Abstract
This study aimed to discover whether egg sharing compromises the chance of donors or recipients achieving a live birth. A descriptive cohort study was performed of 4,545 fertility patients and 5,316 stimulation cycles at a London based fertility clinic between 2010 and 2019. There was no significant difference in clinical pregnancy rate (CPR) or live birth rate (LBR) between egg sharers and standard IVF patients or between egg sharing recipients and non-egg sharing recipients. Both egg sharers and their recipients had fewer oocytes and fewer day 3 embryos available for fresh embryo transfer or cryopreservation than standard IVF patients or non-egg sharing recipients. The cumulative LBR were significantly lower amongst egg sharers than standard IVF patients (p < 0.05), and significantly lower amongst egg sharing recipients than non-egg sharing recipients (p < 0.05). This study demonstrates that egg sharing does not compromise the chances of donors or their recipients achieving a live birth. However, participants may occasionally require additional ovarian stimulation cycles to conceive. With government funding for IVF treatment falling, egg sharing provides a practical option to allow more women access to IVF. Egg sharing is currently the most efficient way of maximising the use of the precious resource of human oocytes.
Introduction
In-vitro fertilisation (IVF) has opened the option of egg donation, a technique first successfully used in 1984, offering an option for women with conditions, such as primary ovarian insufficiency (POI) (Lutjen et al., Citation1984). Additionally, many fertility clinics will advise women over a certain age or with very poor ovarian reserve to consider using donor eggs, due to the very poor chances of conception with their own oocytes (Bracewell-Milnes et al., Citation2018).
The use of donor oocytes is increasing and are currently used in 5% of IVF cycles in the UK, with 67% of cycles using donor eggs and partner sperm (DEPS) being performed in women over 40 years in 2016 (HFEA, Citation2018). With a current worldwide trend towards delaying first time motherhood (Birch Petersen et al., Citation2015; Schmidt et al., Citation2012), oocyte donation offers some women the only realistic chance of achieving a live birth. Indeed, data from the UK shows women aged 40–42 years had a live birth rate (LBR) four times higher using donor rather than autologous eggs (35 vs 9%); and women aged 43–44 years had an eight-fold increase in LBR using donor oocytes compared to their own (32 vs 4%) (HFEA, Citation2018). Unlike other family building options, DEPS cycles allow recipients to experience pregnancy and the offspring to be genetically linked to their partner (Applegarth et al., Citation1995).
The demand for donor eggs has been rising globally, with a 49% increase in DEPS cycles since 2011 in the UK (HFEA, Citation2018); however the annual numbers of newly registered oocyte donors has plateaued and remained stable at 1,600 (HFEA, Citation2019b). In contrast to the straight-forward process of sperm donation, egg donation involves high dose ovarian stimulation and invasive procedures, such as transvaginal oocyte retrieval under sedation, or general anaesthesia. Additionally, the donor must endure the inconvenience of multiple appointments at the fertility clinic to plan the IVF cycle, ultrasound scans and counselling sessions, resulting in missing work and significant travel time. Understandably, few women are willing to donate their eggs on a purely altruistic basis, and therefore supply is falling short of demand in many countries worldwide, including the UK (Dyer, Citation2011). This has resulted in long waiting lists and limited choices, especially among ethnic groups seeking egg donation (Brulliard, Citation2006). This has driven some patients abroad to destinations where donor eggs are more readily available, but where regulations may be less strict (Culley et al., Citation2011), a process known as cross-border reproductive care (CBRC). Identifying this, the HFEA implemented changes aimed at improving the numbers of new donors registering and maximising the use of their gametes (BIONEWS, Citation2011). A significant change was providing £750 as a compensatory payment per cycle, replacing the previous payment of £250 (Bracewell-Milnes et al., Citation2018).
A potential solution to this shortage has been proposed with egg sharing, regulated in the UK since 1998 (Blyth, Citation2002), and now being performed in many western countries including Australia, Belgium, Israel, and the USA (Gürtin et al., Citation2012a). Egg sharing is a process where women already undergoing IVF who give a proportion of their oocytes to an anonymously matched recipient in exchange for subsidised fertility treatment. The scheme has been a heavily debated and controversial method of oocyte donation since its introduction (Blyth, Citation2001, Citation2002). There are clear advantages; firstly, the egg share donor requires IVF for her own needs, so no third party is undergoing this invasive process. Secondly, those who do not qualify for government funded fertility treatment and could not otherwise have funded expensive treatments, gain access to IVF. Thirdly, it provides a fertility patient the chance to help an anonymously matched recipient in a practical way. However, some concerns regarding the egg sharing programme have been raised. Firstly, there is concern for the psychological well-being for the donor if her own treatment is unsuccessful, especially with the knowledge their recipient may have conceived their genetic children (Johnson, Citation1999). Secondly, there is concern that egg sharers are jeopardising their chances of success by donating a proportion of their eggs in their fertility treatment (Bracewell-Milnes et al., Citation2019). Thirdly, theoretical issues have arisen regarding the quality of consent of the donor, in that she is only agreeing to donate so she can access much desired treatment (Lieberman et al., Citation2008). Finally, subsidised fertility treatment could be considered in conflict with a cultural preference for voluntary donation, in countries like the UK (Johnson, Citation1999).
Egg sharing is unquestionably an interesting psychosocial scheme, with significant ethical debate surrounding its practice (Sauer & Kavic, Citation2006). After its introduction, it was considered by some to be no different from ‘paid donation’, at a time when the HFEA was opposed to any payments for donor gametes (Deech, Citation1998). Indeed, shortly after egg sharing was introduced, the HFEA described egg sharing as an ‘unacceptable’ practice (Blyth, Citation2002). In 1998, having concluded that all payments for people donating gametes should be discontinued, the HFEA conducted a consultation to confirm similar views among egg donation patients and healthcare professionals (Blyth, Citation2002). The responses the HFEA received to this consultation indicated only a minority supported the withdrawal of egg sharing, and the HFEA concluded egg sharing should be ‘regulated, not banned’, since the data collected showed that egg sharers were motivated by the desire to have a baby, not by financial reasons (Blyth, Citation2002).
The number of egg donors newly registering at UK fertility clinics has remained consistent since 2013, at around 1,600 per year. However, the number of egg sharers has fallen in recent years. Indeed in 2011, 698 of egg sharers participated as donors, compared to 348 in 2016, which is a 50.1% fall in numbers (HFEA, Citation2019a). The reason for this alarming drop in egg share numbers in the UK are multi-factorial and difficult to explain, requiring further in-depth research.
One of the most consistently raised concerns among egg share donors and healthcare professionals is that egg sharers could compromise their chance of achieving a live birth by sharing their oocytes (Bracewell-Milnes et al., Citation2016, Citation2018, Citation2019). Some healthcare professionals have also hypothesised that fertility clinics administer higher doses of gonadotrophins to egg sharers to obtain more eggs, but subjecting the donor to an increased risk of ovarian hyperstimulation syndrome (OHSS) (Simons & Ahuja, Citation2005). Alongside receiving fewer oocytes, egg share recipients report being apprehensive about receiving oocytes from infertile patients, and that this could impact their chances of achieving a pregnancy (Oyesanya et al., Citation2009). Additionally, egg sharers and recipients can be concerned that their counterpart is favoured by the fertility clinic during their treatment (Ahuja et al., Citation1996).
Studies investigating egg share donor and recipient outcomes have reported contradictory findings, highlighting the need for further research. Earlier studies reported higher pregnancy and live birth rates among recipients compared with egg sharers (Ahuja et al., Citation1996; Check et al., Citation1992). Thum et al. (Citation2003) reported no difference in LBR amongst egg share donors, their recipients and standard IVF patients. Other studies reported no difference in pregnancy rates between egg sharing and non-egg sharing recipients (Check et al., Citation2004, Citation2012; Oyesanya et al., Citation2009); although one of these studies found egg sharing recipients received significantly fewer oocytes (Oyesanya et al., Citation2009). Check et al. (Citation2012) also reported an increased proportion of egg sharers deferred fresh embryo transfer due to an increased risk of OHSS. More recently Malhotra et al. (Citation2013) reported lower pregnancy rates amongst egg share recipients in India. Most recently Braga et al. (Citation2020) reported the outcomes of egg share donors strongly predicted the pregnancy rates of their recipient, but differences between other outcomes were not reported. Overall, these findings are contradictory and do not consistently report LBR.
The primary aim of this study is to investigate whether the egg sharing programme compromises the chance of the donor or recipient achieving a live birth, while also comparing these donor groups to the outcomes of standard IVF patients and non-egg share recipients.
Materials and methods
Study design
This was a retrospective cohort analysis of women undergoing egg sharing, egg donation and standard IVF patients undergoing IVF/intracytoplasmic sperm injection (ICSI) treatment cycles at The Lister Fertility Clinic (London, UK) between January 2010 and December 2019. Patients were divided into four groups: (i) egg sharers; (ii) standard IVF patients; (iii) egg share recipients; (iv) non-egg share recipients. Ethical approval was granted by London Riverside Research Ethics Committee (REC reference 17/LO/1491).
Egg share donors
All egg sharers who participated between 2010 and 2019 were included for analysis. To be eligible to participate in egg sharing patients must be <35 years, have a body mass index (BMI) 18–29kg/m2, and have an anti-müllerian hormone (AMH) of >8 pmol/L. They must have a normal blood screen (HIV, hepatitis B and C, cytomegalovirus, syphilis, cystic fibrosis and normal karyoptype). Prior to donating a proportion of their eggs, egg sharers were assessed by a specialist fertility counsellor. As with altruistic and known egg donors, egg sharers could withdraw from the scheme at any point before the recipient underwent embryo transfer (ET). This means embryos could be created that would then need to be discarded if the egg share donor withdrew her consent. The unit policy is to limit the number of cycles a patient can undergo while egg sharing to four cycles. The aim of the programme is for the egg sharer and her recipient to receive four oocytes each. If an egg sharer produced fewer than eight oocytes, she could donate all oocytes in return for a further free standard IVF cycle, or she could keep all oocytes and be charged as a standard IVF patient.
Standard IVF/ICSI patients
All standard IVF patients who were not egg sharing and underwent fertility treatment in the same time period were matched for age (<35 years), BMI (18–29kg/m2) and egg reserve (AMH >8 pmol/L) and included in data analysis.
Egg-sharing and non-egg-sharing recipients
All patients, aged ≤51 years, receiving oocytes from egg-sharers or non-egg-share donors between 2010 and 2019 were included. Egg share recipients received a 50:50 share of the oocytes from their anonymously matched egg share donor. Non-egg share recipients received all oocytes from known or anonymous donors. Prior to participating in egg donation, recipients and their partners underwent specialist fertility counselling to discuss potential future implications. Recipients had access to certain donor characteristics including, physical appearance, medical history, family history and educational status; the egg donation coordinator anonymously matched donors with recipients according to recipient preferences. If the recipient was not keen on the donor proposed then she could of course decline. If she were to decline three proposed consecutive donors, then the clinic policy is the recipient has a further session with the fertility counsellor, to confirm she is keen to receive fertility treatment using a donor egg.
Stimulation protocols for egg-sharers and standard IVF patients
All patients underwent mid-luteal pituitary downregulation via the long protocol using nafarelin or buserelin, or the antagonist protocol. A gonadotrophin (recombinant FSH, hMG or urinary FSH) was administered for ovarian stimulation. 10,000IU of hCG was administered once follicles reached a pre-ovulatory size (18–22mm); 36 hours later, oocyte aspiration was conducted trans-vaginally with ultrasound guidance. Once embryos had cleaved, the best embryo(s) were selected for ET, conducted on day 3 or 5. All patients received per-vaginum (PV) or per-rectum (PR) progesterone supplementation for two weeks, from the day before ET until the pregnancy test was conducted.
Hormonal replacement for recipients
Women with ovarian function started the oral contraceptive pill from day 2 of the pre-treatment cycle for synchronisation with the egg-sharer or donor undergoing the long protocol. Trans-vaginal ultrasound scan (TVUS) was conducted on day 3 or 4 to check the endometrial thickness and ovaries, and oestradiol supplementation given for 7–10 days. Another scan was then performed to confirm adequate endometrial thickness (>8mm). All patients received PV/PR progesterone supplementation from 4 to 6 days before ET, depending on the age of the embryo transferred, until the pregnancy test was conducted.
Outcome measures
The primary outcome measure was the LBR. Secondary outcomes included the mean numbers of oocytes collected or used; mean gonadotropin doses administered per stimulation cycle; mean numbers of embryos transferred; implantation rate (IR); fertilisation rate (FR); clinical pregnancy rate (CPR) determined by the presence of a gestational sac with a foetal heartbeat at 6 weeks of pregnancy by TVUS; miscarriage rate (MR); and cumulative live birth rate (CLBR), the proportion of fresh and frozen-thawed ETs from one stimulation cycle that resulted in at least one live born neonate.
Statistical analysis
Statistical analyses were conducted with Statistical Package for Social Sciences (SPSS version 26.0, IBM). Descriptive statistics including the mean and standard deviation (SD) were calculated for each continuous variable, and the normal distribution was examined. Analysis of variance (ANOVA) was used to investigate the significance of differences in continuous variables, and Pearson’s χ2 analysis was performed to evaluate categorical outcomes. p values of <0.05 were considered statistically significant.
Results
Between January 2010 and December 2019, a total of 4,545 patients were included for analysis: (i) 670 egg sharers; (ii) 2,777 standard IVF patients; (iii) 765 egg share recipients; and (iv) 333 non-egg share recipients. These patients underwent 5,316 ART treatment cycles: (i) 756 share cycles; (ii) 3,293 standard IVF patients; (iii) 906 egg share recipient cycles; and (iv) 361 non-egg share recipient cycles. shows the cycle characteristics and treatment outcomes of the four cohorts of patients. No donors withdrew their consent during or after treatment, meaning all available embryos were available to transfer for the recipient. There was no difference in age (p = 0.121) or duration of infertility (p = 0.247) between egg share donors and their clinically matched standard IVF patients. Unsurprisingly, both groups of oocyte recipients were older than the egg sharers and the standard IVF patients (p < 0.001) nor was there any difference in the duration of infertility between them (p = 0.247), or between the two recipient groups (p = 0.227). However egg share donors had a statistically significant increased duration of infertility compared to their anonymously matched recipients (p < 0.001).
Table 1. Stimulation characteristics and cycle outcomes.
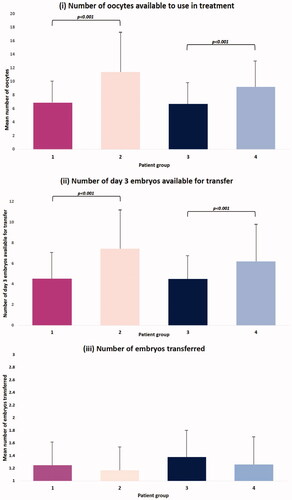
There was no difference in number of oocytes collected between egg sharers (15.34 ± 6.13) or standard IVF patients (14.17 ± 6.21) (p = 0.109). The average number of days taking FSH for ovarian stimulation or the average amount of gonadotrophin used for stimulation were not significantly different between the two groups receiving stimulation (). The average number of oocytes egg sharers donated to their anonymously matched recipients was 7.37 ± 2.92 oocytes. Unsurprisingly, this resulted in standard IVF patients using 66% more oocytes in their IVF treatment than egg sharers (11.41 ± 6.29 vs 6.87 ± 3.27; p < 0.001). This meant standard IVF patients had a higher number of day 3 embryos available for transfer (7.43 ± 4.84 vs 4.52 ± 2.81; p < 0.001) (). Group 3 (egg share recipients) also had a statistically significantly fewer number of oocytes donated to them than non-egg sharing recipients (6.61 ± 2.18 vs 9.31 ± 2.98; p < 0.001) (); and fewer day 3 embryos available for transfer (4.50 ± 2.18 vs 6.20 ± 3.45; p < 0.001) (). Therefore, on average non-egg sharing recipients had 37.3% more oocytes used, and 37.8% more day 3 embryos available for transfer than egg share recipients. Comparing the egg sharers to their anonymously matched recipients, there was no difference in the number of oocytes available (6.87 ± 3.27 vs 6.61 ± 2.18; p = 0.379), or day 3 embryos available for transfer (4.52 ± 2.81 vs 4.50 ± 2.18; p = 0.376) (). Moreover, there was no difference in the number of embryos transferred between any of the four groups ().
Figure 1. Bar graphs comparing (i) the mean number of oocytes used in treatment; (ii) the mean number of day 3 embryos available for transfer; and (iii) the number of embryos transferred for (i) egg sharers; (ii) standard IVF patients; (iii) egg share recipients; (iv) non-egg share recipients. One-way ANOVA tests were conducted to investigate statistical significance. All data is represented as mean ± SD.
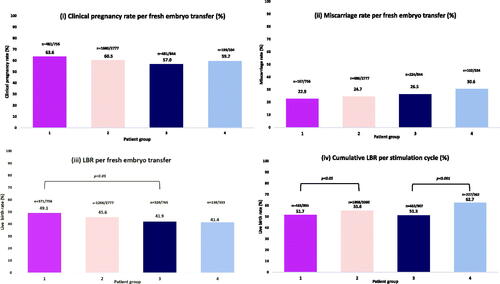
There was no statistically significant difference in fertilisation rates or implantation rates between all four groups (). compares pregnancy outcomes between the patient groups. There was no difference identified in CPR, MR or LBR in egg sharers compared to standard IVF patients. The CLBR was 3.9% higher in the standard IVF patient group, compared to the egg sharers, which was statistically significant (55.6 vs 51.7%; p < 0.05) (). Similarly, there were no significant differences found between CPR, MR or LBR per fresh ET between egg sharing and non-egg sharing recipients. However, non-egg sharing recipients had a significantly higher CLBR compared to egg share recipients (62.7 vs 51.3%, p < 0.001) (). Egg share donors had significantly higher CPR (63.6 vs 57.0%; p < 0.01) and LBR (49.1 vs 41.9%; p < 0.01) per fresh ET compared to their recipients, although there was no statistically significant difference in MR (p = 0.184) or CLBR (p = 0.844) ().
Figure 2. Graphs demonstrating the fertilisation rate and implantation rate between (i) egg sharers; (ii) standard IVF patients; (iii) egg share recipients; (iv) non-egg share recipients. Analysis was performed using Pearson’s chi-squared test. No comparisons reached statistical significance.
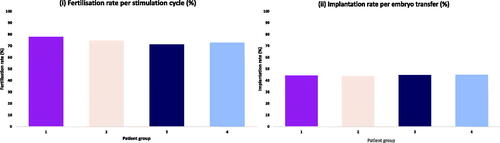
Figure 3. A series of graphs showing the different pregnancy outcomes between (i) egg sharers; (ii) standard IVF patients; (iii) egg share recipients; (iv) non-egg share recipients. Analysis was performed using Pearson’s chi-squared test. Statistically different results were identified in LBR between egg sharers and egg share recipients (p < 0.001) and in CLBR between egg sharers and egg share recipients (p < 0.05), and egg share recipients and non-egg share recipients (p < 0.001).
Discussion
The results obtained from this study revealed that overall the egg sharing programme was not detrimental to egg sharers or recipient’s chances of achieving a live birth per embryo transfer when compared to standard IVF patients and non-egg sharing recipients. However, the CLBR was slightly reduced in the egg share group, meaning a small proportion of egg share donors will need to undergo further IVF cycles to achieve a live birth. This is the first study to analyse these four groups together and is the largest study investigating egg sharing, analysing 5,316 stimulation cycles in total. The study findings for egg sharers and their recipients will be discussed below.
Egg sharer donors
It has been speculated by healthcare professionals that an egg share donor will reduce her chance of successful treatment by giving away a proportion of her eggs (Blyth, Citation2002; Bracewell-Milnes et al., Citation2019). This study demonstrated no difference in FR, IR, CPR, MR or LBR between egg share donors’ and standard IVF patients. Our findings supported the findings from other studies that there was no difference in LBR between the different groups (Check et al., Citation2012; Oyesanya et al., Citation2009; Thum et al., Citation2003).
The number of day 3 embryos available for transfer however was fewer for egg sharers compared to standard IVF patients. This resulted in a higher CLBR for standard IVF patients of 55.60% compared to 51.72% for egg share donors, which was statistically significant. Although perhaps unsurprising, this is the first study to report this finding. It is argued that by increasing this threshold of oocytes required to be eligible to participate in egg sharing, the cumulative outcomes could be improved. One study compared two different egg sharing policies, with either ≥8 or ≥12 oocytes needing to be collected for participants to be eligible to participate in egg sharing (Kolibianakis et al., Citation2003). This study reported no differences in live birth rates between the two groups but there was a cancellation rate of 29.7% in the policy requiring 12 oocytes, compared to 9.7% in the study requiring 8 eggs. The current threshold at The Lister Fertility Clinic is 8 oocytes, to be shared equally between the donor and recipient. In this study, only 3.4% egg sharers collected ≤8 oocytes, meaning 96.6% of participants produced enough to participate. If this threshold were to increase to a minimum number of 12 or 15 oocytes, then the potential cancellation rate would increase to 29.5% and 54.4% respectively (). This would significantly restrict the accessibility of patients for the egg sharing scheme and thus the affordability of IVF, whilst reducing the number of egg share donors (Kolibianakis et al., Citation2003). Egg share donors should be counselled that they have the same chance of LBR per embryo transfer compared to standard IVF patients, but have a 3.9% lower CLBR, meaning there is a small chance they will require further ovarian stimulation because they participated in the egg sharing scheme.
Table 2. A table comparing the numbers of oocytes collected by egg-sharers in stimulation cycles (n = 756), according to different minimum theoretical thresholds.
Another common potential issue raised is that to maximise the number of oocytes collected, fertility clinics will increase the dose of ovarian stimulation to egg share donors, thus exposing them to an increased risk of OHSS (Lieberman, Citation2005). In this study we report no difference in number of days of stimulation, total dose of gonadotrophin or number of oocytes collected compared to standard IVF patients. This is consistent with the findings of other studies (Ahuja et al., Citation2000; Thum et al., Citation2003). This reassuring data shows that egg share donors are not overstimulated to produce more oocytes, but instead put on the same stimulation protocols as standard IVF patients, and therefore not subjected to increased risk of OHSS.
In our unit, all egg share donors and recipients receive counselling as part of their fertility treatment, and to ensure their commitment to and understanding of the egg sharing process. Such counselling needs robust information based on hard data rather than assumption and expert opinion, and this study provides that data. Egg share donors have the right to withdraw from egg sharing treatment at any point prior to the recipient having the embryo transfer. If the egg share donor does not produce sufficient eggs to share equally with her recipient, she has the option of donating all her eggs and then having a free cycle subsequently, or to keep all the eggs for her own treatment, thus leaving the egg sharing programme and becoming a standard IVF patient and being charged for her treatment. Between 2010 and 2019 only 3.4% of egg sharers faced this dilemma.
Studies consistently revealed altruism to be as significant a motivating factor for egg share donors as financial advantages (Bracewell-Milnes et al., Citation2016, Citation2018). Therefore, despite our study finding an overall 3.9% lower CLBR, patients motivated to choose egg sharing for self-interest and altruistic reasons are likely to see the overall benefits of the scheme, considering any surplus embryos a bonus, not an expectation.
Egg share recipients
In the UK the supply falls short of the demand for donor oocytes. Egg sharing is a viable solution to the problem in countries such as the UK, where commercial payments of donors is prohibited and financial compensation is heavily restricted (Faddy et al., Citation2011). Studies have reported that oocyte recipients are reluctant to accept eggs from egg sharers, as they fear that receiving fewer oocytes compared to exclusive oocyte donors will jeopardise their chances of success (Moomjy et al., Citation2000; Oyesanya et al., Citation2009).
Concerns have been raised that the anonymously matched recipient, who is paying for treatment, will be prioritised during oocyte allocation. This study demonstrated that the mean number of oocytes allocated, FR, IR, CPR and MR were no different between the egg share donor and their recipient. In our study oocyte recipients had a statistically significantly lower LBR per ET compared to their donor, which was an unexpected finding (41.90 vs 49.10%; p < 0.05). Oocytes are allocated randomly, with accurate determination of oocyte quality difficult immediately after egg collection, meaning bias in allocation of oocytes is unlikely. Indeed, there was no difference in CLBR between egg share donors and their recipients (51.72 vs 51.30%; p = 0.84). Our data is in contrast to other studies who reported egg share recipients had higher LBR or CLBR than egg share donors, following equal distribution of oocytes (Ahuja et al., Citation1996; Check et al., Citation1992, Citation1994). However, a number of other studies reported no difference in egg share donor and recipient outcomes (Ahuja et al., Citation1996; Sakkas et al., Citation2004; Thum et al., Citation2003).
Another consistently raised concern of egg sharing is that egg sharing recipients will receive poorer quality oocytes from egg sharers because they are infertile, compared to recipients using altruistic donors (Mullin et al., Citation2010; Oyesanya et al., Citation2009). This study demonstrated no difference in FR, IR or LBR of egg share recipients compared to non-egg share recipients. Egg share recipients received significantly fewer oocytes compared to standard recipients (6.61 vs 9.31; p < 0.001), which resulted in them having fewer available day 3 embryos for transfer (4.50 vs 6.20; p < 0.001). This resulted in a lower CLBR for egg share recipients (51.3 vs 62.7%; p < 0.001). Thus, egg share recipients are not disadvantaged per transfer, but because they have fewer oocytes than standard recipient patients, they may require additional cycles to generate sufficient donor oocytes, raising their financial burdens. This needs to be balanced against the advantages of egg sharing, such as addressing a significant supply issue, reducing waiting times for donor eggs and minimising non-patient donors to unnecessary risks. Furthermore, often fertility clinics will divide the retrieved oocytes from altruistic donors between two recipients without affecting success (Ahuja et al., Citation2000; Glujovsky et al., Citation2006; Mullin et al., Citation2010). This practice, similar to egg sharing, improves treatment efficacy, diminishes donor shortages and avoids potential wastage of donor eggs (Glujovsky et al., Citation2006). However, these studies did not report CLBR.
Overall, egg share recipients should be reassured by an overall high LBR per embryo transfer of 41.9% and a CLBR of 51.3%. It appears because egg share donors are highly selected for age and adequate AMH, the smaller number of oocytes available for their recipient are still adequate to offer excellent outcomes for both the egg share donor and her recipient.
Current egg share numbers in the UK
Despite the reassuring findings from this study regarding egg share outcomes, in recent years there has been a significant decrease in the number of fertility patients participating in egg sharing, from 698 in 2011, to 348 in 2016 (HFEA, Citation2019a). A recent study in the UK found very little knowledge amongst the general public of egg sharing; however once an explanation was given, 70.4% approved of the practice (Bracewell-Milnes et al., Citation2021). Another study investigating the views towards egg sharing of healthcare professionals in the UK found 78.2% thought egg sharing should take place, however, 63.1% of respondents had no knowledge of the scheme (Bracewell-Milnes et al., Citation2019). This meant only 16.5% of healthcare professionals who can refer patients for fertility treatments had referred a patient for egg sharing, with the majority citing a lack of knowledge for the reason they hadn’t referred (Bracewell-Milnes et al., Citation2019). Another study examined how egg share donors and recipients found out about the option of egg sharing; the IVF clinic was the main source of information, followed by personal internet research, with very little knowledge acquired from general practioners (GPs) and general gynaecologists (Gürtin et al., Citation2012b). This is highly significant as we know these medical practitioners are a widely consulted source of information for couples trying to conceive (Gürtin et al., Citation2012b). Indeed, the same study reported numerous participants were significantly frustrated by the time delay of finding out about egg sharing, and were disappointed not to have been informed by their relevant healthcare professionals earlier (Gürtin et al., Citation2012b). This is a reasonable position, since the time from initially seeing a GP to being referred for IVF treatment is 1–2 years on average, and often there is an age cut off for when women would qualify to participate in the egg sharing programme. Additionally, the nature of the information that women are accessing is of concern, since it is known the internet holds a lot of inaccurate, unregulated, and biased information for patients. These data imply that a significant majority of both healthcare professionals, fertility patients and the general public support egg sharing, meaning a lack of knowledge of this practice is contributing to its declining numbers, rather than a lack of support for it.
Conclusion
In the UK, the number of donor oocytes available falls well short of demand, and egg sharing has the potential to significantly increase the egg donor pool. This study demonstrates that egg sharing does not compromise the chances of donors or their recipients achieving a live birth per embryo transferred. However, participants may occasionally require additional ovarian stimulation cycles to conceive. With decreasing government funding for IVF treatment, egg sharing provides a practical option to allow a greater number of patients to access IVF, while not putting themselves at any additional risk of complications. This data should reassure and encourage prospective egg share donors and recipients, fertility clinics and other healthcare professionals who would potentially refer patients for this treatment. The egg sharing programme is currently the most efficient way of maximising the use of the precious resource of human oocytes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahuja, K. K., Simons, E. G., Fiamanya, W., Dalton, M., Armar, N. A., Kirkpatrick, P., Sharp, S., Arian-Schad, M., Seaton, A., & Watters, W. J. (1996). Egg-sharing in assisted conception: Ethical and practical considerations. Human Reproduction, 11(5), 1126–1131. https://doi.org/10.1093/oxfordjournals.humrep.a019310
- Ahuja, K. K., Simons, E. G., Rimington, M. R., Nair, S., Gill, A., Evbuomwan, I., & Bowen-Simpkins, P. (2000). One hundred and three concurrent IVF successes for donors and recipients who shared eggs: Ethical and practical benefits of egg sharing to society. Reproductive Biomedicine Online, 1(3), 101–105. https://doi.org/10.1016/S1472-6483(10)61947-5
- Applegarth, L., Goldberg, N. C., Cholst, I., McGoff, N., Fantini, D., Zellers, N., Black, A., & Rosenwaks, Z. (1995). Families created through ovum donation: A preliminary investigation of obstetrical outcome and psychosocial adjustment. Journal of Assisted Reproduction and Genetics, 12(9), 574–580. https://doi.org/10.1007/BF02212577
- BIONEWS. (2011). Drastic changes to sperm and egg donation policy made by the HFEA. HFEA. Retrieved February, 2022, from https://www.hfea.gov.uk/media/2808/trends-in-egg-and-sperm-donation-final.pdf: HFEA.
- Birch Petersen, K., Hvidman, H. W., Sylvest, R., Pinborg, A., Larsen, E. C., Macklon, K. T., Andersen, A. N., & Schmidt, L. (2015). Family intentions and personal considerations on postponing childbearing in childless cohabiting and single women aged 35–43 seeking fertility assessment and counselling. Human Reproduction, 30(11), 2563–2574. https://doi.org/10.1093/humrep/dev237
- Blyth, E. (2001). Guidance for egg sharing arrangements: Redefining the limits of information-giving in donor assisted conception. Reproductive Biomedicine Online, 3(1), 45–47. https://doi.org/10.1016/S1472-6483(10)61965-7
- Blyth, E. (2002). Subsidized IVF: The development of ‘egg sharing’ in the United Kingdom. Human Reproduction, 17(12), 3254–3259. https://doi.org/10.1093/humrep/17.12.3254
- Bracewell-Milnes, T., Holland, J. C., Jones, B. P., Saso, S., Almeida, P., Maclaran, K., Norman-Taylor, J., Nikolaou, D., Shah, N. M., Johnson, M., & Thum, M. Y. (2021). Exploring the knowledge and attitudes of women of reproductive age from the general public towards egg donation and egg sharing: A UK-based study. Human Reproduction, 36(8), 2189–2201. https://doi.org/10.1093/humrep/deab157
- Bracewell-Milnes, T., Rajendran, S., Saso, S., Jones, B., Platts, S., Cato, S., & Thum, M. Y. (2019). Investigating knowledge and perceptions of egg sharing among healthcare professionals in the United Kingdom. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 236, 98–104. https://doi.org/10.1016/j.ejogrb.2019.03.003
- Bracewell-Milnes, T., Saso, S., Abdalla, H., & Thum, M. Y. (2018). A systematic review investigating psychosocial aspects of egg sharing in the United Kingdom and their potential effects on egg donation numbers. Human Fertility, 21(3), 163–173. https://doi.org/10.1080/14647273.2017.1329554
- Bracewell-Milnes, T., Saso, S., Bora, S., Ismail, A. M., Al-Memar, M., Hamed, A. H., Abdalla, H., & Thum, M. Y. (2016). Investigating psychosocial attitudes, motivations and experiences of oocyte donors, recipients and egg sharers: A systematic review. Human Reproduction Update, 22(4), 450–465. https://doi.org/10.1093/humupd/dmw006
- Braga, D., Setti, A. S., Iaconelli, A., Jr, & Borges, E. Jr(2020). Predictive factors for successful pregnancy in an egg-sharing donation program. JBRA Assisted Reproduction, 24(2), 163–169. https://doi.org/10.5935/1518-0557.20190087
- Brulliard, K. (2006). In competitive marketplace, Asian egg donors in demand. The Washington Post, A1–A7. https://www.washingtonpost.com/archive/politics/2006/11/19/in-competitive-marketplace-asian-egg-donors-in-demand
- Check, J. H., Askari, H. A., Fisher, C., & Vanaman, L. (1994). The use of a shared donor oocyte program to evaluate the effect of uterine senescence. Fertility and Sterility, 61(2), 252–256. https://doi.org/10.1016/S0015-0282(16)56512-1
- Check, J. H., Fox, F., Choe, J. K., Krotec, J. W., & Nazari, A. (2004). Sharing of oocytes from infertile versus paid donors results in similar pregnancy and implantation rates. Fertility and Sterility, 81(3), 703–704. https://doi.org/10.1016/j.fertnstert.2003.07.026
- Check, J. H., Nowroozi, K., Chase, J., Nazari, A., & Braithwaite, C. (1992). Comparison of pregnancy rates following in vitro fertilization-embryo transfer between the donors and the recipients in a donor oocyte program. Journal of Assisted Reproduction and Genetics, 9(3), 248–250. https://doi.org/10.1007/BF01203822
- Check, J. H., Wilson, C., Jamison, T., Choe, J. K., & Cohen, R. (2012). The sharing of eggs by infertile women who are trying to conceive themselves with an egg recipient for financial advantages does not jeopardize the donor’s chance of conceiving. Clinical and Experimental Obstetrics & Gynecology, 39(4), 432–433. https://doi.org/10.31083/j.ceog.2020.03.5077
- Culley, L., Hudson, N., Rapport, F., Blyth, E., Norton, W., & Pacey, A. A. (2011). Crossing borders for fertility treatment: Motivations, destinations and outcomes of UK fertility travellers. Human Reproduction, 26(9), 2373–2381. https://doi.org/10.1093/humrep/der191
- Deech, R. (1998). Legal and ethical responsibilities of gamete banks. Human Reproduction, 13(Suppl 2), 80–83. discussion 84–89. https://doi.org/10.1093/humrep/13.suppl_2.80
- Dyer, C. (2011). Payment to egg donors is to be tripled to remedy shortage. BMJ, 343, d6865. https://doi.org/10.1136/bmj.d6865
- Faddy, M., Gosden, R., Ahuja, K., & Elder, K. (2011). Egg sharing for assisted conception: A window on oocyte quality. Reproductive BioMedicine Online, 22(1), 88–93. https://doi.org/10.1016/j.rbmo.2010.08.009
- Glujovsky, D., Fiszbajn, G., Lipowicz, R., Lavolpe, M., & Sueldo, C. (2006). Practice of sharing donated oocytes among several recipients. Fertility and Sterility, 86(6), 1786–1788. https://doi.org/10.1016/j.fertnstert.2006.04.042
- Gürtin, Z. B., Ahuja, K. K., & Golombok, S. (2012a). Emotional and relational aspects of egg-sharing: Egg-share donors’ and recipients’ feelings about each other, each others’ treatment outcome and any resulting children. Human Reproduction, 27(6), 1690–1701. https://doi.org/10.1093/humrep/des085
- Gürtin, Z. B., Ahuja, K. K., & Golombok, S. (2012b). Egg-share donors’ and recipients’ knowledge, motivations and concerns: Clinical and policy implications. Clinical Ethics, 7(4), 183–192. https://doi.org/10.1258/ce.2012.012024
- HFEA. (2018). Fertility Treatment 2014–2016: Trends and figures. Retrieved October 13, 2022, from https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2018-trends-and-figures
- HFEA. (2019a). Fertility treatment 2018: Trends and figures. Retrieved October 13, 2022, from https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2019-trends-and-figures
- HFEA. (2019b). Trends in egg and sperm donation. Retrieved from https://www.hfea.gov.uk/media/2808/trends-in-egg-and-sperm-donation-final.pdf: HFEA.
- Johnson, M. H. (1999). The medical ethics of paid egg sharing in the UK. Human Reproduction, 14(7), 1912–1918. https://doi.org/10.1093/humrep/14.7.1912
- Kolibianakis, E. M., Tournaye, H., Osmanagaoglu, K., Camus, M., Van Waesberghe, L., Van Steirteghem, A., & Devroey, P. (2003). Outcome for donors and recipients in two egg-sharing policies. Fertility and Sterility, 79(1), 69–73. https://doi.org/10.1016/S0015-0282(02)04406-0
- Lieberman, B. (2005). Egg-sharing: A critical view. The Obstetrician & Gynaecologist, 7(2), 109–111. https://doi.org/10.1576/toag.7.2.109.27068
- Lieberman, J. A., Moscicki, A. B., Sumerel, J. L., Ma, Y., & Scott, M. E. (2008). Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clinical and Vaccine Immunology, 15(1), 49–54. https://doi.org/10.1128/CVI.00216-07
- Lutjen, P., Trounson, A., Leeton, J., Findlay, J., Wood, C., & Renou, P. (1984). The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature, 307(5947), 174–175. https://doi.org/10.1038/307174a0
- Malhotra, N., Shah, D., Pai, R., Pai, H. D., & Bankar, M. (2013). Assisted reproductive technology in India: A 3 year retrospective data analysis. Journal of Human Reproductive Sciences, 6(4), 235–240. https://doi.org/10.4103/0974-1208.126286
- Moomjy, M., Mangieri, R., Beltramone, F., Cholst, I., Veeck, L., & Rosenwaks, Z. (2000). Shared oocyte donation: Society’s benefits. Fertility and Sterility, 73(6), 1165–1169. https://doi.org/10.1016/S0015-0282(00)00539-2
- Mullin, C. M., Fino, M. E., Talebian, S., Keegan, D., Grifo, J. A., & Licciardi, F. (2010). Comparison of pregnancy outcomes in anonymous shared versus exclusive donor oocyte in vitro fertilization cycles. Fertility and Sterility, 93(2), 574–578. https://doi.org/10.1016/j.fertnstert.2009.07.1669
- Oyesanya, O. A., Olufowobi, O., Ross, W., Sharif, K., & Afnan, M. (2009). Prognosis of oocyte donation cycles: A prospective comparison of the in vitro fertilization-embryo transfer cycles of recipients who used shared oocytes versus those who used altruistic donors. Fertility and Sterility, 92(3), 930–936. https://doi.org/10.1016/j.fertnstert.2008.07.1769
- Sakkas, D., D'Arcy, Y., Percival, G., Sinclair, L., Afnan, M., & Sharif, K. (2004). Use of the egg-share model to investigate the paternal influence on fertilization and embryo development after in vitro fertilization and intracytoplasmic sperm injection. Fertility and Sterility, 82(1), 74–79. https://doi.org/10.1016/j.fertnstert.2003.11.054
- Sauer, M. V., & Kavic, S. M. (2006). Oocyte and embryo donation 2006: Reviewing two decades of innovation and controversy. Reproductive Biomedicine Online, 12(2), 153–162. https://doi.org/10.1016/S1472-6483(10)60855-3
- Schmidt, L., Sobotka, T., Bentzen, J. G., & Nyboe Andersen, A. (2012). Demographic and medical consequences of the postponement of parenthood. Human Reproduction Update, 18(1), 29–43. https://doi.org/10.1093/humupd/dmr040
- Simons, E. G., & Ahuja, K. K. (2005). Egg-sharing: An evidence based solution to donor egg shortages. The Obstetrician & Gynaecologist, 7(2), 112–116. https://doi.org/10.1576/toag.7.2.112.27069
- Thum, M. Y., Gafar, A., Wren, M., Faris, R., Ogunyemi, B., Korea, L., Scott, L., & Abdalla, H. I. (2003). Does egg-sharing compromise the chance of donors or recipients achieving a live birth? Human Reproduction, 18(11), 2363–2367. https://doi.org/10.1093/humrep/deg464
