Abstract
Clinical genomics is a system of multiple stakeholders and institutions. Yet, studies focusing on the comparative perspectives of these stakeholders are limited. This study engages four groups of professionals (clinical geneticists, genetic counselors, laboratory professionals, and researchers) working in clinical genomics to investigate their perceptions of the benefits and risks of using genomics in Australian healthcare. The study is underpinned by a risk governance approach. For data collection, qualitative semi-structured interviews were used. Our results show that all professionals unanimously identified that the benefit of clinical genomics lies in improving health outcomes for patients. However, the risks associated with delivering this benefit differed by professional category. We found that the further the profession was from the patient (e.g. researcher) the narrower the perceived risks were amongst the individuals interviewed. However, “privacy” as a perceived risk was ranked highly by all professions indicating a shared desire for responsible data governance practices.
Introduction
It is well-argued in the existing literature that next-generation sequencing (NGS) (Gaff et al. Citation2017; Zhao et al. Citation2021) as well as advanced bioinformatics analysis and data interpretation (Crockett and Voelkerding Citation2015; Tempini Citation2021; Lewis and Bartlett Citation2013) have significant potential to transform how genetic and rare diseases are diagnosed, treated, and contribute towards precision medicine (Ashley Citation2016; Tutton Citation2014). For example, due to advances in genomic technologies, genetic conditions such as Alport syndrome can now be diagnosed more efficiently (Savige et al. Citation2019; Kashtan Citation2021) and there is a new gene therapy available for treatment (Daga et al. Citation2020). Additionally, the ability to detect breast cancer susceptibility genes (BRCA 1 and 2) has helped numerous families with a history of breast cancer to identify whether they carry these inherited genes (Gorodetska, Kozeretska, and Dubrovska Citation2019).
The use of these new and emerging genomic technologies in healthcare, however, requires a holistic approach to assessing, categorizing, communicating, and managing any associated risks (Gottweis Citation2005). Our review of the genomics literature suggests the most common sources of risks associated with clinical genomics are routinely identified as: (i) clinical uncertainty (Char et al. Citation2018; Butterfield et al. Citation2019; Robinson et al. Citation2017; Lincoln et al. Citation2021); and (ii) data governance (Prince and Berkman Citation2018; Selita et al. Citation2020; Lowrance and Collins Citation2007). These two sources are inherently technological in nature. Yet, their impacts extend beyond technical risks to pose social and ethical implications. For example, it has been argued that the uncertainties related to the accuracy of NGS arising from false positives or negatives could put the psychological safety and well-being of patients at risk (Char et al. Citation2018) (Butterfield et al. Citation2019). For example, Robinson et al. (Citation2017) report the existence of false positives in the use of NGS in cancer research. Further, given the level of sensitive information genome data holds, poor data governance could compromise patient’s and family’s privacy, and raise questions about the ownership of genome data (Prince and Berkman Citation2018; Tabor et al. Citation2012). Suyash and Bustamante D (Citation2015), for example, demonstrate the susceptibility of re-identification of individuals based on genome data in a web-based data storage service.
In this context, as with any other, risk can be characterized as objective or subjective in nature (Hansson Citation2010). The former generally refers to the quantitative probability of a risk occurring, which measures the risk in relation to its likelihood and severity. The latter is more often associated with qualitative descriptions of risk, as perceived and characterized by relevant stakeholders and informed by their own perspectives and experiences. Our aim in this study is to explore subjective risks (also referred as “risk perceptions”) along with the perceived benefits associated with clinical genomics from the perspectives of four professional groups working in the Australian healthcare system. Renn (Citation2008) writes that perceived risks are equally important as objective risks for identifying, understanding and managing risks in a holistic way. He notes that in some cases it is irrelevant whether these perceptions have a direct correspondence with the physical world as long as those concerned believe that these perceptions matter to them or to objects, processes or people they care about. The relevance is that these perceptions can powerfully shape how people behave and understand the world around them and their role within it. Renn (Citation2008) further argues that developing an understanding of how risks are perceived by various actors within a system can play a central role in a robust risk governance approach.
Although studies examining risk perceptions exist in the broader ethical, legal, and social implications (ELSI) literature (e.g. Henderson et al. Citation2012; Burke et al. Citation2015), there are three gaps that this study aims to address. First, the majority of existing studies have been conducted in either clinical (Johnson et al. Citation2017; Chen Citation2021; Wright et al. Citation2019; Koay and Sharp Citation2014) or research settings (Butterfield et al. Citation2019; Burke et al. Citation2015) only, with the role of laboratories largely overlooked (Vears, Sénécal, and Borry Citation2020). In this study, we aim to explore perceptions across research, laboratory and clinical settings to understand how stakeholder perceptions may be shaped. Second, we can find no evidence of studies that compare how risks in clinical genomics are perceived by different professionals operating within that system. The value of identifying any differences in risk perceptions among professionals working across a broader system is that it can assist with identifying any potential for miscommunication or misinterpretation of the value proposition of clinical genomics. Such variation poses its own risk of creating a barrier to the integrated delivery of genomics into healthcare. Third, most risk-focused studies have concentrated on the application side of clinical genomics (O’Shea et al. Citation2020; Veenstra et al. Citation2010) (i.e. post technology development), without examining the potential risks during the technology development phase (e.g. sequencing, data analytics and reporting). To address these gaps, using a risk governance approach, we study the risk perceptions of professionals working across the clinical genomics system; that is, clinical geneticists, genetic counselors, laboratory professionals (comprised of bioinformaticians and pathologists) and researchers. Against this backdrop, this study aims to investigate how professionals working in the clinical genomics system perceive the benefits and risks of using genomics in Australian healthcare.
Risk governance is a well-established theory that has been used to assess a number of emerging and potentially disruptive technologies (Renn and Roco Citation2006; Malakar, Lacey, and Bertsch Citation2022). It is underpinned by the idea that decisions about risks do not occur in silos but arise from multi-actor and multi-institutional processes, where decision-making power is distributed (Renn Citation2008). That is why the involvement of multiple actors and institutions from a shared system is critical to identifying the breadth of benefits and risks (Renn and Schweizer Citation2009). For this reason, risk governance is a useful approach for our research because clinical genomics operates as a complex adaptive system (Long et al. Citation2019), comprised of a network of actors and institutions (Long et al. Citation2021; Best, Stark, Phillips, et al. Citation2020; Bowdin et al. Citation2014), each having crucial roles in shaping and making decisions about the benefits and risks, and working collaboratively to balance the two (Delaney et al. Citation2016). We therefore argue that engaging with multiple actors that play pivotal roles in clinical genomics provides an opportunity to broaden our knowledge of the benefits and risks associated with clinical genomics. In so doing, we focus on the perspectives of professionals working in the field of clinical genomics in Australian healthcare.
In Australia, healthcare is publicly funded by the Australian health insurance scheme, Medicare. The States, Territories, and the Commonwealth Government collectively provide funding to deliver healthcare (Burns et al. Citation2019). The Medicare Benefits Schedule (MBS) provides financial assistance towards the cost of medical services that are listed on the MBS. The Medical Services Advisory Committee (MSAC) assesses new medical tests and technologies for their safety, effectiveness, and cost-effectiveness and provides recommendations about those tests and technologies to the federal Minister for Health (Norris et al. Citation2022). Clinical genomics in Australia, traditionally the purview of clinical genetics services, is increasingly recognized by Medicare as a range of genomic tests is already listed on the MBS (Stark et al. Citation2019).
Australia is making rapid progress in integrating genomics into clinical practice, with the Australian Genomics Health Alliance (AGHA) promoting collaborative research and partnerships across various domains in the sector (Stark et al. Citation2019). In 2017, Australia issued its first National Health Genomics Policy Framework 2018-2021, developed to facilitate the integration of genomics into the Australian health system (Australian Government Citation2017). Along with a range of benefits, the Framework also identifies the possibility of genomics presenting risks to individuals and society, thereby indicating the need for carefully managing both. At the time of writing this paper, a nationally coordinated approach to managing genomic data was underway, with genomic data in Australia protected by the Privacy Act 1988 (Cth).
The complexity any risk-benefit assessment increases as the number of key players and institutions in the system increases (Renn Citation2008). A recent study demonstrates the presence of a complex clinical genomics landscape in Australia’s health system, with the participation of diverse stakeholders and the lack of clarity of each other’s roles within the system (Long et al. Citation2021). By bringing the perspectives of stakeholders from laboratory, research, and clinical settings together, we believe this study sheds new light on the insights and experiences of these stakeholders, especially with regard to their perceptions of the key benefits and risks of clinical genomics in Australian healthcare. With the increasing use of genomics in clinical practice globally, we also see potential to translate the lessons from this research to other country contexts, with similar healthcare systems.
Methods
Participant selection
We applied two methods to identify potential participants for the study. First, we identified research institutions, hospitals, clinics, and laboratories involved in clinical genomics in Australia via publicly available information. A list of potential participants belonging to the four professional categories, clinical geneticists, genetic counselors, laboratory professionals, and researchers, were identified from these institutions. Invitations to participate in this study were then sent out to 46 purposefully selected potential participants of which 19 agreed to participate. Second, a snowball sampling method (Marshall and Rossman Citation2011) was used to identify additional participants. A total of 10 potential participants were identified via this method, of which six agreed to participate. In total, 25 professionals participated in this study. We acknowledge that other stakeholders are critical to clinical genomics, including administrators, patients, insurance officers, and policymakers. These stakeholders were not included in this study and so our findings are limited in this aspect. However, we believe that the perceptions of the selected four groups of stakeholders were more comparable for the purpose of this initial study of stakeholder perceptions.
The four professional categories in this research have a unique set of roles. Clinical geneticists are medical doctors specializing in genomics. They are frontline professionals working closely with patients and families. They help patients and families to diagnose and treat genetic conditions. Their primary responsibilities include but are not limited to ordering genomic tests, interpreting, and communicating genomic test results. The clinical geneticists who participated in this study worked at hospitals or research institutions.
Similarly, genetic counselors are frontline professionals working closely with patients and families. Their primary role is to help patients and families understand genetic conditions and their causes through pre- and post-test counseling. Note that genetic counseling is not necessarily available for all genetic tests, and there are other methods available for providing education, such as interactive online platforms. Genetic counseling is prioritized for those interactions that have potential sensitivity, such as tests with reproductive implications and with predictive outcomes. The genetic counselors who participated in this study worked with genetic services teams at hospitals or research institutions.
Laboratory professionals work closely with clinical geneticists and genetic counselors. This category is comprised of pathologists and bioinformaticians. Pathologists are medical specialists who help analyzing genetic tests and interpreting results. Bioinformaticians perform informatics analysis of genome data to produce genomic test results, using computational tools. The laboratory professionals who participated in this study were employed by hospitals or public laboratories.
Researchers work closely with all other professional groups. They conduct scientific studies to develop new computational tools for informatic analysis or improve patient care in clinical genomics in other ways. Two clinical geneticists, one genetic counselor, and one laboratory professional conducted research as their secondary role. However, we directed questions to them according to their primary role.
Data collection and analysis
We used in-depth interviews to collect data for this study. All interviews were conducted by the lead author via telephone, and all participants provided their informed consent to participate. All participants, except one, were unknown to the interviewer. For interviews, a pretested protocol was used, which covered a range of topics including but not limited to perceived benefits and risks of clinical genomics, societal implications of genomic technologies, and any challenges they experienced in their roles. Questions related to perceived benefits and risks were structured and asked to all participants. No follow-up interviews were conducted for this research. On average, interviews were 45 min in duration. With permission, all interviews were audio recorded and transcribed for data analysis. This research was approved by CSIRO’s Social Science and Interdisciplinary Human Research Ethics Committee (093_20).
For data analysis, we used thematic analysis using a deductive approach (Braun and Clarke Citation2006). Themes were deductively organized into two groups of perceived benefits and risks, using the R package for Qualitative Data Analysis (RQDA) (Huang Citation2018) in the R Software (R Core Team Citation2018). The data was analyzed in the following four stages:
The transcripts were cleaned, and files converted into.txt format for upload into RQDA for thematic analysis.
For coding, two researchers read the transcripts separately and segments (i.e. sentences and paragraphs) of transcripts were assigned to codes (Bradley, Curry, and Devers Citation2007) associated with perceived benefits and risks.
After the first round of coding, meetings to discuss the initial analysis led to further coding. These meetings were useful to deal with discrepancies and finalize a framework for analysis (Braun and Clarke Citation2006). Themes were identified by grouping similar meaning codes, resulting in two and five themes under the perceived benefits and the perceived risks categories respectively and are reported in this paper. The final themes were reviewed and confirmed by both researchers. The involvement of multiple researchers to identifying themes, de-briefing and comparing notes, correcting errors to harmonize the coding process, and developing a metadata of codes helped to ensure reliability and validity and added rigor to the data analysis (Morse et al. Citation2002).
Once themes were identified, we used supplementary counting to supplement the qualitative thematic analysis (Hannah and Lautsch Citation2011) in presenting the results. Increasingly, the method of counting has been gaining traction among social science and medical scholars, for example, Emery et al. (Citation2021), Reiss, Greene, and Ford (Citation2017), and Best, Stark, Brown, et al. (Citation2020). We documented number of times each theme has been discussed by participants in all professional categories. Doing so helped us to compare the prominence of themes (Sandelowski Citation2001) by each professional category.
Results
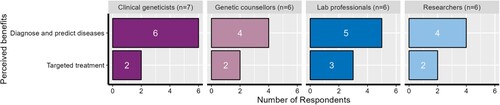
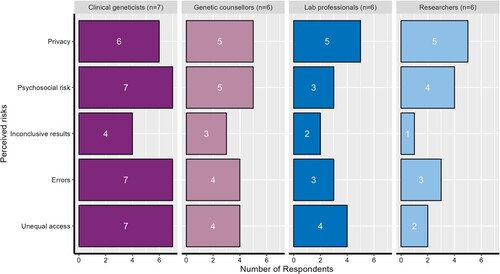
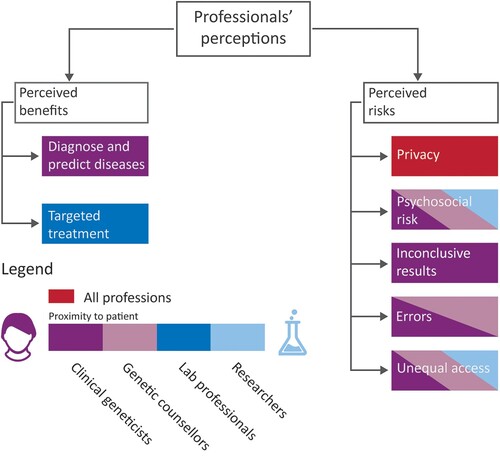
Participants described their perceptions of benefits and risks in the interviews. From the thematic analysis, we identified two themes associated with benefits and five themes associated with risks. The identified benefits and risks were based on participants’ perceptions, informed by their professional experiences, with several themes relating to patients and their care. shows a summary of the benefits and risks and how they were ranked by the four professional groups interviewed (i.e. the color of the theme reflects which professional group ranked that theme most highly). In the following sections, we discuss these observations in more detail.
Figure 1. Benefits and risks perceived by Australian professionals across the clinical genomics system.
Perceived benefits
Participants across all professional categories identified the benefits of clinical genomics for patients with genetic conditions. Although clinical genomics is a system comprising multiple institutions and stakeholders with varying roles, the benefits identified in this research unanimously related to patients, suggesting strong agreement about the main purpose of clinical genomics. The two themes that emerged from our analysis of perceived benefits were: “diagnose and predict disease risks” and “targeted treatment.” shows the frequency with which the four types of professionals perceived these benefits.
Diagnose and predict disease risks
Within this theme, participants described the increased capability of genomics to diagnose genetic diseases and predict genetic risk factors. Participants across all four groups agreed that the diagnostic rates, particularly in relation to rare diseases and cancers, had increased significantly in the last decade, and genomic tests were now able to predict the risk of inherited genetic conditions in families more accurately. All participants emphasized that this level of accuracy in genetic disease diagnosis had not previously been possible but had been made possible by the ability to sequence the whole human genome. One laboratory professional expressed this as follows:
We are seeing diagnostic rates go from five or 10 percent to 40–50 percent. So that has been fantastic for patients and families in the rare disease space. We couldn’t have done that by looking at individual genes. Laboratory professional (L16)
… there’s a huge disconnect between what’s happening in academia and how it is actually shaping medicine. There are particular cases that really make a lot of sense for doing the NGS […] say in the instance of a neonate, they are born with some sort of metabolic abnormality […] In that case, having an unbiased screen of the entire genome makes a lot of sense […] The system [however] hasn’t caught up and adapted to [new research findings]. Researcher (R2)
Targeted treatment
“Targeted treatment” was also identified as a benefit arising from clinical genomics. Although this theme was identified with less frequency, at least two participants across all groups generally described genomics being used to develop targeted approaches for treating genetic mutations by, for example, tailoring surveillance strategies to the genetic predispositions of patients. Due to genomic sequencing, participants reported, it is possible to monitor specific changes in patients’ conditions, which allows clinicians to make treatment decisions. Participants indicated that being able to diagnose patients’ illness is a pre-requisite to targeted treatment.
There were, however, some differences in how “targeted treatment” was expressed across the professional categories. Clinical geneticists and laboratory professionals focused on the available genomic tests and genomic sequencing in tailoring treatment for patients. Particularly in the treatment of cancer, they reported that they use genomic sequencing to study mutations in tumors and based on those mutations, patients were treated. The following quote from a clinical geneticist working in the field of colorectal cancer illustrates this:
Tailoring chemo preventative options based on a genetic disposition is a clear advantage for going ahead in that direction of genetic profiling … which was never available before and offers great prospects moving forward for individual patients based on their genomic tumour profiling. Clinical geneticist (C5)
It [Genomic testing] could outline an existing treatment, in some cases which could be very simple even, and that could make a big difference […] there’s a whole bunch of metabolic disorders where the metabolite can be supplemented, and the disease can be avoided completely. Researcher (R3)
Perceived risks
Participants were asked about risks they perceived in the application of clinical genomics in Australian healthcare. In response, participants discussed risks concerning genomic technologies, data security, the mental health of patients, genomic sequencing processes, lack of available skilled human resources, existing genomic databases, and access to genomic tests. During the data analysis, we grouped their responses into five themes that captured a range of multidimensional risks. These themes are “privacy”, “psychosocial risks,” “inconclusive results,” “errors,” and “unequal access.”
shows the number of participants reporting these perceived risks by professional category. At face value, it appears that there is a potential pattern emerging between frontline professionals (clinical geneticists and genetic counselors) and support professionals (laboratory professionals and researchers). That is, clinical geneticists discussed all categories of risk with the highest frequency, followed by genetic counselors. Laboratory professionals and researchers discussed these risks less frequently in their interviews.
Privacy
“Privacy” was identified almost unanimously across the professional categories. Participants associated privacy risks with data leakage, identifiability, misuse, discrimination, and the potential for unintended data reuse, confirming some key issues that have been reported in the literature. All identified genomic data as “sensitive data” in accordance with Australian privacy regulations and requiring protection. Participants expressed their concerns about the possibility of discrimination against patients by insurance companies or employers based on their genetic conditions, and that such discrimination could affect patients’ mental well-being, which extended privacy beyond technical risk toward the more normative-oriented risks and their effects. Within this theme, we also identified the view that sharing data is critical to advancing science in clinical genomics. However, to achieve that, it was agreed by participants that stringent policies to safeguard patients’ information was an important step. The need for data sharing in a responsible manner was explained by one clinical geneticist who worked in the field of padiatric rare disease.
… data sharing is critical in order for us to maximise the outcomes of the test, what are the implications for individuals … to be re-identified from genomic data? So, how do we do this in a way that’s responsible and maximises the health benefits but yet protects privacy? Clinical geneticist (C6)
We need to have a federated approach to genomic data, which will control access and will allow people to recognise the value of it, whilst at the same time protecting it. Genetic counsellor (G19)
… from my point of view, these [privacy issues] are somewhat overblown … we’ve received legal advice that says that genomic data in isolation is not considered to be reasonably re-identifiable. It is re-identifiable, but only if you’ve got the keys necessary to open that backup … any patient identifying information, such as date of birth … Laboratory professional (L22)
Psychosocial risk
Under the theme of “psychosocial risks,” participants discussed how genomic tests could potentially affect the psychological and social well-being of patients. From the data analysis, this theme was discussed by the majority of clinical geneticists, followed by genetic counselors, researchers, and laboratory professionals. Participants identified that psychosocial risk can be caused by a range of factors including incidental findings and patients’ unrealistic expectations.
Both clinical geneticists and genetic counselors perceived incidental findings as the major factor for causing psychological distress to patients, explaining there can be a possibility of revealing results that are not the focus of the initial testing and to which patients may not have indicated to be made aware of. A genetic counselor, working in the field of familial cancer, explained how incidental findings could cause a risk to patients’ psychological well-being because patients may not be prepared for such findings:
I guess people if they’re having some sort of genomic testing, they’re likely doing it for a particular reason. There’s a particular diagnosis or something that they want to try or that the clinical team want to get to the bottom of it … so [for example] coming in for a cholesterol based inherited condition and then they [patients] find out incidentally that they’ve got a BRCA mutation. It might not be something that they’re necessarily prepared for or wanted to know. Genetic counsellor (G20)
You also could view incidental findings as a benefit, in a way, because it sort of gives you the ability to maybe take action sooner than you would have been able to, had you not known you were predisposed to a certain condition. Laboratory professional (L10)
Sometimes there can be challenges around managing expectations for patients. Sometimes people think that this is definitely going to give them an answer … Ensuring that people really understand and are really informed when they provide consent for testing. Genetic counsellor (G4)
Inconclusive results
In this theme, participants reported improvements in diagnostic rates, but also discussed the possibility of not receiving a confirmed diagnosis from a genomic test. Clinical geneticists identified this risk with most frequency followed by genetic counselors, lab professionals, and researchers. All participant groups discussed this theme and explained limitations in both technology and reference databases as contributing factors. According to participants, not all pathogenic variants in genes have been identified, meaning current testing may not yet have the capacity to identify all variants, thus, leading to inclusive results in some circumstances. Participants suggested that having a larger and more representative genome database could address this. This was expressed by one researcher as follows:
It comes down to the more diverse your data is the more robust and accurate your model will be […] we don’t have enough data to train these models [yet] … Researcher (R1)
… [for] families who thought they were going to get an answer and don’t, they will often have questions as to why. Where we’ve thought that we were going to find an answer, but we didn’t … Clinical geneticist (C18)
… that [inconclusive results] is always a risk. That’s the risk of all the research that we do, there’s always the risk the findings are inconclusive. You’re probably more surprised if you find something that is conclusive. Researcher (R15)
Errors
In describing the theme of “errors,” participants discussed the possibility of getting false positives or negatives due to human and technological error. This could arise, for example, as a result of mixing up samples, miscalculating or misinterpreting the results. Clinical geneticists discussed this risk with most frequency followed by genetic counselors. An equal number of lab professionals and researchers identified this risk.
Although all participants agreed that the risk of “errors” existed, some participants emphasized that this risk was currently a more secondary concern. However, they believed that this risk would likely increase in the future as genomic testing processes increasingly rely on computational and algorithms. This potential for increasing risk was described by one clinical geneticist as follows:
… in our service, that [risk of errors] is not a problem … because we do a mixture of the computational stuff, but it’s all checked by humans as well and the curation is a human process … But I can certainly see that in the future, [when] we’ll probably be relying significantly more on algorithm-based stuff … we’re not at that stage yet. Clinical geneticist (C18)
it’s a risk, but it’s not a unique risk to genomics … if you got a whole genome or a whole exome, you would only ever get that once in your life and the results that you’d get out of that are what inform you from that point forward. You don’t typically go and repeat that again, so the risks associated with a false positive or a false negative are quite high but the likelihood of that risk actually happening are quite low. Laboratory professional (L22)
Unequal access
The theme of “unequal access” captures how participants perceived potential risk associated with inequitable distribution and access to genomic tests in Australia. All professional groups discussed this risk, and all clinical geneticists and the majority of genetic counselors spoke of it in their interviews. Participants described a range of contributing factors to this risk including a lack of doctors (general practitioners and specialists) trained in genomics, the cost of testing itself, and regional availability of genomic testing and services. Clinical geneticists spoke of the lack of doctors trained in genomics and the cost of testing most frequently, whereas genetic counselors, lab professionals, and researchers emphasized the costs associated with testing as contributing to unequal access.
Clinical geneticists indicated that the demand for genomic tests was increasing, but that this was not necessarily accompanied by the availability of trained professional to meet this demand. A clinical geneticist described this challenge as follows:
There’re many challenges in delivering genomic tests successfully. But one of the principal challenges is the number of skilled people that you have that are able to interpret the data and produce a meaningful result. Clinical geneticist (C6)
Discussion
We aimed to understand the perceptions of benefits and risks of processionals working across the clinical genomics system. In so doing, we also intended to examine whether their professional roles influence their perceptions of benefits and risks. Based on the results, we present three key findings.
Shared view of benefits and a multidimensional view of risks
In the last decade, the benefits of using clinical genomics have been discussed widely in the healthcare and medicine scholarship. Most of these studies indicate that the application of genomics has the greatest benefit to patients via improved diagnosis and treatment outcomes (Char et al. Citation2018; Delaney et al. Citation2016). Similarly, the Australian National Health Genomics Policy Framework envisions that genomics has potential to fundamentally change how diseases are diagnosed, treated, monitored, and prevented (Australian Government Citation2017). Our study echoes these previous claims, as there was strong agreement among all four professional groups in our study on the benefits of clinical genomics in improving patients’ diagnosis, treatment and health outcomes. This demonstrates how the narrative around the benefits of clinical genomics is consistent across multiple professional categories, established literature, and policy documents.
However, what our research also revealed is that there is a much more nuanced and multidimensional view of the potential risks associated with clinical genomics across different professional groups working in this field. These risks comprised ethical (privacy and psychosocial risks), technical (inconclusive results and errors), and social considerations (unequal access). Ethical risks such as privacy (Rodriguez et al. Citation2013; Selita et al. Citation2020) and psychosocial risks (Johnson et al. Citation2017; Hammer Citation2019) have been identified in other studies. Although some studies suggest psychological impacts to cancer patients from genomic tests are not significant (Heshka et al. Citation2008; Meiser Citation2005; Schlich-Bakker, ten Kroode, and Ausems Citation2006), our participants widely acknowledged that genomic tests in general can affect patients’ psychological well-being. Technical risks associated with clinical genomics have not been examined to any great extent in the existing literature, and we believe the identification of these risks is one of the contributions of this study. The limited discussion on technical risks, as argued by O’Shea et al. (Citation2020), is likely because most risk-related studies focus on the application side of clinical genomics. We were able to identify these risks because our study adopted a systems perspective, which included potential risks associated with the development and use of genomic technologies. Potential unequal access to genomics tests has also been discussed in the literature (Burns et al. Citation2019; Reynolds Citation2020). In line with these studies, our participants also described the potential risk of some patients being deprived of access to these tests for various reasons. By applying a risk governance approach, we were able to identify multidimensional risks perceived by professionals in the clinical genomics system.
Influence of professional roles on risk perception
An individual’s perception of risk and health is affected by factors such as socio-cultural backgrounds, educational qualification, and gender (Qin et al. Citation2021). Our findings suggest that perceptions of risk are influenced by the participants’ professional roles as well. In relation to the identification of risks, our study shows that across four professional groups a diverse and multidimensional set of risks were identified. The results of our analysis indicated some clear differences emerging between how risks are perceived between frontline professionals (i.e. clinical geneticists and genetic counselors, who are interfacing with patients) and professionals that support those frontline roles (i.e. laboratory professionals and researchers, who do not have direct contact with patients). This finding suggests professionals who work closely with patients tend to perceive a more diverse range of potential risks than those working “behind the scenes.” This characterization of risk is likely because frontline professionals are most involved with patient care and have direct experience with the range of potential impacts of genomic tests at the individual and family level (Pedersen and Vedsted Citation2015).
Although all four professional groups in this study identified all of the risks, there were some subtle differences in their interpretation of those risks. For example, clinical geneticists primarily identified the limited number of doctors specializing in genomics as contributing to the risk of errors and unequal access. Genetic counselors discussed managing patients’ expectations to mitigate psychosocial risks. Given genetic counselors are primarily responsible for communicating with patients before and after genomic tests (Middleton et al. Citation2017), their role also enabled them to understand the nature of these impacts on patients’ psychological well-being. Laboratory professionals perceived the issues around privacy risk as potentially being overblown and expressed the view that there was limited risk of such data being used to reidentify individuals. Their view differed quite markedly from the other professional groups in this study. This perception of laboratory professionals appears to be influenced by their professional background, as laboratories perform genomic sequencing (Vears, Sénécal, and Borry Citation2017) and play a lead role in managing patients’ data. The role of privacy risks in this study is of interest and is explored further in the next section.
While other studies highlight a need for the involvement of multiple professions in clinical genomics (Best, Stark, Brown, et al. Citation2020; Long et al. Citation2021), mapping how these four professional groups perceived risks and the differences in their perceptions allowed us to capture a wider set of perceived risks, from a systems perspective. This also suggests that building a collective understanding of the roles and boundaries of professionals (Wright et al. Citation2019) across the clinical genomics system will help building a greater awareness of the interconnected nature of risks and responsibilities that exist within that system (Bowdin et al. Citation2014; Vears, Sénécal, and Borry Citation2020; Douglas, Lacey, and Howard Citation2022).
The role of privacy in unlocking responsible data governance
While this study highlights the value of developing an understanding of the nuanced and diverse range of perceived risks within a system, privacy was identified with the most frequency and by almost all participants, irrespective of their professional category. For example, most participants expressed concerns about data leakage, identifiability, misuse, and potential for discrimination based on patients’ genetic data/conditions (e.g. by insurance companies or employers). The risk of privacy also has a potential ripple-effect, which means that one risk has the potential to trigger several other risks (Renn Citation2021) (e.g, potential for privacy risk to trigger psychosocial risk). Further to this, uncertainty about how data may be used in the future can lead to patients’ hesitancy to undertake genomic tests (Middleton et al. Citation2020; Sanderson et al. Citation2016). Our participants recognized that managing the privacy risk was not only important for protecting patient privacy and their confidence in clinical genomics, but it also had the potential to impact on the development of the field (Tutton Citation2007).
The realization of the benefits of clinical genomics is highly data dependent (Tempini Citation2021; Tempini and Leonelli Citation2021). It could be argued that the notion that all see privacy as a critical risk to the success of clinical genomics may be shaped by the fact that all professionals depend on access to data in order to improve their capacities to fulfill their respective professional roles. However, studies examining the nuance of this ethical tension between privacy and genome data sharing are growing, most highlighting that absolute privacy limits data sharing and affects the pace of biomedical research (Rodriguez et al. Citation2013; Shabani, Thorogood, and Borry Citation2016). Advances in clinical genomics rely on how much data is available and how frictionlessly the data can be shared. While privacy is a frequently recognized risk in clinical genomics, the priority that it receives across different professional groups suggests it may represent an area of focus that could unlock potential benefits in research and clinical practice (Malakar et al. Citation2023). As data collection and sharing are building blocks for the success of clinical genomics, having a structure that patients’ trust and safeguards their privacy could realistically contribute to enacting a risk governance approach in clinical genomics. Our study indicates that a deeper exploration of the privacy risks posed by clinical genomics and data sharing for medical advancements, not only the views of professionals but also that of patients, will be critical to develop appropriate policy-relevant actions.
Conclusion
The use of genomics in clinical practice delivers significant benefits to patients worldwide and in Australia. The advent of NGS and bioinformatics platforms opened new pathways to diagnose and treat genetic and rare diseases. Along with these benefits, concerns about potential risks associated with clinical genomics have also been raised. As clinical genomics is a system of multiple professionals and institutions, we aimed to understand how various professionals working in a range of roles and institutions perceived benefits and risks in clinical genomics in Australian healthcare, and whether understanding those perceptions would reveal a more nuanced set of considerations about perceived risks and benefits than currently presented in the genomics literature.
Our results show that professionals across all categories perceived improvement in healthcare for patients with genetic conditions as the primary benefit. These professionals also perceived multiple dimensions of risks across ethical, technical, and social domains. Our findings also suggest that risks are perceived differently depending on how directly the professionals' work brings them into contact with patients. That is, those working directly with patients have professional experience with the range of ways clinical genomics may impact upon patients directly (in a clinical setting), whereas researchers may view risk more theoretically or simply within the context of their own professional role and engagement with the data (and not with an actual patient with lived experience of some of the risks explored). The diversity of these perceptions can help to create how risk and responsibility may be managed by various professionals in their own roles and in collaboration with each other. Further, the existing literature recognizes the vulnerability posed by data storage systems and the associated risks. Our finding regarding the shared prioritization of privacy as a key risk validates existing calls for a responsible data governance platform that not only enables safeguarding patients’ privacy but promotes protected data sharing across Australia. We also believe that having a diverse set of professionals in this study helped us to understand a broader range of risks perceived by key professionals in clinical genomics.
There are some limitations of this research, which warrant further examination. We interviewed four groups of professionals only. We acknowledge that these professionals are parts of a (much) larger system. Therefore, the findings do not necessarily represent all perceived risks that might be associated with the clinical genomics system. Rather, this study revealed the complexity and subtleties of risk perceptions that arise, even among a relatively small subset of stakeholders, and the influence of their professional contexts on their perceptions. Future research might usefully explore the perceptions of responsibilities these professionals have in addressing these perceived risks in the clinical genomics system.
Finally, the ethical risks associated with data governance in clinical genomics is a well-established in the literature, so it is perhaps unsurprising that privacy was unanimously identified as a potential risk. In considering this result, it might also be argued that stricter privacy protocols could lead to limited data access and, eventually, limited success of clinical genomics, which would limit the roles of the professionals interviewed for this study and how they also characterized the benefits to patients. Further, a more comprehensive interrogation of potential privacy risks that includes the views and lived experiences of patients is required to build a more robust understanding of the nature of those risks. The benefits and risks identified in this study are based on the perceptions of the four groups of professionals, and in some cases those perceptions vary between professional groups and from the well-established benefits and risks that have been identified in the literature. However, such perceptions provide a foundation for identifying a broader range of benefits and risks among stakeholders that may be necessary for identifying more comprehensive and responsive risk mitigation approaches in the delivery clinical genomics.
Supplemental Material
Download MS Word (22.6 KB)Acknowledgements
We would like to thank the Australian Genomics Health Alliance for their support in data collection. Thanks to all participants for taking part in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ashley, Euan A. 2016. “Towards Precision Medicine.” Nature Reviews Genetics 17 (9): 507–522. doi:10.1038/nrg.2016.86.
- Australian Government. 2017. “National Health Genomics Policy Framework.” In, edited by Department of Health, 1-14. Canberra, ACT.
- Best, Stephanie, Zornitza Stark, Helen Brown, Janet C. Long, Kushani Hewage, Clara Gaff, Jeffrey Braithwaite, and Natalie Taylor. 2020. “The Leadership Behaviors Needed to Implement Clinical Genomics at Scale: A Qualitative Study.” Genetics in Medicine 22 (8): 1384–1390. doi:10.1038/s41436-020-0818-1.
- Best, Stephanie, Zornitza Stark, Peta Phillips, You Wu, Janet C Long, Natalie Taylor, Jeffrey Braithwaite, John Christodoulou, and Ilias Goranitis. 2020. “Clinical Genomic Testing: What Matters to key Stakeholders?” European Journal of Human Genetics 28 (7): 866–873. doi:10.1038/s41431-020-0576-1.
- Bowdin, Sarah, Peter N. Ray, Ronald D. Cohn, and M. Stephen Meyn. 2014. “The Genome Clinic: A Multidisciplinary Approach to Assessing the Opportunities and Challenges of Integrating Genomic Analysis Into Clinical Care.” Human Mutation 35 (5): 513–519. doi:10.1002/humu.22536.
- Bradley, Elizabeth H., Leslie A. Curry, and Kelly J. Devers. 2007. “Qualitative Data Analysis for Health Services Research: Developing Taxonomy, Themes, and Theory.” Health Services Research 42 (4): 1758–1772. doi:10.1111/j.1475-6773.2006.00684.x.
- Braun, Virginia, and Victoria Clarke. 2006. “Using Thematic Analysis in Psychology.” Qualitative Research in Psychology 3 (2): 77–101. doi:10.1191/1478088706qp063oa.
- Burke, Wylie, Paul Appelbaum, Lauren Dame, Patricia Marshall, Nancy Press, Reed Pyeritz, Richard Sharp, and Eric Juengst. 2015. “The Translational Potential of Research on the Ethical, Legal, and Social Implications of Genomics.” Genetics in Medicine 17 (1): 12–20. doi:10.1038/gim.2014.74.
- Burns, Belinda L., Gemma A. Bilkey, Emily P. Coles, Faye L. Bowman, John P. Beilby, Nicholas S. Pachter, Gareth Baynam, Hugh J. S. Dawkins, Tarun S. Weeramanthri, and Kristen J. Nowak. 2019. “Healthcare System Priorities for Successful Integration of Genomics: An Australian Focus.” Frontiers in Public Health 7: 41. doi:10.3389/fpubh.2019.00041.
- Butterfield, Rita M, James P Evans, Christine Rini, Kristine J Kuczynski, Margaret Waltz, R Jean Cadigan, Katrina AB Goddard, Kristin R Muessig, and Gail E Henderson. 2019. “Returning Negative Results to Individuals in a Genomic Screening Program: Lessons Learned.” Genetics in Medicine 21 (2): 409–416. doi:10.1038/s41436-018-0061-1.
- Char, D. S., S. S. Lee, D. Magnus, and M. Cho. 2018. “Anticipating Uncertainty and Irrevocable Decisions: Provider Perspectives on Implementing Whole-Genome Sequencing in Critically ill Children with Heart Disease.” Genetics in Medicine 20 (11): 1455–1461. doi:10.1038/gim.2018.25.
- Chen, Haidan. 2021. “Privacy in Breast Cancer Biobank: Chinese Patients’ Perceptions.” Social Science & Medicine 282: 114134. doi:10.1016/j.socscimed.2021.114134.
- Crockett, David K., and Karl V. Voelkerding. 2015. “Bioinformatics Tools in Clinical Genomics.” In Genomic Applications in Pathology, edited by George Jabboure Netto and Iris Schrijver, 177–196. New York, NY: Springer New York.
- Daga, Sergio, Francesco Donati, Katia Capitani, Susanna Croci, Rossella Tita, Annarita Giliberti, Floriana Valentino, et al. 2020. “New Frontiers to Cure Alport Syndrome: COL4A3 and COL4A5 Gene Editing in Podocyte-Lineage Cells.” European Journal of Human Genetics 28 (4): 480–490. doi:10.1038/s41431-019-0537-8.
- Delaney, Susan K., Michael L. Hultner, Howard J. Jacob, David H. Ledbetter, Jeanette J. McCarthy, Michael Ball, Kenneth B. Beckman, et al. 2016. “Toward Clinical Genomics in Everyday Medicine: Perspectives and Recommendations.” Expert Review of Molecular Diagnostics 16 (5): 521–532. doi:10.1586/14737159.2016.1146593.
- Douglas, David M., Justine Lacey, and David Howard. 2022. “Ethical Responsibility and Computational Design: Bespoke Surgical Tools as an Instructive Case Study.” Ethics and Information Technology 24 (1): 11. doi:10.1007/s10676-022-09641-2.
- Emery, Rebecca L., Sydney T. Johnson, Melissa Simone, Katie A. Loth, Jerica M. Berge, and Dianne Neumark-Sztainer. 2021. “Understanding the Impact of the COVID-19 Pandemic on Stress, Mood, and Substance use among Young Adults in the Greater Minneapolis-St. Paul Area: Findings from Project EAT.” Social Science & Medicine 276: 113826. doi:10.1016/j.socscimed.2021.113826.
- Gaff, Clara L., Ingrid M Winship, Susan M Forrest, David P. Hansen, Julian Clark, Paul M Waring, Mike South, and Andrew H. Sinclair. 2017. “Preparing for Genomic Medicine: A Real World Demonstration of Health System Change.” npj Genomic Medicine 2 (1): 16. doi:10.1038/s41525-017-0017-4.
- Gorodetska, Ielizaveta, Iryna Kozeretska, and Anna Dubrovska. 2019. “BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance.” Journal of Cancer 10 (9): 2109–2127. doi:10.7150/jca.30410.
- Gottweis, Herbert. 2005. “Governing Genomics in the 21st Century: Between Risk and Uncertainty.” New Genetics and Society 24 (2): 175–194. doi:10.1080/14636770500184818.
- Hammer, Marilyn J. 2019. “Beyond the Helix: Ethical, Legal, and Social Implications in Genomics.” Seminars in Oncology Nursing 35 (1): 93–106. doi:10.1016/j.soncn.2018.12.007.
- Hannah, DavidR, and BrendaA Lautsch. 2011. “Counting in Qualitative Research: Why to Conduct it, When to Avoid it, and When to Closet it.” Journal of Management Inquiry 20 (1): 14–22. doi:10.1177/1056492610375988.
- Hansson, Sven Ove. 2010. “Risk: Objective or Subjective, Facts or Values.” Journal of Risk Research 13 (2): 231–238. doi:10.1080/13669870903126226.
- Henderson, Gail E, Eric T Juengst, Nancy MP King, Kristine Kuczynski, and Marsha Michie. 2012. “What Research Ethics Should Learn from Genomics and Society Research: Lessons from the ELSI Congress of 2011.” Journal of Law, Medicine & Ethics 40 (4): 1008–1024. doi:10.1111/j.1748-720X.2012.00728.x.
- Heshka, Jodi T., Crystal Palleschi, Heather Howley, Brenda Wilson, and Philip S. Wells. 2008. “A Systematic Review of Perceived Risks, Psychological and Behavioral Impacts of Genetic Testing.” Genetics in Medicine 10 (1): 19–32. doi:10.1097/GIM.0b013e31815f524f.
- Huang, Ronggui. 2018. “RQDA: R-Based Qualitative Data Analysis. R Package Version 0.3.1.” In.
- Johnson, L. M., Jessica M Valdez, Emily A Quinn, April D Sykes, Rose B McGee, Regina Nuccio, Stacy J Hines-Dowell, et al. 2017. “Integrating Next-Generation Sequencing Into Pediatric Oncology Practice: An Assessment of Physician Confidence and Understanding of Clinical Genomics.” Cancer 123 (12): 2352–2359. doi:10.1002/cncr.30581.
- Kashtan, Clifford E. 2021. “Alport Syndrome: Achieving Early Diagnosis and Treatment.” American Journal of Kidney Diseases 77 (2): 272–279. doi:10.1053/j.ajkd.2020.03.026.
- Koay, P. P., and R. R. Sharp. 2014. “Managing Expectational Language: Translational Genetic Professionals Consider the Clinical Potential of Next-Generation Sequencing Technologies.” New Genetics and Society 33 (2): 126–148. doi:10.1080/14636778.2014.910448.
- Lewis, Jamie, and Andrew Bartlett. 2013. “Inscribing a Discipline: Tensions in the Field of Bioinformatics.” New Genetics and Society 32 (3): 243–263. doi:10.1080/14636778.2013.773172.
- Lincoln, Stephen E., Tina Hambuch, Justin M. Zook, Sara L. Bristow, Kathryn Hatchell, Rebecca Truty, Michael Kennemer, et al. 2021. ““One in Seven Pathogenic Variants Can be Challenging to Detect by NGS: An Analysis of 450,000 Patients with Implications for Clinical Sensitivity and Genetic Test Implementation.” Genetics in Medicine. doi:10.1038/s41436-021-01187-w.
- Long, Janet C., Hossai Gul, Elise McPherson, Stephanie Best, Hanna Augustsson, Kate Churruca, Louise A. Ellis, and Jeffrey Braithwaite. 2021. “A Dynamic Systems View of Clinical Genomics: A Rich Picture of the Landscape in Australia Using a Complexity Science Lens.” BMC Medical Genomics 14 (1): 63. doi:10.1186/s12920-021-00910-5.
- Long, Janet C., Chiara Pomare, Stephanie Best, Tiffany Boughtwood, Kathryn North, Louise A. Ellis, Kate Churruca, and Jeffrey Braithwaite. 2019. “Building a Learning Community of Australian Clinical Genomics: A Social Network Study of the Australian Genomic Health Alliance.” BMC Medicine 17 (1): 44. doi:10.1186/s12916-019-1274-0.
- Lowrance, William W., and Francis S. Collins. 2007. “Identifiability in Genomic Research.” Science 317 (5838): 600–602. doi:10.1126/science.1147699.
- Malakar, Yuwan, Justine Lacey, and Paul M. Bertsch. 2022. “Towards Responsible Science and Technology: How Nanotechnology Research and Development is Shaping Risk Governance Practices in Australia.” Humanities and Social Sciences Communications 9 (1): 17. doi:10.1057/s41599-021-01028-w.
- Malakar, Yuwan, Justine Lacey, Natalie A. Twine, Rod McCrea, and Denis C. Bauer. 2023. “Balancing the Safeguarding of Privacy and Data Sharing: Perceptions of Genomic Professionals on Patient Genomic Data Ownership in Australia.” European Journal of Human Genetics. doi:10.1038/s41431-022-01273-w.
- Marshall, Catherine, and Gretchen B. Rossman. 2011. Designing Qualitative Research, 5 Vols. London: SAGE.
- Meiser, Bettina. 2005. “Psychological Impact of Genetic Testing for Cancer Susceptibility: An Update of the Literature.” Psycho-Oncology 14 (12): 1060–1074. doi:10.1002/pon.933.
- Middleton, Anna, Peter Marks, Anita Bruce, Liwsi K. Protheroe-Davies, Cath King, Oonagh Claber, Catherine Houghton, et al. 2017. “The Role of Genetic Counsellors in Genomic Healthcare in the United Kingdom: A Statement by the Association of Genetic Nurses and Counsellors.” European Journal of Human Genetics 25 (6): 659–661. doi:10.1038/ejhg.2017.28.
- Middleton, Anna, Richard Milne, Mohamed A Almarri, Shamim Anwer, Jerome Atutornu, Elena E Baranova, Paul Bevan, Maria Cerezo, Yali Cong, and Christine Critchley. 2020. “Global Public Perceptions of Genomic Data Sharing: What Shapes the Willingness to Donate DNA and Health Data?” The American Journal of Human Genetics 107 (4): 743–752. doi:10.1016/j.ajhg.2020.08.023.
- Morse, Janice M., Michael Barrett, Maria Mayan, Karin Olson, and Jude Spiers. 2002. “Verification Strategies for Establishing Reliability and Validity in Qualitative Research.” International Journal of Qualitative Methods 1 (2): 13–22. doi:10.1177/160940690200100202.
- Norris, Sarah, Andrea Belcher, Kirsten Howard, and Robyn L. Ward. 2022. “Evaluating Genetic and Genomic Tests for Heritable Conditions in Australia: Lessons Learnt from Health Technology Assessments.” Journal of Community Genetics, doi:10.1007/s12687-021-00551-2.
- O’Shea, Rosie, Nicole M. Rankin, Maira Kentwell, Margaret Gleeson, Lucinda Salmon, Katherine M. Tucker, Sarah Lewis, and Natalie Taylor. 2020. “How Can Australia Integrate Routine Genetic Sequencing in Oncology: A Qualitative Study Through an Implementation Science Lens.” Genetics in Medicine 22 (9): 1507–1516. doi:10.1038/s41436-020-0838-x.
- Pedersen, Anette, and Peter Vedsted. 2015. “General Practitioners’ Anticipated Risk of Cancer at Referral and Their Attitude to Risk Taking and to Their Role as Gatekeeper.” Journal of Health Services Research & Policy 20 (4): 210–216. doi:10.1177/1355819615601822.
- Prince, Anya E. R, and Benjamin E. Berkman. 2018. “Reconceptualizing Harms and Benefits in the Genomic age.” Personalized Medicine 15 (5): 419–428. doi:10.2217/pme-2018-0022.
- Qin, Hua, Christine Sanders, Yanu Prasetyo, Muh Syukron, and Elizabeth Prentice. 2021. “Exploring the Dynamic Relationships Between Risk Perception and Behavior in Response to the Coronavirus Disease 2019 (COVID-19) Outbreak.” Social Science & Medicine 285: 114267. doi:10.1016/j.socscimed.2021.114267.
- R Core Team. 2018. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Reiss, Michael, Carolyn A. Greene, and Julian D. Ford. 2017. “Is it Time to Talk? Understanding Specialty Child Mental Healthcare Providers’ Decisions to Engage in Interdisciplinary Communication with Pediatricians.” Social Science & Medicine 175: 66–71. doi:10.1016/j.socscimed.2016.12.036.
- Renn, Ortwin. 2008. Risk Governance: Coping with Uncertainty in a Complex World. London: Earthscan.
- Renn, Ortwin. 2021. “New Challenges for Risk Analysis: Systemic Risks.” Journal of Risk Research 24 (1): 127–133. doi:10.1080/13669877.2020.1779787.
- Renn, Ortwin, and Mike Roco. 2006. White Paper on Nanotechnology Risk Governance. Switzerland: International Risk Governance Council in Geneva.
- Renn, Ortwin, and Pia-Johanna Schweizer. 2009. “Inclusive Risk Governance: Concepts and Application to Environmental Policy Making.” Environmental Policy and Governance 19 (3): 174–185. doi:10.1002/eet.507.
- Reynolds, Joel Michael. 2020. “Health for Whom? Bioethics and the Challenge of Justice for Genomic Medicine.” Hastings Center Report 50 (S1): S2–S5. doi:10.1002/hast.1149.
- Robinson, James T., Helga Thorvaldsdóttir, Aaron M. Wenger, Ahmet Zehir, and Jill P. Mesirov. 2017. “Variant Review with the Integrative Genomics Viewer.” Cancer Research 77 (21): e31–ee4. doi:10.1158/0008-5472.CAN-17-0337.
- Rodriguez, Laura L, Lisa D Brooks, Judith H Greenberg, and Eric D Green. 2013. “The Complexities of Genomic Identifiability.” Science 339 (6117): 275–276. doi:10.1126/science.1234593.
- Sandelowski, Margarete. 2001. “Real Qualitative Researchers do not Count: The use of Numbers in Qualitative Research.” Research in Nursing & Health 24 (3): 230–240. doi:10.1002/nur.1025.
- Sanderson, Saskia C., Michael D. Linderman, Sabrina A. Suckiel, George A. Diaz, Randi E. Zinberg, Kadija Ferryman, Melissa Wasserstein, Andrew Kasarskis, and Eric E. Schadt. 2016. “Motivations, Concerns and Preferences of Personal Genome Sequencing Research Participants: Baseline Findings from the HealthSeq Project.” European Journal of Human Genetics 24 (1): 14–20. doi:10.1038/ejhg.2015.118.
- Savige, Judy, Francesca Ariani, Francesca Mari, Mirella Bruttini, Alessandra Renieri, Oliver Gross, Constantinos Deltas, et al. 2019. “Expert Consensus Guidelines for the Genetic Diagnosis of Alport Syndrome.” Pediatric Nephrology 34 (7): 1175–1189. doi:10.1007/s00467-018-3985-4.
- Schlich-Bakker, Kathryn J., Herman F. J. ten Kroode, and Margreet G. E. M. Ausems. 2006. “A Literature Review of the Psychological Impact of Genetic Testing on Breast Cancer Patients.” Patient Education and Counseling 62 (1): 13–20. doi:10.1016/j.pec.2005.08.012.
- Selita, Fatos, Vanessa Smereczynska, Robert Chapman, Teemu Toivainen, and Yulia Kovas. 2020. “Judging in the Genomic era: Judges’ Genetic Knowledge, Confidence and Need for Training.” European Journal of Human Genetics 28 (10): 1322–1330. doi:10.1038/s41431-020-0650-8.
- Shabani, Mahsa, Adrian Thorogood, and Pascal Borry. 2016. “Who Should Have Access to Genomic Data and how Should They be Held Accountable? Perspectives of Data Access Committee Members and Experts.” European Journal of Human Genetics 24 (12): 1671–1675. doi:10.1038/ejhg.2016.111.
- Stark, Zornitza, Tiffany Boughtwood, Peta Phillips, John Christodoulou, David P. Hansen, Jeffrey Braithwaite, Ainsley J. Newson, Clara L. Gaff, Andrew H. Sinclair, and Kathryn N. North. 2019. “Australian Genomics: A Federated Model for Integrating Genomics Into Healthcare.” The American Journal of Human Genetics 105 (1): 7–14. doi:10.1016/j.ajhg.2019.06.003.
- Suyash, Shringarpure S., and Carlos Bustamante D. 2015. “Privacy Risks from Genomic Data-Sharing Beacons.” The American Journal of Human Genetics 97 (5): 631–646. doi:10.1016/j.ajhg.2015.09.010.
- Tabor, Holly K., Jacquie Stock, Tracy Brazg, Margaret J. McMillin, Karin M. Dent, Joon-Ho Yu, Jay Shendure, and Michael J. Bamshad. 2012. “Informed Consent for Whole Genome Sequencing: A Qualitative Analysis of Participant Expectations and Perceptions of Risks, Benefits, and Harms.” American Journal of Medical Genetics Part A 158A (6): 1310–1319. doi:10.1002/ajmg.a.35328.
- Tempini, Niccolò. 2021. “Data Curation-Research: Practices of Data Standardization and Exploration in a Precision Medicine Database.” New Genetics and Society 40 (1): 73–94. doi:10.1080/14636778.2020.1853513.
- Tempini, Niccolò, and Sabina Leonelli. 2021. “Actionable Data for Precision Oncology: Framing Trustworthy Evidence for Exploratory Research and Clinical Diagnostics.” Social Science & Medicine 272: 113760. doi:10.1016/j.socscimed.2021.113760.
- Tutton, Richard. 2007. “Constructing Participation in Genetic Databases.” Science, Technology, & Human Values 32 (2): 172–195. doi:10.1177/0162243906296853.
- Tutton, R. 2014. Genomics and the Reimagining of Personalized Medicine.
- Vears, Danya F., Karine Sénécal, and Pascal Borry. 2017. “Reporting Practices for Unsolicited and Secondary Findings from Next-Generation Sequencing Technologies: Perspectives of Laboratory Personnel.” Human Mutation 38 (8): 905–911. doi:10.1002/humu.23259.
- Vears, Danya F., Karine Sénécal, and Pascal Borry. 2020. “Exploration of Genetic Health Professional - Laboratory Specialist Interactions in Diagnostic Genomic Sequencing.” European Journal of Medical Genetics 63 (3): 103749. doi:10.1016/j.ejmg.2019.103749.
- Veenstra, David L., Joshua A. Roth, Louis P. Garrison, Scott D. Ramsey, and Wylie Burke. 2010. “A Formal Risk-Benefit Framework for Genomic Tests: Facilitating the Appropriate Translation of Genomics Into Clinical Practice.” Genetics in Medicine 12 (11): 686–693. doi:10.1097/GIM.0b013e3181eff533.
- Wright, Sarah, Mary Porteous, Diane Stirling, Oliver Young, Charlie Gourley, and Nina Hallowell. 2019. “Negotiating Jurisdictional Boundaries in Response to new Genetic Possibilities in Breast Cancer Care: The Creation of an ‘Oncogenetic Taskscape’.” Social Science & Medicine 225: 26–33. doi:10.1016/j.socscimed.2019.02.020.
- Zhao, Sen, Xi Cheng, Wen Wen, Guixing Qiu, Terry Jianguo Zhang, Zhihong Wu, and Nan Wu. 2021. “Recent Advances in Clinical Genetics and Genomics.” Intelligent Medicine, doi:10.1016/j.imed.2021.03.005.