Daar and Singer Citation2011), global inequality regarding the availability, quality and use of genomic medicine technology is growing for many reasons. Despite this, many authors consider the development of genomics capacities in developing countries a way of producing technologies adapted to the needs of their populations and improving available local health care. These changes could also have a positive impact on the economic development of developing countries (Pang and Weatherall Citation2012, 1853–1854; Séguin et al. Citation2008, 487; WHO Citation2002, 4–12). In order to foster the development of medical genomics capabilities in these countries however, efficient innovation systems and participation in international research undertakings in the field are critical. Moreover, genomic medicine needs to be evaluated through a realistic lens so that expectations are not exaggerated and attention is not turned away from other promising approaches to improving health in developing countries. The translation of genomic discoveries into the clinical setting will be especially problematic according to experts (Evans et al. Citation2011; Williams-Jones and Corrigan Citation2003).
In addition, as technological development is strongly influenced by social, political and cultural factors (Jasanoff Citation2004), it is important that developing countries establish a socio-cultural framework regarding the development of genomics that reflects the cultural and social underpinnings of a particular area (Kumar Citation2012). The integration of genomic medical applications in health care raises many socio-ethical issues and hence the ethical analysis should not be based only on external philosophies or worldviews. Clinicians and society at large are concerned about the impact of genetic information on the well-being of individuals and groups (Clayton Citation2003, 562). For example, genetic testing is susceptible to lead to an unanticipated finding that an individual is at risk for a medical condition other than the one for which the test was prescribed (McGuire et al. Citation2013). Moreover, finding that an individual is at risk for a medical condition has consequences for his relatives as they may have genetic mutations in common. Genomic medical applications could also increase the risk of an individual to be discriminated against based on his genetic information. An individual with known disease susceptibility could, for instance, be denied access to health insurance, education and employment (Clayton Citation2003, 562). Finally, genomic medicine might not fit well within traditional conceptions of medicine. Nevertheless, we believe that if genomic medicine functions within a socio-culturally adequate healthcare framework, the impact of these concerns can be minimized. Genomic medicine should not be seen as being in opposition to traditional medicine, but as a complementary tool that may improve its efficiency (Joshi, Ghodke and Shintre Citation2010, 26–32). On this regard, scholars have noted that traditional medicine and modern medicine have increasingly borrowed from each other in the last decades, notably in the field of genomic medicine (Bhardwaj Citation2012; Yun, Song, and Wang Citation2012).
The challenges of the innovation system
The preceding section showed that amidst the possible pitfalls surrounding genomic medicine there is considerable potential for positive impacts on the health of populations in developing countries. However, established innovation policies, traditionally based on commercialization of research, strong intellectual property protection and closed innovation, seem to have impeded the development and accessibility of genomic medicine applications in developing countries. The same applies to the involvement of researchers from developing countries in international genomic research projects (Barton Citation2002, 121). This has led to widespread criticism, both in developed and developing countries, of the public policy on innovation currently encouraged in developed countries (Koutouki Citation2010; TIP Citation2008a, 7).
The present model for developing technological products is based on the funding of basic research in universities through public research money. Technology transfer offices are then charged with promoting commercialization of discoveries flowing from the research with the help of the private sector (CST Citation2005). Commercialization of the fruit of research, classically defined as the process that consists in extracting economic value from new innovations and knowledge using patents, licencing agreements and the creation of spin-off companies, has thus become the dominant paradigm all around the world (De Jonge and Louwaars Citation2009, 535).
Intellectual property plays a preponderant role in the biotechnology innovation system, and the dominant current of thought holds that a high level of protection leads to the highest levels of innovation (TIP Citation2008a, 7). However, it has been demonstrated that patents in the field of biotechnologies can sometimes be an obstacle to setting up new research projects, particularly with respect to patents on gene sequences (Gold et al. Citation2010, 1–2; Joly Citation2010, 417). A number of recent studies conducted in developed countries have shown that the accent placed on commercialization of biotechnologies as an objective of research funded by the public sector has had disappointing results, with stagnation and even decline in this economic sector in certain countries (Joly, Livingstone, and Dove Citation2012, 3).
The recent results of the genomics innovation sector do indeed show that there are elements slowing down R&D of new therapeutic applications (Gold et al. Citation2010, 2). For example, the funds invested in R&D of new forms of medication double each year, yet those investments lead to the production of fewer drugs, and the new drugs are less innovative than they used to be (Gold et al. Citation2010, 2; Sampogna Citation2008). While it was frequent for more than 40 new drugs to be approved each year by the Food and Drug Administration in the United States before the turn of the millennium, that number has never been reached since (E&Y Citation2014). In the United Kingdom, only 26% of the new drugs launched between 2001 and 2012 were very innovative, involving the discovery of new chemical entities, and over 50% of the new drugs were not very innovative, involving only incremental changes to pre-existing drugs (Ward et al. Citation2014, 1).
University research does not seem to fare any better. In Canada, studies have shown that university research in genomics in recent years has produced only a modest number of invention disclosures and in general not much high economic value intellectual property and very little revenue. The research commercialization strategy promoted by American universities has also had disappointing results since most of the profits made by the universities have come from a very small number of star technologies (Ward et al. Citation2014, 3). The data collected in the biotechnology sector since the emergence of the research commercialization paradigm show that research commercialization policies have had modest socio-economic benefits (Colyvas et al. Citation2002, 62–63; Mowery et al. Citation2004, 184). Some experts on innovation now argue that we need to go beyond traditional technology transfer strategies based on obtaining patents and licences, and establish research valorization policies, based on a concept much less restrictive than commercialization (Joly, Livingstone, and Dove Citation2012, 3).
Valorization encompasses the whole spectrum of channels that ensure that the results of research activities have positive effects outside of academic circles. It involves, for example, training researchers and professionals through inclusive participation in research projects, holding conferences and colloquia, networking, the creation of research partnerships, but also licences, patents and company creation (CST Citation2005, 9–12). Indeed, the main difference between valorization and commercialization is located at the level at which indicators are chosen to measure the performance of an innovation system (Joly, Livingstone, and Dove Citation2012, 3). The goal of valorization is to implement broadly defined social and economic indicators defined in broadly. They include the concept of collaborative innovation, characterized by a will to facilitate knowledge sharing so as to accelerate innovation (Joly Citation2012). The research valorization paradigm has inspired the development of the indicators that will be described in the next part of this text.
New indicators benefitting developing countries
The relevance of introducing new indicators
Nobel Prize laureates, such as Randy Schekman and Peter Higgs, have been critical of commonly used methods for assessing healthcare research performance based on achieving short-term objectives (Kleinert and Horton Citation2014, 197). In genomics, the social value of scientific progress is currently seen as secondary to the central emphasis given to commercialization. Hence the right balance between social and commercial research objectives is needed, as well as identifying the mechanisms that will make it possible to meet both goals as effectively as possible (De Jonge and Louwaars Citation2009, 538). However, this task is made especially complex by the small amount of data available to measure the performance of research projects on genomics. Such data are of significant importance in order for policymakers to make informed decisions regarding the development of innovation. Indeed, it has already been demonstrated that innovation policies based on performance indicators favoring commercialization of research have had a major impact on the way university researchers obtain patents (Langford et al. Citation2006, 1590).
Thus, we lack data needed to address critical issues to ensure the performance of the innovation system, for example, to be able to tell if, how and when intellectual property leads to increased investment in R&D of new therapeutic applications (TIP Citation2008a, 10). Very little information is available on which research results make it to the market, how long R&D takes and concerning the marketing of therapeutic applications, as well as the positive and negative factors that influence the process (Joly, Livingstone, and Dove Citation2012, 4). Such data are simply not collected or are unusable because the various institutions involved in research have no shared standards at either the national or the international level. Moreover, the limited data available mainly concern intellectual property, and overlook the social benefits of research and innovations through its impact on knowledge-sharing activities that are not related to technology transfer (Langford et al. Citation2006, 1589–1590). At present, public decision-makers mainly use data in the form of incomplete lists of invention disclosures, patent applications, patents granted, licences and spin-off companies (Guebert and Bubela Citation2014, 3).
For example, in Canada, a set of indicators developed by the Association of University Technology Managers is used by public decision-makers to establish research valorization policies and measure the performance of research projects and the national innovation system. These indicators are based on two success models: (1) the first is based on the idea that granting licences and creating spin-off companies necessarily generates profits; (2) the second is based on the income generated by the university, in particular through granting licences. Such indicators assume a linear relationship between variables, which has already been proven to be problematic in the biomedical field (TIP Citation2008b, 2).
The innovation system involves, in fact, many interactions among different players with specific objectives and motivations in the framework of a circular, dynamic process. New indicators of the performance of a research project or innovation system taking inspiration from the richer concept of valorization have much to contribute to this more realistic social view of innovation processes. Progress has already been made in this respect with the importance of fostering the development of R&D capacities and capturing the social value of innovation making its way into the innovation system recently. Innovation experts have begun suggesting new indicators and establishing new criteria for assessing research performance with objectives that are broader than the simple commercialization of research. These indicators include publications, conferences, student and employee exchanges, networking, teaching, consultation and collaboration (Joly, Livingstone, and Dove Citation2012, 3–4). The adoption of innovation policies based on valorization is likely to favor the development of R&D capacities in genomics and access to genomic medicine applications in developing countries.
Methodology: the choice of indicators
Assessment of performance in innovation, which involves studying and developing indicators, is a scientific discipline in and of itself. According to Fred Gault, a researcher affiliated with the United Nations University, this discipline is especially complex (Citation2013, 7–8). The primary objective of developing performance indicators is to provide decision-makers with information giving them the power to take action on the results. A set of indicators therefore must be re-useable in order to measure the evolution of the performance of a research project over time. The development and use of indicators by decision-makers can, and often does, have social implications (11–15), as well as influence on research practices and the themes studied by researchers in the biomedical field (Macleod et al. Citation2014, 101–102). With respect to innovation, the old adage that what you measure is what you get is generally well founded (TIP Citation2008b, 2).
While the discipline of research performance assessment is already more than 50 years old, very little is known about the socio-economic impact of science. This is particularly due to the fact that studies in this field focus mainly on the economic impact of research, which is easier to quantify than its social impact (Godin and Dore Citation2005, 1–5). This is even truer in the field of medical genomics, notably because this discipline is still very young, and has been gaining importance only over the last 20 years, following the completion of the Human Genome Project. This makes it more complicated to measure its real benefits on the social level (Evans Citation2014a). For example, it is easy to quantify the number of publications flowing from a research project or to list therapeutic applications brought to market in the framework of a research project, but it is more difficult to measure their concrete impact on the health of a population. Moreover, often many years go by before the impact of a scientific discovery becomes tangible and observable (Rank Citation2009). Although it is easy to calculate the number of patents resulting from a research project, the results of that calculation reveal little as to their value and importance on the levels of scientific progress and development of therapeutic applications (OECD Citation2013). Hence, frequently, assessment of the long-term performance of a research project remains approximate owing to methodological limitations and due to the difficulty in collecting the data necessary to establish appropriate indicators. In genomics, those responsible for assessing research project performance have turned mainly to indicators based on quantifiable research results, such as patents, licence granting, spin-off companies, publications, students who have been trained and third-party citations of patents and publications. The data for such indicators are generally more easily accessible, but they provide an incomplete portrait of the value of genomics research (Evans Citation2014a; TIP Citation2008b, 2–3).
The indicators proposed in this article aim to provide data on the benefits of international genomics projects on genomics R&D capacity building and access to genomic medicine applications in developing countries. In order to develop these indicators, we first performed a scoping review of the literature on research performance assessment. We performed our research via the Google Scholar and McGill University library databases, using the following key word combinations: research AND (performance OR success) AND (evaluation OR review OR analysis). We also searched policy documents and research reports on the websites of various national and international organizations, such as the Organization for Economic Cooperation and Development, Canada’s Science, Technology and Innovation Council, the Canadian Academy of Health Sciences, the United States’ National Institutes of Health, the United States’ National Human Genome Research Institute, the United Kingdom’s Medical Research Council and the Innovation Partnership. Finally, we retrieved external performance assessment reports. We have adapted examples from a book (Gault Citation2013), scientific articles (Salman et al. Citation2014;
Donovan and Hanney Citation2011; Godin and Dore Citation2005; Hanney et al. Citation2003; Ioannidis and Khoury Citation2014), policy documents (CAHS Citation2009; Evans Citation2014a; MRC Citation2016; NIH Citation2016; OECD Citation2005, Citation2008; STIC Citation2012; TIP Citation2008b) and performance assessment reports (DRA Citation2010; KPMG Citation2009) to our objective of documenting the impact of international genomics projects in developing countries.
The development of the indicators was also informed by the theoretical foundations of research valorization, which favors knowledge sharing and collaboration to accelerate innovation. The social and commercial channels of valorization, as described by researchers Joly, Livingstone, and Dove (Citation2012) (see ), provide broad directions concerning measures for capturing the socio-economic value of research. The social channels encompass all activities designed to maximize research benefits outside of the realm of commercialization. The first social channel, namely, exchanges between individuals, takes the form of internships, cooperative research programs and consultation mandates that provide researchers with the means to develop their skills through contact with other researchers. The second social channel is exchange of knowledge. This channel emphasizes free or low-cost sharing of more overt knowledge, such as patents and genetic data, in order to favor innovation. Application of knowledge, the third social channel, relates to activities of researchers in medical genomics linked with the development of public policy, guidelines and information documents on best practices. The fourth and last social channel is collaborative research. This refers to the creation of research groups, bringing together experts in a single area from different research institutions to significantly accelerate research on a predefined issue (Joly, Livingstone, and Dove Citation2012).
Figure 1. Main channels for research valorization (Joly, Livingstone, and Dove Citation2012).
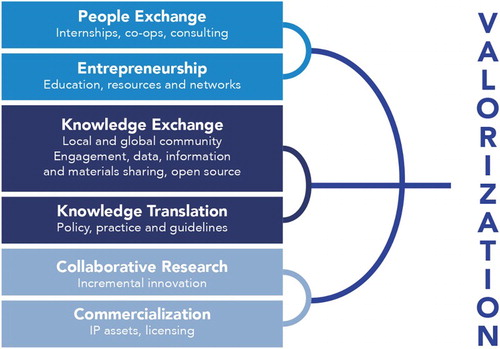
The goal of commercial valorization channels is to maximize the economic benefits of a research project or, on a larger scale, the innovation system. The first commercial channel is entrepreneurship, which refers to commercialization of researchers’ expertize through contract research, research partnerships and various consulting activities, generally for a company or government institution. The second commercial channel, which is also at the foundation of the innovation system, is based on intellectual property protection and the granting of user licences for patented technologies. The third commercial channel is based on entrepreneurship and refers to technology transfer through the creation of spin-off companies (CST Citation2005, 13–19).
Proposed indicators
This section proposes a set of indicators, inspired by the valorization channels, designed to measure the performance of research projects in genomics with respect to both (1) R&D capacity building in genomics and (2) access to genomic medicine applications in developing countries. Indicator nos. 1 and 2 are related to the relevance of the project for the health of people in developing countries. Indicator nos. 3–5 are related to the involvement of researchers from developing countries in selected projects and their ability to use the data from those projects. Indicator nos. 6–8 are related to the development of research infrastructure, involvement of researchers from developing countries in project management activities and the training of researchers in developing countries. Finally, indicator no. 9 is related to the management of intellectual property emerging from the project.
Indicator no. 1: the data collected in the framework of the project include data collected from populations of developing countries
The great majority of genome-wide association studies conducted to date have targeted populations of European descent. This tendency can also be seen in the most recent studies involving sequencing of the complete genome of participants (Bustamante, Burchard, and De La Vega Citation2011, 163). However, it has been established that the frequency of genetic variants contributing to the occurrence of diseases differs from one population to the next (Rotimi and Jorde Citation2012, 48–49). In other words, genetic variants are not unique to specific populations, but the frequency with which they are found in specific populations differs (De Vries and Pepper Citation2012). Knowledge of rare genetic variants, those present in less than 5% of the world population but accounting for the great majority of existing variations, are especially useful for identifying the risk that a person will have a disease and for predicting how he or she will react to medication. Biases with respect to the importance of certain variants could thus be created if the populations found in developing countries are not included in the framework of major population-based studies in genomics (Bustamante, Burchard, and De La Vega Citation2011, 164). Moreover, the environment in which developing countries’ populations live is often different from that of populations in developed countries. For researchers to be able to address the health problems affecting individuals in developing countries, it is therefore imperative that the data collected in the framework of international genomics projects include data collected from populations in developing countries.
It is, however, important to note that the concept of population as currently defined by genomic researchers on the basis of geographical and biological criteria has been subject to criticism from scholars from diverse social science fields for being ambiguous and failing to take into account social, economic, cultural, behavioral and political determinants of population classification (Panofsky and Bliss Citation2017). The use of the population concept was initially adopted in genomic research to replace classification of research participants on a racial basis. The inherent difficulties involved in the classification of individuals in function of imprecise and not yet well-understood social and biological determinants traditionally associated to race have, however, not been resolved by switching to the concept of population as there remains a lack of consensus concerning an appropriate definition of population (Reardon Citation2005). Difficulties associated with the classification of individuals according to populational criteria have been documented in genomic research in general (Ali-Khan et al. Citation2011; Panofsky and Bliss Citation2017) as well as in the specific context of international genomic research projects, such as the International HapMap Project (Reardon Citation2008). Scholars have thus suggested that genomic researchers should be as precise as possible in their description of the population groups involved in their studies. Relevant information to define involved populations could include, among other things, socio-economic status, social class, personal or family wealth, insurance status, age, diet and nutrition, language spoken, religion and tribal affiliation (Caulfield et al. Citation2009). In addition, some of the methods used to determine the frequency of genetic variants across populations have also been subject to criticism (Bolnick Citation2008). Nevertheless, as geographical criteria constitute the current standard for population classification and these criteria have been positively associated with differences in occurrence of genetic variants, the assessment of this indicator could be done by looking at the geographic origin of the populations from which the data were gathered for the selected projects. It should eventually be modified to reflect a future better understanding of the determinants of population classification.
Indicator no. 2: part of the project’s research is on a disease significantly affecting the health of populations of developing countries
Although developing countries are now also affected by a myriad of diseases that are generally associated with developed countries, people in developing countries nonetheless remain disproportionately vulnerable to diseases such as malaria and tuberculosis. Overlooked tropical diseases, which include dengue fever and Chagas disease, are responsible for much of the burden of disease in developing countries (WHO Citation2015). These infectious diseases remain understudied since the pharmaceutical R&D sector pays little interest to them due to a lack of economic incentive (Moon, Bermude, and Hoen Citation2012, 1). The impact of genomic medical applications on the extent and frequency of these diseases could be limited by other important factors such as the inefficient healthcare systems, consumption of contaminated water and levels of poverty. Moreover, as previously mentioned in this text, genomic medicine and genomics research raise many socio-ethical issues, especially in developing countries where their implementation is not always rigorously supervised. For example, patients and participants in developing countries should always be asked for their informed consent. Among other things, they should be informed about the risks associated with their participation as well as who will have access to their genetic information (De Vries et al. Citation2011, 5–14; Ramsay et al. Citation2014, 15–20). Policies should also be developed to ensure that developing countries’ populations are not at risk of being discriminated against. Once these issues have been addressed, research in genomic medicine could provide strong assistance in controlling infectious diseases through the development of drugs, vaccines and diagnostic testing at points of service (Daar et al. Citation2002, 230; Pang Citation2012, 153), especially if the research is conducted in a socio-cultural framework that is acceptable to the local population. However, progress in research on these diseases requires gathering genetic data on humans, parasites and viruses, as well as vectors of transmission in the framework of international genomics projects. This indicator could be assessed by identifying areas of research (i.e. specific diseases) covered by the selected projects and their relevance for developing countries.
Indicator no. 3: researchers from developing countries are involved in the project
Researchers in developing countriesFootnote3 often have difficulty integrating into the international innovation system in genomics because of a lack of financial resources, inadequate research infrastructure or insufficient expertize (UNMPSTTF Citation2004, 39). These obstacles can prevent them from becoming involved in established international genomics projects and therefore gaining access to the shared knowledge of researchers involved in such projects, who are generally influential researchers in the international community of research on medical genomics. Moreover, their participation in international genomics projects generally provides access to explicit knowledge produced by such projects, such as the genetic data collected. These data are needed to set up local research projects that will directly benefit the populations of developing countries, and access to them is not always open to all in the research community. The knowledge and skills acquired by active participation in international genomics projects can go far in establishing local genomic research and contributing to the capacity building of the scientific community in developing countries as well as students who would not otherwise be privy to such information and training. Hence we propose an indicator to verify the involvement of researchers from developing countries in international genomics projects. This indicator could be assessed by identifying the research centers and researchers in developing countries who are identified as participating members in the selected projects.
Indicator no. 4: researchers in developing countries have access to the data collected in the framework of the project
Access to the explicit knowledge produced in the context of major research projects in genomics, such as human genetic data, is now crucial to researchers in genomic medicine, who need to have access to comprehensive sets of data in order to infer statistically valid findings (Green, Guyer and NHGRI Citation2011, 204). Researchers in developing countries thus need to have access to the data collected in international genomics projects in order to conduct research in genomic medicine that could benefit the populations of developing countries. This indicator could be assessed by verifying project web sites and policy documents, including data access agreements, to see whether the access policies of the selected projects provide researchers in developing countries free or equitable access to the data collected.
Indicator no. 5: the data collected in the framework of the project are used by researchers in developing countries
Following our verification of the capacity of researchers in developing countries to access the data collected in international genomics projects, thanks to Indicator No. 4, it is necessary to verify whether the researchers in developing countries do indeed use the data in the framework of research projects designed to find solutions to health problems in populations of developing countries. As most researchers using a specific genomic information database will mention the use of the database in the methodology or acknowledgements sections of the scientific publication to which their project will have led, the indicator could be assessed by searching citations of the selected projects in genomics publications accessible through the Web of Science database of scientific publications (TR, Citationn.d.). It would also be possible to review lists of researchers who have obtained access to these genomics projects’ data.
Indicator no. 6: the project contributes to the development of research infrastructure in developing countries
The collection, processing, storage and sharing of genetic data require large financial resources and appropriate research infrastructure (NHGRI, Citationn.d.; Sboner et al. Citation2011, 125–134). For example, the sequencing systems produced by Illumina that are commonly used by genomic research centers, such as the Harvard University and Massachusetts Institute of Technology Broad Institute, alone cost many hundreds of thousands of American dollars, and up to US$1 million for the most recent system (Krol Citation2014; MUGQIC, Citationn.d.). Moreover, genetic data stored in electronic form can take up a lot of space and require extremely powerful computers or access to an online cloud data storage service that is also very costly. Furthermore, the online platforms for exchanging the data collected are also expensive to develop (Sboner et al. Citation2011, 129–132).
The development of genomic research infrastructures in developing countries is hindered by the prohibitive costs associated to such infrastructures (UNMPSTTF Citation2004, 39). Furthermore, initiatives designed to encourage the development of research infrastructure in the context of international genomics projects play a crucial role in developing R&D capacities in genomics in developing countries. We therefore suggest an indicator for documenting initiatives designed to develop genomic research infrastructures in developing countries in the framework of international genomics projects. This indicator could be assessed by identifying initiatives designed to develop genomics research infrastructures in developing countries in the framework of the selected projects. Such initiatives are likely to be presented on the projects’ web sites, in related marker papers published in scientific journals and in other associated official documents.
Indicator no. 7: decision-making positions are given to researchers and managers in developing countries
International genomics projects emerge mainly from the work of researchers in developed countries, and are generally funded by government agencies in those countries. Decision-making positions, such as those held by members of the board of directors and the scientific committee, are thus often attributed to researchers and managers in developed countries. Moreover, appointing researchers and managers from developing countries to decision-making positions may contribute to positioning the research programs of such projects toward healthcare issues that are important to those countries. Lastly, entrusting decision-making positions to researchers and managers in developing countries can also contribute to the development of genomics R&D capabilities in those countries through the acquisition of competencies with respect to the management of large-scale scientific projects and improved networking opportunities by those researchers and managers. Hence, it is crucial that decision-making positions be given to researchers and managers from developing countries in the framework of international genomics projects in order to accelerate innovation. This indicator could be assessed by identifying decision-making positions that have been given to researchers and managers from developing countries in the framework of the selected projects.
Indicator no. 8: the project includes training opportunities accessible and relevant to researchers/students in developing countries
Developing countries lack qualified individuals able to contribute to the genomics innovation system (UNMPSTTF Citation2004, 51). Training researchers and students through involvement in research projects is considered an important channel for genomics research valorization in Western countries (Evans Citation2014a, Citation2014b, 10–11). Encouraging researchers and students from developing countries to participate in international genomics projects is an initiative that has already been suggested (Rotimi Citation2014; UNMPSTTF Citation2004, 51). Some research projects in genomics go further, however, by establishing programs designed specifically to train researchers and students, which is crucial to favoring the development of genomics R&D capabilities in developing countries. This indicator is assessed by identifying the relevant training programs available to researchers and students from developing countries in the framework of the selected projects.
Indicator no. 9: the project’s intellectual property management policies are favorable to developing countries
The obstacles to sharing knowledge that are created by strong intellectual property protection in genomics research can impede R&D of new therapeutic applications (TIP Citation2008a, 8–9). The difficulties facing researchers in developing countries who have access to limited financial and administrative resources may include having trouble obtaining permission to use patented technologies needed to carry out research projects in genomics (Barton Citation2002, 122–123). Strong intellectual property protection can also hinder the circulation of therapeutic applications resulting from genomics medical research, such as drugs and genetic tests, in developing countries.
However, in recent years, intellectual property management models favoring the sharing of knowledge in order to accelerate innovation and the circulation of new technologies have emerged (Bubela, FitzGerald, and Gold Citation2012, 2; Joly Citation2010, 418). For example, model clauses have been drafted for managing intellectual property in ways that encourage sharing the benefits of research with the populations of developing countries (Guebert and Bubela Citation2014, 3; Hotez et al. Citation2013, 23–24; Joly, Allen, and Knoppers Citation2012, 143–146; Mimura Citation2010, ch. 9). Greater willingness to manage IP portfolio in ways that are less disadvantageous to least developed economies has also been witnessed in the private pharma sector. For example, GlaxoSmithKline recently toned down its approach to filing and enforcing patents so that intellectual property protection is more attuned to a country’s economic maturity. For instance, the company states that it will not file patents for its medicines in least developed countries and low-income countries in the future. In low-to-middle income countries, it will file for patents, but will seek to offer and agree on licences to allow supplies of generic versions of its medicines for 10 years (GSK Citation2016). It remains to be seen whether this proposed scheme will effectively lead to greater access and whether it could eventually be expanded to include expensive biological drugs and diagnostic tests such as those developed through genomics research.
In order to foster access to genomic medicine applications in developing countries, it is important that intellectual property models favoring the sharing of knowledge and research benefits with developing countries be integrated into the research valorization policies of international genomics projects. The indicator could be assessed by identifying whether the project has intellectual property management policies designed to promote equitable access to innovation in developing countries in the research valorization policies of the selected projects.
Conclusion
Genomic medicine can have a major positive impact on the health of populations in developing countries in coming years. In particular, medical applications, such as new, faster diagnostic tests that can be used at the bedside and new drugs, could help to reduce the morbidity and mortality rates in these countries. However, the emphasis on commercialization in the genomics innovation system, notably by public research funding agencies, impedes the implementation of these technologies in developing countries. At this point in time, decision-makers base their assessments of genomics research project performance on limited data that mainly concern intellectual property and commercialization of research outcomes, thus overlooking the social benefits of research and the impact of knowledge-sharing activities on innovation. In the preceding pages, we have therefore suggested a set of key indicators inspired by the research valorization paradigm and designed to measure the performance of international genomics projects, which are research infrastructures vital to enabling developing countries to benefit from progress in genomic medicine. The purpose of these indicators is to facilitate assessment of the impact of international genomics projects on the development of R&D capabilities in genomics and access to genomic medicine applications in developing countries. The proposed next step would be to test them by using them in the performance assessment of major international genomics projects.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
1. For the purposes of this study, the term “developed country” refers to the 35 countries in the list of advanced countries published annually by the IMF (Citation2014, 161–164).
2. For the purposes of this study, the term “developing country” refers to the 153 countries in the list of emerging and developing countries published annually by the IMF (Citation2014, 161–167).
3. For the purposes of our study, researchers from developing countries are those attached to a research institution located in a developing country.
References
- Ali-Khan, Sarah E., Tomasz Krakowski, Rabia Tahir, and Abdallah S. Daar. 2011. “The Use of Race, Ethnicity and Ancestry in Human Genetic Research.” The HUGO Journal 5: 47–63. doi:10.1007/s11568-011-9154-5.
- Barton, John H. 2002. “Research-tool Patents: Issues for Health in the Developing World.” Bulletin of the World Health Organization 80: 121.
- Bhardwaj, Minakshi. 2012. “The Progress of Genomics in the Developing World: Rising Social, Economic, and Ethical Concerns.” In Genomics and Health in the Developing World, edited by Dhavendra Kumar, 90–102. Oxford: Oxford University Press.
- Bolnick, Deborah A. 2008. “Individual Ancestry Inference and the Reification of Race as a Biological Phenomenon.” In Revisiting Race in a Genomic Age, edited by Barbara A. Koenig, Sandra Soo-Jin Lee, and Sarah S. Richardson, 70–85. New Brunswick, NJ: Rutgers University Press.
- Brown, Stuart M., John G. Hay, and Harry Ostrer. 2008. Essentials of Medical Genomics. 2nd ed. Oxford: Wiley-Blackwell.
- Bubela, Tania, Garret A. FitzGerald, and Richard Gold. 2012. “Recalibrating Intellectual Property Rights to Enhance Translational Research Collaborations.” Science Translational Medicine 4: 122. doi:10.1126/scitranslmed.3003490.
- Bustamante, Carlos D., Esteban Gonzalez Burchard, and Francisco M. De La Vega. 2011. “Genomics for the World.” Nature 475: 163–165. doi:10.1038/475163a.
- CAHS (Canadian Academy of Health Sciences). 2009. Making an Impact: A Preferred Framework and Indicators to Measure Returns on Investment in Health Research. Ottawa: CAHS.
- Caulfield, Timothy, Stephanie M. Fullerton, Sarah E. Ali-Khan, Laura Arbour, Esteban G. Burchard, Richard S. Cooper, Billie-Jo Hardy, et al. 2009. “Race and Ancestry in Biomedical Research: Exploring the Challenges.” Genome Medicine 1: 8. doi:10.1186/gm8.
- Clayton, Ellen Wright. 2003. “Ethical, Legal, and Social Implications of Genomic Medicine.” The New England Journal of Medicine 349: 562–569. doi: 10.1056/NEJMra012577
- Colyvas, Jeannette, Michael Crow, Annetine Gelijns, Roberto Mazzoleni, Richard R. Nelson, Nathan Rosenberg, and Bhaven N. Sampat. 2002. “How Do University Inventions Get Into Practice.” Management Science 48: 61–72. doi: 10.1287/mnsc.48.1.61.14272
- CST (Conseil de la science et de la technologie), Quebec. 2005. La Valorisation de la Recherche Universitaire: Clarification Conceptuelle. Sainte-Foy: Conseil de la science et de la technologie.
- Daar, Abdallah S., and Peter A. Singer. 2011. The Grandest Challenge: Bringing Life-saving Science from Lab to Village. Toronto: Doubleday Canada.
- Daar, Abdallah S., Halla Thorsteindottir, Douglas K. Martin, Alyna C. Smith, Shauna Nast, and Peter A. Singer. 2002. “Top Ten Biotechnologies for Improving Health in Developing Countries.” Nature Genetics 32: 229–232. doi: 10.1038/ng1002-229
- De Jonge, Bram, and Niels Louwaars. 2009. “Valorizing Science: Whose Values?” EMBO Reports 10: 535–539. doi:10.1038/embor.2009.113.
- De Vries, Jantina, Susan J. Bull, Ogobara Doumbo, Muntaser Ibrahim, Odile Mercereau-Puijalon, Dominic Kwiatkowski, and Michael Parker. 2011. “Ethical Issues in Human Genomics Research in Developing Countries.” BMC Medical Ethics 12: 356.
- De Vries, Jantina, and Michael Pepper. 2012. “Genomic Sovereignty and the African Promise: Mining the African Genome for the Benefit of Africa.” Journal of Medical Ethics 38: 474–478. doi: 10.1136/medethics-2011-100448
- Donovan, Claire, and Stephen Hanney. 2011. “The ‘Payback Framework’ Explained.” Research Evaluation 20: 181–183. doi: 10.3152/095820211X13118583635756
- DRA (Dennis Rank and Associates). 2010. Evaluation of Genome British Columbia. Vancouver: Dennis Rank and Associates.
- E&Y (Ernst & Young). 2014. Beyond Border: Unlocking Value – Biotechnology Industry Report 2014. London: E&Y.
- Evans, James P., Eric M. Meslin, Theresa M. Marteau, and Timothy Caulfield. 2011. “Deflating the Genomic Bubble.” Science 331: 861–862. doi: 10.1126/science.1198039
- Evans, Samantha. 2014a. “Evaluating Genomic Science: A Conversation with Dr. Samantha Evans.” University of British Columbia conference, Vancouver, May 21.
- Evans, Samantha. 2014b. Handout – Evaluating Genomic Science: A Conversation with Dr. Samatha Evans, Director of Evaluation, Genome Canada. Ottawa: Genome Canada.
- Gault, Fred, ed. 2013. Handbook of Innovation Indicators and Measurement. Cheltenham: Edward Elgar.
- Godin, Benoit, and Christian Dore. 2005. Measuring the Impact of Science: Beyond the Economic Dimension. Montreal: Institut national de la recherche scientifique.
- Gold, Richard, Warren Kaplan, James Orbinski, Sarah Harland-Logan, and Sevil N.-Marandi. 2010. “Are Patents Impeding Medical Care and Innovation?” PLoS Medicine 7: e1000208. doi:10.1371/journal.pmed.1000208.
- Green, Eric D., Mark S. Guyer, and National Human Genome Research Institute. 2011. “Charting a Course for Genomic Medicine from Base Pairs to Bedside.” Nature 470: 204–213. doi:10.1038/nature09764.
- GSK (GlaxoSmithKline). 2016. “GSK Expands Graduated Approach to Patents and Intellectual Property to Widen Access to Medicines in the World’s Poorest Countries.” Accessed May 12, 2016. http://www.gsk.com/en-gb/media/press-releases/2016/gsk-expands-graduated-approach-to-patents-and-intellectual-property-to-widen-access-to-medicines-in-the-world-s-poorest-countries/.
- Guebert, Jenilee M., and Tania Bubela. 2014. “Implementing Socially Responsible Licensing for Global Health: Beyond Neglected Diseases.” Science Translational Medicine 6: 260cm11. doi:10.1126/scitranslmed.3009422.
- Hanney, Stephen R., Miguel A. Gonzalez Block, Martin J. Buxton, and Maurice Kogan. 2003. “The Utilization of Health Research in Policy-making: Concepts, Examples and Methods of Assessment.” Health Research Policy and Systems 1: 717. doi: 10.1186/1478-4505-1-2
- Hotez, Peter, Rachel Cohen, Carol Mimura, Tadataka Yamada, and Stephen L. Hoffman. 2013. Strengthening Mechanisms to Prioritize, Coordinate, Finance, and Execute R&D to Meet Health Needs in Developing Countries. Washington, DC: Institute of Medicine of the National Academies.
- IMF (International Monetary Fund). 2014. World Economic Outlook. Washington, DC: IMF.
- Ioannidis, John P. A., and Muin J. Khoury. 2014. “Assessing Value in Biomedical Research: The PQRST of Appraisal and Reward.” Journal of the American Medical Association 312: 483. doi:10.1001/jama.2014.6932.
- Jasanoff, Sheila. 2004. “Ordering Knowledge, Ordering Society.” In States of Knowledge: The Co-production of Science and Social Order, edited by Sheila Jasanoff, 13–45. London: Routledge.
- Joly, Yann. 2010. “Open Biotechnology: Licenses Needed.” Nature Biotechnology 28: 417–419. doi:10.1038/nbt0510-417.
- Joly, Yann. 2012. “Le rôle des modèles ouverts dans la valorisation de la recherche biomédicale.” Département de physiologie-biophysique de l’Université de Sherbrooke conference, Sherbrooke, May 17.
- Joly, Yann, Clarissa Allen, and Bartha Maria Knoppers. 2012. “Open Access as Benefit Sharing? The Example of Publicly Funded Large-scale Genomic Databases.” Journal of Law, Medicine & Ethics 40: 143–146. doi:10.1111/j.1748-720X.2012.00652.x.
- Joly, Yann, Angus Livingstone, and Edward S. Dove. 2012. Moving Beyond Commercialization: Strategies to Maximize the Economic and Social Impact of Genomics Research. Ottawa: Genome Canada.
- Joshi, Kalpana, Yogita Ghodke, and Pooja Shintre. 2010. “Traditional Medicine and Genomics.” Journal of Ayurveda and Integrative Medicine 1: 26. doi: 10.4103/0975-9476.59824
- Kleinert, Sabine, and Richard Horton. 2014. “How Should Medical Science Change?” The Lancet 383: 197–198. doi:10.1016/S0140-6736(13)62678-1.
- Knoppers, Bartha M. 2013. “Genomics: From Persons to Populations and Back Again.” Genome 56: 537–539. doi:10.1139/gen-2013-0148.
- Koutouki, Konstantia. 2010. “A Legal Placebo: The Role of International Patent Law in the Protection of Indigenous Traditional Knowledge of Medicinal Plants.” Canadian Intellectual Property Review 26: 19.
- KPMG. 2009. Evaluation of Genome Canada – Final Report. Ottawa: KPMG.
- Krol, Aaron. 2014. “What You Need to Know about Illumina’s New Sequencers.” Bio-IT World, January 15. Accessed November 26, 2014. http://www.bio-itworld.com/2014/1/15/what-you-need-know-about-illuminas-new-sequencers.html.
- Kumar, Dhavendra. 2012. “Transcultural Perspectives on Genetics and Genomics.” In Genomics and Health in the Developing World, edited by Dhavendra Kumar, 66–72. Oxford: Oxford University Press.
- Langford, Cooper H., Jeremy Hall, Peter Josty, Stelvia Matos, and Astrid Jacobson. 2006. “Indicators and Outcomes of Canadian University Research: Proxies Becoming Goals?” Research Policy 35: 1586–1598. doi: 10.1016/j.respol.2006.09.021
- Macleod, Malcolm R., Susan Michie, Ian Roberts, Ulrich Dirnagl, Iain Chalmers, John P. A. Ioannidis, Rustam Al-Shahi Salman, An-Wen Chan, and Paul Glasziou. 2014. “Biomedical Research: Increasing Value, Reducing Waste.” The Lancet 383: 101–104. doi:10.1016/S0140-6736(13)62329-6.
- McCarty, Jeanette J., Howard L. McLeod, and Geoffrey S. Ginburg. 2013. “Genomic Medicine: A Decade of Successes, Challenges and Opportunities.” Science Translational Medicine 5: 189sr4. doi:10.1126/scitranslmed.3005785.
- McGuire, Amy L., Steven Joffe, Barbara A. Koenig, Barbara B. Biesecker, Laurence B. McCullough, Jennifer S. Blumenthal-Barby, Timothy Caulfied, Sharon F. Terry, and Robert C. Green. 2013. “Ethics and Genomic Incidental Findings.” Science 340: 1047–1048. doi: 10.1126/science.1240156
- Mimura, Carol. 2010. “Nuanced Management of IP Rights: Shaping Industry-university Relationships to Promote Social Impact.” In Working Within the Boundaries of Intellectual Property: Innovation Policy for the Knowledge Society, edited by Rochelle C. Dreyfuss, Diane L. Zimmerman, and Harry First, 293–304. Oxford: Oxford University Press.
- Moon, Suerie, George Bermude, and Ellen’T Hoen. 2012. “Innovation and Access to Medicines for Neglected Populations: Could a Treaty Address a Broken Pharmaceutical R&D System?” PLoS Medicine 9: e1001218. doi:10.1371/journal.pmed.1001218.
- Mowery, David C., Richard R. Nelson, Bhaven N. Sampat, and Arvids A. Ziedonis. 2004. Ivory Tower and Industrial Innovation: University-industry Technology Transfer Before and After the Bayh-Dole Act. Stanford, CA: Stanford University Press.
- MRC (Medical Research Council). 2016. Outputs, Outcome and Impact of MRC Research: 2014/2015 Report. London: MRC.
- MUGQIC (McGill University and Genome Quebec Innovation Centre). n.d. “Illumina HiSeq and MiSeq Technologies.” Accessed November 28, 2014. http://gqinnovationcenter.com/services/sequencing/technoMPSIlluminaHiSeq.aspx?l=f.
- NHGRI (National Human Genome Research Institute). n.d. DNA Sequencing Costs. NHGRI. Accessed November 28, 2014. http://www.genome.gov/sequencingcosts/.
- NIH (National Institutes of Health). 2016. NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instruction Guide. Bethesda, MD: NIH.
- OECD (Organisation for Economic Co-operation and Development). 2005. Oslo Manual: Guidelines for Collecting and Interpreting Innovation Data. 3rd ed. Paris: OECD.
- OECD (Organisation for Economic Co-operation and Development). 2008. Open Innovation in a Global Perspective – What Do Existing Data Tell Us? Paris: OECD.
- OECD (Organisation for Economic Co-operation and Development). 2013. Measuring Patent Quality: Indicators of Technological and Economic Value. Paris: OECD.
- Pang, Tikki. 2012. “Infections, Genomics and Global Public Health.” In Genomics and Health in the Developing World, edited by Dhavendra Kumar, 152–156. Oxford: Oxford University Press.
- Pang, Tikki, and David Weatherall. 2012. “Genomics and World Health: A Decade on.” The Lancet 379: 1853–1854. doi:10.1016/S0140-6736(12)60562-5.
- Panofsky, Aaron, and Catherine Bliss. 2017. “Ambiguity and Scientific Authority: Population Classification in Genomic Science.” American Sociological Review 82: 59–87. doi:10.1177/0003122416685812.
- Ramsay, Michele, Jantina De Vries, Himla Soodyall, Shane A. Norris, and Osman Sankoh, as Members of the H3Africa Consortium. 2014. “Ethical Issues in Genomic Research on the African Continent: Experiences and Challenges to Ethics Review Committees.” Human Genomics 8: 15. doi: 10.1186/s40246-014-0015-x
- Rank, Dennis. 2009. “Measuring the Impacts of Science and Technology.” Canadian Association of Research Administrators, conference, Ottawa, December 17.
- Reardon, Jenny. 2005. Race to the Finish: Identity and Governance in an Age of Genomics. Princeton, NJ: Princeton University Press.
- Reardon, Jenny. 2008. “Race Without Salvation: Beyond the Science/Society Divide in Genomic Studies of Human Diversity.” In Revisiting Race in a Genomic Age, edited by Barbara A. Koenig, Sandra Soo-Jin Lee, and Sarah S. Richardson, 304–319. New Brunswick, NJ: Rutgers University Press.
- Rotimi, Charles. 2014. “H3Africa: Human Heredity and Health in Africa.” Conference to eliminate heath disparities in genomic medicine, the role of policy, Washington, DC, September 4–5.
- Rotimi, Charles, and Lynn Jorde. 2012. “Ancestry, Disease and Variable Drug Response in the Genomic Era.” In Genomics and Health in the Developing World, edited by Dhavendra Kumar, 47–54. Oxford: Oxford University Press.
- Salman, Rustam Al-Shahi, Elaine Beller, Jonathan Kagan, Elina Hemminki, Robert S. Phillips, Julian Savulescu, Malcolm Macleod, Janet Wisely, and Iain Chalmers. 2014. “Increasing Value and Reducing Waste in Biomedical Research Regulation and Management.” The Lancet 383: 176–185. doi:10.1016/S0140-6736(13)62297-7.
- Sampogna, Christina. 2008. Collaborative Mechanisms for IPR Management: Theory & Practice. Stockholm Network Workshop. Paris: Organisation for Economic Co-operation and Development, September 22.
- Sboner, Andra, Xinmeng Jasmine Mu, Dov Greenbaum, Raymond K. Auerbach, and Mark B. Gerstein. 2011. “The Real Cost of Sequencing: Higher Than you Think!” Genome Biology 12: 125. doi:10.1186/gb-2011-12-8-125.
- Séguin, Béatrice, Billie-Jo Hardy, Peter A. Singer, and Abdallah S. Daar. 2008. “Genomic Medicine and Developing Countries: Creating a Room of Their own.” Nature Reviews Genetics 9: 487–493. doi:10.1038/nrg2379.
- Seung, Sebastian. 2012. Connectome. Boston, MA: Houghton Mifflin Harcourt.
- Singer, Peter A., Archana Bhatt, Sarah Frew, Heather Greenwood, Deepa L. Persad, Fabio Salamanca-Buentello, Béatrice Séguin, Andrew D. Taylor, Halla Thorsteindottir, and Abdallah S. Daar. 2005. “The Critical Role of Genomics in Global Health.” Global Forum Update on Research for Health 2: 113.
- Smith, Richard D., Halla Thorsteindottir, Abdallah S. Daar, Richard Gold, and Peter A. Singer. 2004. “Genomics Knowledge and Equity: A Global Public Goods Perspective of the Patent System.” Bulletin of the World Health Organization 82: 385.
- STIC (Science, Technology and Innovation Council). 2012. State of the Nation 2012 – Canada’s Science, Technology and Innovation System: Aspiring to Global Leadership. Ottawa: STIC.
- TIP (The Innovation Partnership), International Expert Group on Biotechnology, Innovation and Intellectual Property. 2008a. Toward a New Era of Intellectual Property: From Confrontation to Negotiation. Montreal: TIP.
- TIP (The Innovation Partnership), International Expert Group on Biotechnology, Innovation and Intellectual Property. 2008b. Designing Metrics to Assess the Impacts and Social Benefits of Publicly Funded Research in Health and Agricultural Biotechnology. Montreal: TIP.
- TR (Thomson Reuters). n.d. Web of Science. TR. Accessed April 7, 2016. http://ipscience.thomsonreuters.com/product/web-of-science/?utm_source=false&utm_medium=false&utm_campaign=false.
- UNICEF (United Nations Children’s Fund). 2014. Generation 2030/Africa: Child Demographics in Africa. New York: UNICEF.
- UNMPSTTF (United Nations Millennium Project Science and Technology Task Force), Genomics Working Group. 2004. Genomics and Global Health. Toronto: University of Toronto Joint Centre for Bioethics.
- Ward, Derek J., Angharad Slade, Tracey Genus, Orsolina I. Martino, and Andrew J. Stevens. 2014. “How Innovative Are New Drugs Launched in the UK? A Retrospective Study of New Drugs Listed in the British National Formulary (BNF) 2001–2012.” BMJ Open 4: e006235. doi:10.1136/bmjopen-2014-006235.
- WHO (World Health Organization). 2002. Genomics and World Health. Geneva: WHO.
- WHO (World Health Organization). n.d. Genomics and the Global Health Divide. WHO. Accessed April 7, 2014. http://www.who.int/genomics/healthdivide/en/.
- WHO (World Health Organization). 2015. Investing to Overcome the Global Impact of Neglected Tropical Diseases. Geneva: WHO.
- Williams-Jones, Bryn, and Oonagh P. Corrigan. 2003. “Rhetoric and Hype: Where’s the ‘Ethics’ in Pharmacogenomics.” American Journal of Pharmacogenomics 3: 375–383. doi: 10.2165/00129785-200303060-00004
- Yun, Hong-Min, Manshu Song, and Wei Wang. 2012. “Traditional Chinese Medicine in the Area of Genomics.” In Genomics and Health in the Developing World, edited by Dhavendra Kumar, 842–845. Oxford: Oxford University Press.
