Abstract
The aim of this study was to test whether women who conceived after a period of subfertility are less likely to undergo invasive prenatal testing (IPT) and determine factors of influence in that decision. We conducted a retrospective study at the Maastricht University Medical Centre (MUMC+) to compare the rates of IPT following abnormal results of combined first trimester screening (cFTS) or second trimester screening (STS), or because of advanced maternal age among women tested for the effect of type and duration of subfertility and history of fertility investigations and/or treatment. We included 977 women who underwent IPT between January 2010 and December 2013. The women who conceived after fertility investigations and/or treatment had lower rates of IPT following abnormal STS (12.6% vs. 20.0%, OR = 0.58, 95% CI; 0.34–0.97). The difference was not statistically significant after correction for maternal age and severity of the foetal anomaly. Maternal age was, in contrast to fertility treatment or duration of subfertility, related to the choice of IPT among formerly subfertile women. Therefore, the lower uptake of IPT in women conceiving after a period of subfertility is dependent on the indication for IPT and maternal age and less on the type and duration of subfertility.
Introduction
Subfertility is a complex global health problem affecting 10 to 15% of all couples who are trying to conceive (Evers, Citation2002). The considerable psychological burden on couples unable to conceive is understudied and frequently underestimated. The fact that pregnancies conceived after a period of subfertility take more effort, both from an emotional and an economic perspective, would lead many couples to consider such pregnancies as more precious. The term ‘precious’ is used in literature (Harris, Citation1999, Minkoff & Berkowitz, Citation2005; Shalev et al., Citation1999) to describe pregnancies and babies considered to be of higher value or at higher risk of obstetric complications than other pregnancies and babies. High value in this sense is a subjective judgement and refers to pregnancies conceived after great investment, i.e. by assisted reproductive technologies (ART) and/or after an extended time period of ‘attempting to conceive’. In addition, pregnancies conceived at an advanced maternal age (AMA) are considered to be of higher value since the chances of getting pregnant again decreases with the years. Both these groups, comprising patients with worthy pregnancies and an increased risk of obstetric complications, represent pregnancies that might be ‘irreplaceable’, and therefore seen as ‘precious’. Although the ‘preciousness’ of a pregnancy is a highly subjective experience, it probably has a great impact on the choices made by a couple during pregnancy. One of these intricate choices is undergoing prenatal screening to detect structural and genetic abnormalities in an unborn foetus and, even more intricate a choice, invasive prenatal tests to establish the diagnosis as they carry along a risk of miscarriage of about 1% (Alfirevic, Mujezinovic, & Sundberg, Citation2003).
Prenatal screening aims to find complications, diseases and disabilities during pregnancy (RIVM, Citation2017). In the Netherlands, prenatal ultrasound screening consists of the combined first trimester screening (cFTS) for foetal aneuploidy, and the second trimester screening (STS) for foetal structural anomalies (Baardman et al., Citation2014; Lichtenbelt et al., Citation2013). In cFTS, maternal serum concentrations of pregnancy-associated plasma protein A and the free β-subunit of human chorionic gonadotropin are measured between 8 and 14 weeks of gestation in combination with foetal nuchal translucency to calculate foetal risks for trisomy 21, 18 and 13. In STS detailed foetal structural scanning is carried out around 20 weeks of gestation to exclude the presence of various anomalies. If an abnormality is found at either occasion, women are counselled regarding the possibility of undergoing invasive prenatal testing (IPT) by amniocentesis or chorionic villus biopsy to establish the genetic cause of the anomaly (Engels, Bhola, Twisk, Blankenstein, & Van Vugt, Citation2014). The most common indications for undergoing IPT in the Netherlands are abnormal screening results after cFTS and STS (Engels et al., Citation2014; Larion et al., Citation2014; NVOG, Citation2000; Siljee et al., Citation2014). In addition, women can opt for IPT simply because of advanced maternal age (35 years and older) (AMA). When faced with a decision to undergo IPT, parents-to-be need to consider a lot of health aspects, such as the safety of the procedure, the choices they have in the case of an abnormal test result, the psychological burden of either terminating a much-anticipated pregnancy or accepting a child affected with a structural or genetic abnormality.
For this study, we hypothesized that women who conceived after subfertility are less likely to undergo IPT after an abnormal cFTS, after an abnormal ultrasound finding during STS and because of AMA than women without subfertility. Furthermore, we hypothesized that women with a longer duration of subfertility, a longer duration of treatment and lower parity were less likely to opt for IPT. We also hypothesized that women who conceived after subfertility and got pregnant after fertility treatment would be less likely to undergo IPT compared to subfertile women who did not undergo fertility treatment.
Materials and methods
Data were retrospectively collected from the hospital electronic database of women who underwent prenatal ultrasound scans in Maastricht University Medical Centre (MUMC+) between January 2010 and December 2013 and cross-referenced with those who attended the fertility department between January 2009 and December 2013 for fertility investigations and treatment.
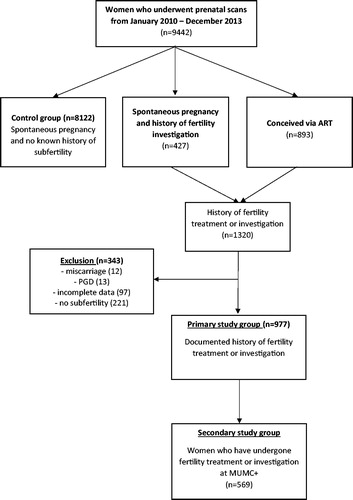
The main outcome was the rate of invasive prenatal testing (amniocentesis or chorionic villus sampling) for each indication (abnormal cFTS, abnormal STS or AMA). The control group consisted of women conceiving spontaneously with no known history of subfertility. The primary study group consisted of women with a history of subfertility based on undergoing fertility investigations and/or treatment. In addition, the following data could be retrieved from the subgroup of women with fertility investigation at the MUMC+ (secondary study group): (i) duration of subfertility (duration between the self-reported first attempt to pregnancy until the date of conception); (ii) duration of treatment (duration between the date of first diagnostic test at the fertility clinic and conception); (iii) parity; and (iv) the cause of subfertility and whether it was primary or secondary. We excluded from the analysis women with incomplete data on pregnancy outcome, women in whom subfertility was not confirmed, women without an ongoing pregnancy (miscarriage), and women who had undergone pre-implantation genetic diagnosis.
The indications for IPT after abnormal STS were assessed by two gynaecologists (SA and CW) and subsequently assigned to two different groups, categorized by severity of foetal anomaly and the likelihood of an underlying genetic cause, following EUROCAT guidelines (Kaasen et al., Citation2010). Anomalies were considered severe when carrying a significant morbidity or mortality risk with limited expectation from available treatment (e.g. acrania, skeletal dysplasia, bilateral renal agenesis, myelomeningocele with hydrocephalus, hypoplastic left heart syndrome). Mild-to-moderate anomalies were those in which the available treatment options are usually expected to give good results (e.g. bilateral clubfoot, cleft lip, gastroschisis, unilateral renal cysts, unilateral clubfoot) (EUROCAT, Citation2005).
The choice for invasive prenatal tests in both the primary and secondary study group was compared versus the control group for each of the three indications (abnormal cFTS, abnormal STS and AMA) using a chi-square test. Odds ratios were calculated using a binary logistic regression analysis and were corrected for maternal age and risk of trisomy 21 (in the abnormal cFTS group), for maternal age and risk of chromosomal anomalies (in the abnormal STS group), and for maternal age (in the AMA group). In the secondary test group, bivariate logistic regression analyses and chi-square tests were used to calculate odds ratios for factors possibly influencing the choice for IPT: maternal age, duration of subfertility, whether women had a fertility treatment, duration of fertility treatment and whether there was primary or secondary subfertility. A p value lower than 0.05 was considered statistically significant. All analyses were performed using SPSS Statistics 23 (IBM Corp, Armonk, NY).
The study was approved by the local ethical committee of the MUMC+ (reference number 144044). Procedures followed were in accordance with institutional guidelines and adhered to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects (revised 13 November 2001, effective 13 December 2001). Consent to participate was waived by the medical ethical committee of the MUMC because of the retrospective study design.
Results
Study population
Out of a total of 9442 women who attended the prenatal screening department in the MUMC + during the studied period, 8122 reported spontaneous conception and no history of subfertility (control group), 427 reported spontaneous conception with a history of fertility investigations and 893 reported conception by fertility treatment (). Thus, a total of 1320 women had subfertility based on undergoing fertility investigations and/or treatment, of whom 343 were further excluded because of incomplete data on pregnancy outcome, unconfirmed subfertility, having a miscarriage or undergoing pre-implantation genetic diagnosis. The remaining 977 were included as the primary study group, of whom 569 constituted the secondary study group (subfertile undergoing investigations and/or treatment at MUMC+).
Patient characteristics were compared between the primary study group and the control group (). Maternal age did not differ significantly between both groups (p = 0.065). Maternal weight, BMI and smoking status differed significantly in the primary study group compared to the control group, maternal weight and BMI were higher in the control group and there were fewer smokers in the primary study group. The method of conception in the primary study group is also shown in .
Table 1. Population characteristics of the primary study group (women with a documented history of fertility treatment or investigation) and the control group (women with a spontaneous pregnancy and no known history of subfertility).
A total of 138 women had an indication for IPT because of an abnormal cFTS, 1636 because of an abnormal STS and 2014 women because of AMA. Women who had more than one indication for IPT at different stages of pregnancy were assigned to the indication that occurred last.
Choice for invasive prenatal tests after abnormal cFTS
Subfertile women (primary study group) had lower rates of invasive prenatal tests after an abnormal cFTS compared to the control group (55.6% vs. 75.8%), although the difference was not statistically significant (OR = 0.40, 95% CI; 0.14–1.10, p = 0.088), even after adjustment for the risk on trisomy 21 and maternal age (OR = 0.52, 95% CI; 0.14–1.10, p = 0.243). Similarly, there was no difference between the secondary study group compared to the control group (OR = 0.38, 95% CI; 0.11–1.35, p = 0.152), even after adjustment for the risk on trisomy 21 and maternal age (OR = 0.56, 95% CI; 0.14–2.23, p = 0.413) ().
Table 2. Uptake of invasive prenatal test (IPT) for different indication (abnormal cFTS, abnormal STS and AMA) expressed in odds ratio’s (OR) and 95% CI and adjusted odds ratio’s (AdjOR) for different confounders per indication.
Choice for invasive prenatal tests after abnormal STS
Subfertile women (primary study group) had lower rates of invasive prenatal tests following abnormal STS (12.6% vs. 20.0%, OR = 0.58, 95% CI; 0.34–0.97, p = 0.040). The difference was not statistically significant after correction for maternal age and severity of the foetal anomaly (OR = 0.59, 95% CI; 0.32–1.08, p = 0.087). Similarly, the secondary study group had a lower uptake rate compared to the control group (OR 0.52, 95% CI; 0.29–0.92, p = 0.023), and the difference became statistically marginally significant after adjustment for maternal age and severity of the found anomaly (OR = 0.52, 95% CI; 0.27–1.00, p = 0.050) ().
Choice for invasive prenatal tests because of AMA
The rates for invasive prenatal tests because of AMA did not differ significantly between the primary study group and the control group (OR = 0.78, 95% CI; 0.53–1.15), even after adjustment for maternal age (OR = 0.91, 95% CI; 0.62–1.35). Similarly, the rates did not differ between the secondary group compared to the control group (OR = 0.74, 95% CI; 0.48–1.13), even after adjustment for maternal age (OR = 0.91, 95% CI; 0.62–1.35). Neither did the rates differ between the secondary study group compared to the control group (OR = 0.74, 95% CI; 0.48–1.13), even after adjustment for maternal age (OR = 0.86, 95% CI; 0.56–1.33) ().
Factors influencing choice for invasive diagnostic test
In women undergoing fertility investigations and treatment at the MUMC+ (secondary study group), patient characteristics possibly influencing the choice for invasive diagnostic tests were analysed. Only maternal age was significantly related to the choice for invasive diagnostic tests (p < 0.001). The duration of treatment, the duration of subfertility, the fertility treatment itself and whether it concerned a primary or secondary subfertility were not significantly related to the uptake of an invasive diagnostic test in this group ().
Table 3. Bivariate logistic regression analyses of factors possibly influencing the choice for IPT in the secondary study group.
Discussion
In this study, there was a clear trend for lower rates of IPT in women who conceived after fertility treatment or investigations, compared to women with no known history of subfertility, after all three indications (STS, cFTS and AMA). The difference in IPT rates was only statistically significant following an abnormal STS, and not significant after abnormal cFTS, for AMA, and after adjustment for maternal age and severity of the foetal anomaly. Maternal age was a more important determinant for undergoing IPT than the type and duration of subfertility and fertility treatment.
This study was carried out in a large sample size of women, in a regional referral centre for prenatal diagnosis where subfertility investigations and treatment have reliable registration of rates of IPT in electronic hospital databases. However, there are certain limitations due to the retrospective design of the study that should be addressed. First, we assume that spontaneously conceiving women who had no self-reported history of subfertility represent a healthy control group to our subfertile study group. This is an assumption based on standard history taking during prenatal assessment without actual knowledge of the duration of actively seeking conception. On the other hand, information on the method of conception is considered reliable as this has been validated from the fertility clinic database. We are currently undergoing a prospective study, in which a more robust method of checking the duration of seeking pregnancy, is implemented. Second, we could not test the influence of other possible confounders on women’s choices, such as education, ethnicity and socio-economic status, as well as the role played by the partner and counsellor during the process of choosing to undergo IPT (Alsulaiman et al., Citation2012; Caleshu, Shiloh, Price, Sapp, & Biesecker, Citation2010; Srebnik, Miron-Shatz, Rolison, Hanoch, & Tsafrir, Citation2013). Third, ultrasound details of prenatal assessments (e.g. the thickness of nuchal translucency and number of associated structural anomalies or softmarkers) were too diverse as parameters to include as possible confounders. Alternatively, we chose to correct for more measurable and objective parameters such as the calculated risk for trisomy 21.
Our results suggest that the uptake of IPT in subfertile women is dependent on the indication for testing. After abnormal first trimester screening, two large studies have shown reduced uptake of IPT in women who conceived by fertility treatment, after correction for AMA and risk at trisomy 21 (Hunt et al., Citation2012; Lichtenbelt et al., Citation2013). In contrast to our study, these articles focused on fertility treatment and did not address the global effect of subfertility. One study performed in the USA among 2544 women found no influence of IVF on the acceptance of amniocentesis (Elimian et al., Citation2003). Little is published about the effect of subfertility on the uptake of invasive diagnostic testing after an ultrasound anomaly found during STS. It could be expected that the uptake of IPT depends on the severity of the diagnosed foetal anomaly and ranged between 26 and 40% (Ahman et al., Citation2014; Sharda & Phadke, Citation2007). Both studies did not take the method of conception into account. In our study, an uptake of 20.3% was found and it was more influenced by the severity of foetal anomaly than the history of subfertility.
Before the introduction of modern screening modalities for foetal aneuploidies, maternal age was the only factor used by clinicians for risk stratification. This meant that until recently, AMA was one of the main indications for undergoing IPT in many countries, and also in our academic centre (Johnson & Tough, Citation2012). The International Federation of Gynecology and Obstetrics (FIGO) has defined AMA as 35 years old or over for primipara and 40 years old or over for parous women, however, whether parity or other factors have an influence on the uptake of prenatal screening or invasive tests has not been widely reported. The uptake of invasive diagnostic testing of women with AMA is variable and ranges between 48 and 70% after a positive down screening (Godino, Turchetti, & Skirton, Citation2013; Kuppermann et al., Citation2006; Marini, Sullivan, & Naeem, Citation2002). The uptake in our study population was 37.8%, indicating that the majority of women with AMA declined invasive testing. Also, women with AMA in our study did not have significantly different rates of invasive tests compared to younger women. The acceptance rate of prenatal screening among women with advanced age has increased over the past few years and was found to be significantly higher among women aged 35–36 years compared to women over 37 years old, suggesting that advanced maternal age is positively correlated with a higher uptake of IPT (Lam et al., Citation2000). The emerging trend in western societies of women with advanced age seeking pregnancy has brought with it complex counselling issues, especially when taking into account the available assisted reproductive techniques and increased obstetric risks. It is still unclear how AMA interacts with other aspects of subfertility to affect women’s choices regarding prenatal screening. Our data suggest that, in women undergoing fertility investigations and treatment at our centre, only maternal age was significantly related to the choice for invasive diagnostic tests, and not the duration of treatment or subfertility. Many external factors (the opportunity for screening and use of genetic counselling) and psychosocial aspects (ethnicity, socio-demographic status and attendance of partners during counselling) play a vital role in women’s choices and need to be addressed during prenatal counselling and is taken into consideration in future studies (Godino et al., Citation2013).
It is also still unknown how the advent of non-invasive prenatal test (NIPT) will affect screening policies and consequently the counselling received, and choices made by women in general and women with history of subfertility in particular. This technique utilizes cell-free foetal DNA in maternal blood to detect foetal aneuploidies, and therefore carries no risk of miscarriage to pregnant women (Buchanan, Sachs, Toler, & Tsipis, Citation2014). At large referral centres in the USA, the introduction of NIPT has had a huge impact on traditional diagnostic screening with a decrease in the monthly performed CVS and amniocentesis procedures of 77.2 and 52.5%, respectively (Larion et al., Citation2014).
In conclusion, in this retrospective study, women who conceived after fertility treatment or investigations, had a trend for a lower rate of invasive prenatal tests, compared to women with no known history of subfertility, mainly following abnormal second trimester screening. Advanced maternal age was a more important determinant for undergoing invasive prenatal tests than the type and duration of subfertility and fertility treatment. The concept of the ‘precious baby’ due to a history of subfertility needs further exploration in prospective studies focusing on the type of foetal anomaly as well as ethnic, cultural and socio-economic factors possibly influencing the choices made by future parents.
Acknowledgements
We would like to thank Dr G. Dunselman, Dr R. van Golde, Dr J. Dumoulin and Dr S van Kuijk for their aid and constructive remarks during the set-up of the study.
Disclosure statement
For all the authors, no potential conflicts of interest do exist. There are no financial or personal relationships that could be viewed as presenting a potential conflict of interest.
References
- Ahman, A., Axelsson, O., Maras, G., Rubertsson, C., Sarkadi, A., & Lindgren, P. (2014). Ultrasonographic fetal soft markers in a low-risk population: Prevalence, association with trisomies and invasive tests. Acta Obstetricia et Gynecologica Scandinavica, 93, 367–373. doi: 10.1111/aogs.12334.
- Alfirevic, Z., Mujezinovic, F., & Sundberg, K. (2003). Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database of Systematic Reviews, 2003, (3), CD003252. doi: 10.1002/14651858.CD003252.
- Alsulaiman, A., Hewison, J., Abu-Amero, K., Ahmed, S., Green, J., & Hirst, J. (2012). Attitudes to prenatal diagnosis and termination of pregnancy for 30 conditions among women in Saudi Arabia and the UK. Prenatal Diagnosis, 32, 1109–1113. doi: 10.1002/pd.3967.
- Baardman, M.E., Du Marchie Sarvaas, G.J., de Walle, H.E.K., Fleurke-Rozema, H., Snijders, R., Ebels, T., … Bakker, M.K. (2014). Impact of introduction of 20-week ultrasound scan on prevalence and fetal and neonatal outcomes in cases of selected severe congenital heart defects in The Netherlands. Ultrasound in Obstetrics & Gynecology, 44, 58–63. doi: 10.1002/uog.13269.
- Buchanan, A., Sachs, A., Toler, T., & Tsipis, J. (2014). NIPT: Current utilization and implications for the future of prenatal genetic counseling. Prenatal Diagnosis, 34, 850–857. doi: 10.1002/pd.4382.
- Caleshu, C., Shiloh, S., Price, C., Sapp, J., & Biesecker, B. (2010). Invasive prenatal testing decisions in pregnancy after infertility. Prenatal Diagnosis, 30, 575–581. doi: 10.1002/pd.2529.
- Elimian, A., Demsky, M., Figueroa, R., Ogburn, P., Spitzer, A.R., & Quirk, J.G. (2003). The influence of IVF, multiple gestation and miscarriage on the acceptance of genetic amniocentesis. Prenatal Diagnosis, 23, 501–503. doi: 10.1002/pd.633.
- Engels, M.A., Bhola, S.L., Twisk, J.W., Blankenstein, M.A., & Van Vugt, J.M. (2014). Evaluation of the introduction of the national Down syndrome screening program in the Netherlands: Age-related uptake of prenatal screening and invasive diagnostic testing. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 174, 59–63. doi: 10.1016/j.ejogrb.2013.12.009.
- EUROCAT. (2005). Guide 1.3 and reference documents: Instructions for the Registration and Surveillance of Congenital Anomalies. Retrieved from http://www.eurocat-network.eu/content/EUROCAT-guide-1.3.pdf
- Evers, J.L. (2002). Female subfertility. Lancet, 360, 151–159. doi: 10.1016/S0140-6736(02)09417-5..
- Godino, L., Turchetti, D., & Skirton, H. (2013). A systematic review of factors influencing uptake of invasive fetal genetic testing by pregnant women of advanced maternal age. Midwifery, 29, 1235–1243. doi: 10.1016/j.midw.2012.11.009.
- Harris, J. (1999). Precious fertility and third-trimester tests. Prenatal Diagnosis, 19, 753–754. doi: 10.1002/(SICI)1097-0223(199908)19:8<753::AID-PD620>3.0.CO;2-S.
- Hunt, L., Peterson, M., Sinnott, S., Sutton, B., Cincotta, R., Duncombe, G., …, McInerney-Leo, A. (2012). Uptake of invasive prenatal tests in pregnancies conceived via assisted reproductive technologies: the experience in Queensland, Australia. Prenatal Diagnosis, 32, 1049–1052. doi: 10.1002/pd.3953.
- Johnson, J., & Tough, S., SOGC Genetics Committee. (2012). Delayed child-bearing. Journal of Obstetrics and Gynaecology Canada, 34, 80–93. doi: 10.1016/S1701-2163(16)35138-6.
- Kaasen, A., Helbig, A., Malt, U.F., Naes, T., Skari, H., & Haugen, G. (2010). Acute maternal social dysfunction, health perception and psychological distress after ultrasonographic detection of a fetal structural anomaly. BJOG: An International Journal of Obstetrics & Gynaecology, 117, 1127–1138. doi: 10.1111/j.1471-0528.2010.02622.x.
- Kuppermann, M., Learman, L.A., Gates, E., Gregorich, S.E., Nease Jr R.F., Lewis, J., & Washington, A.E. (2006). Beyond race or ethnicity and socioeconomic status: predictors of prenatal testing for Down syndrome. Obstetrics and Gynecology, 107, 1087–1097. doi: 10.1097/01.AOG.0000214953.90248.db.
- Lam, Y.H., Tang, M.H., Lee, C.P., Sin, S.Y., Tang, R., Wong, H.S., & Wong, S.F. (2000). Acceptability of serum screening as an alternative to cytogenetic diagnosis of Down syndrome among women 35 years or older in Hong Kong. Prenatal Diagnosis, 20, 487–490. doi: 10.1002/1097-0223(200006)20:6<487::AID-PD853>3.0.CO;2-2.
- Larion, S., Warsof, S.L., Romary, L., Mlynarczyk, M., Peleg, D., & Abuhamad, A.Z. (2014). Uptake of non-invasive prenatal testing at a large academic referral center. American Journal of Obstetrics & Gynecology, 211, 651.e1– 651.e7. doi: 10.1016/j.ajog.2014.06.038.
- Lichtenbelt, K.D., Schuring-Blom, G.H., van der Burg, N., Page-Christiaens, G.C., Knoers, N.V., Schielen, P.C., & Koster, M.P. (2013). Factors determining uptake of invasive testing following first-trimester combined testing. Prenatal Diagnosis, 33, 328–333. doi: 10.1002/pd.4067.
- Marini, T., Sullivan, J., & Naeem, R. (2002). Decisions about amniocentesis by advanced maternal age patients following maternal serum screening may not always correlate clinically with screening results: need for improvement in informed consent process. American Journal of Medical Genetics, 109, 171–175. doi: 10.1002/ajmg.10319.
- Minkoff, H.L., & Berkowitz, R. (2005). The myth of the precious baby. Obstetrics and Gynecology, 106, 607–609. doi: 10.1097/01.AOG.0000174585.08884.59.
- NVOG. (2000). Richtlijn: Indicaties voor prenatale diagnostiek. Retrieved from: http://nvog documenten.nl/uploaded/docs/richtlijnen_pdf/28_indica_prenatale_diagno.pdf
- RIVM. (2017). Zwangerschapsscreeningen. Retrieved from: http://www.rivm.nl/Onderwerpen/Z/Zwangerschapsscreeningen
- Shalev, J., Meizner, I., Rabinerson, D., Mashiach, R., Peleg, D., Orvieto, R., … , Rafael, Z. (1999). Elective cytogenetic amniocentesis in the third trimester for pregnancies with high risk factors. Prenatal Diagnosis, 19, 749–752. doi: 10.1002/(SICI)1097-0223(199908)19:8<749::AID-PD619>3.0.CO;2-#.
- Sharda, S., & Phadke, S.R. (2007). Uptake of invasive prenatal diagnostic tests in women after detection of soft markers for chromosomal abnormality on ultrasonographic evaluation. Journal of Perinatology, 27, 550–555. doi: 10.1038/sj.jp.7211787.
- Siljee, J.E., Knegt, A.C., Knapen, M.F., Bekker, M.N., Visser, G.H., & Schielen, P.C. (2014). Positive predictive values for detection of trisomies 21, 18 and 13 and termination of pregnancy rates after referral for advanced maternal age, first trimester combined test or ultrasound abnormalities in a national screening programme (2007-2009). Prenatal Diagnosis, 34, 259–264. doi: 10.1002/pd.4302.
- Srebnik, N., Miron-Shatz, T., Rolison, J.J., Hanoch, Y., & Tsafrir, A. (2013). Physician recommendation for invasive prenatal testing: The case of the 'precious baby'. Human Reproduction, 28, 3007–3011. doi: 10.1093/humrep/det354.