ABSTRACT
Background: This comparison of PRAsugrel and clopidogrel in Japanese patients with ischemic STROke (PRASTRO)-I trial investigates the noninferiority of prasugrel to clopidogrel sulfate in the prevention of recurrence of primary events (ischemic stroke, myocardial infarction, and death from other vascular causes), and the long-term safety of prasugrel in Japanese patients with non-cardioembolic stroke.
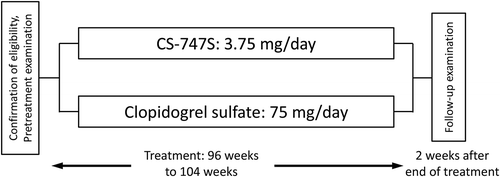
Research design and methods: This was an active-controlled, randomized, double-blind, double-dummy, parallel-group study conducted between July 2011 and March 2016 at multiple centers around Japan. Patients had to meet eligibility criteria before receiving 3.75 mg prasugrel or 75 mg clopidogrel orally once daily for a period of 96–104 weeks.
Results: A total of 3747 patients were included in this trial; 1598 in the 3.75 mg prasugrel group and 1551 in the 75 mg clopidogrel group completed the study. During the study period, 287 (15.2%) patients in the prasugrel group and 311 (16.7%) in the clopidogrel group discontinued treatment. Baseline characteristics, safety, and efficacy results are forthcoming and will be published separately.
Conclusions: This article presents the study design and rationale for a trial investigating the noninferiority of prasugrel to clopidogrel sulfate with regards to the inhibitory effect on primary events in patients with non-cardioembolic stroke.
1. Introduction
The primary therapeutic target for patients with non-cardioembolic stroke is the prevention of recurrence and to do so requires careful management of risk factors to avoid bleeding complications and antiplatelet therapy [Citation1–Citation3]. According to the Guidelines for the Management of Stroke, use of aspirin and clopidogrel sulfate is strongly recommended as antiplatelet therapy for secondary prevention [Citation4,Citation5]. The results of a meta-analysis by the Antiplatelet Trialists’ Collaboration indicated that antiplatelet therapies involving aspirin, clopidogrel sulfate, and ticlopidine hydrochloride reduced vascular events (vascular death, myocardial infarction, and stroke) by approximately 20% in patients with strokes and transient ischemic attacks (TIA) [Citation6]. However, there are poor responders to the standard antiplatelet therapies of aspirin or clopidogrel sulfate [Citation7,Citation8], and data suggest that these poor responders have elevated ischemic event risks [Citation9,Citation10].
Research also shows that a high proportion of patients with clearly poor responsiveness to aspirin or clopidogrel sulfate are among the growing population of people with diabetes [Citation11]. Insufficient production of the active drug metabolites of clopidogrel sulfate is implicated as a possible cause for this poor responsiveness. Studies have reported that the suppression of platelet aggregation by clopidogrel sulfate is influenced by cytochrome P450 (CYP) polymorphisms [Citation12,Citation13]. Furthermore, ethnic variations in the frequencies of CYP2C19 variant alleles have been reported, with poor metabolizers (PMs) and intermediate metabolizers (IMs) accounting for about 20% of Caucasians and more than 50% of the Japanese population [Citation14,Citation15]. These findings suggest that a large proportion of Japanese patients might be incapable of achieving a satisfactory anti-aggregation effect with clopidogrel sulfate owing to their low production of active clopidogrel metabolites.
Prasugrel is a thienopyridine antiplatelet agent and adenosine diphosphate (ADP) receptor antagonist. The active metabolite of prasugrel selectively inhibits the P2Y12 receptor located on the platelet membrane, thereby suppressing platelet aggregation. Nonclinical and clinical trials conducted to date have indicated that prasugrel inhibits platelet aggregation more potently, exerts therapeutic action faster, and is less influenced by CYP2C19 polymorphisms than clopidogrel sulfate. In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI 38), prasugrel (loading/maintenance doses: 60/10 mg) reduced the rates of ischemic events (a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) significantly more than clopidogrel sulfate in patients who had undergone percutaneous coronary intervention (PCI) for the treatment of acute coronary syndrome and were concomitantly administered aspirin [Citation16]. Of note is that prasugrel suppressed ischemic events effectively in the subgroup of patients with diabetes as well as in CYP2C19 PM and IM patients [Citation17,Citation18]. Based on those results, prasugrel has gained regulatory approval in the US and European countries for the treatment of acute coronary syndrome in patients undergoing PCI. However, patients with a history of stroke may have an increased frequency of developing bleeding complications, such as intracranial hemorrhage. Therefore, we considered that lower doses of prasugrel could diminish the risk of bleeding in stroke patients.
The present trial investigated the non-inferiority of prasugrel versus clopidogrel sulfate using a lower dose of prasugrel (approximately one-third [3.75 mg] the dose level used in TRITON-TIMI 38). Clopidogrel sulfate, which has been strongly recommended by the Japanese Guidelines for the Management of Stroke 2015 [Citation19] as the antiplatelet therapy of choice for secondary stroke prevention, was chosen as a comparator at a dose of 75 mg per day. The prasugrel dosage of 3.75 mg per day was chosen for two reasons: First, the tolerability of this dosage has been confirmed in non-cardioembolic stroke patients. Second, 3.75 mg prasugrel showed more potent platelet inhibition than 75 mg clopidogrel without increasing bleeding risk, irrespective of the CYP2C19 genotype (unpublished data on file; Daiichi Sankyo Co., Ltd., Tokyo, Japan).
The main objectives of this trial were to compare the efficacy and safety between prasugrel and clopidogrel sulfate in patients with non-cardioembolic stroke, and thus examine an indication of prasugrel for treating cerebrovascular disease. The primary end point was the composite of ischemic stroke, myocardial infarction, and death from other vascular causes. This end point was selected to precisely evaluate the suppressive effects expected from prasugrel on ischemic stroke, other vascular events, and deaths. The reduced prasugrel dose would likely make it difficult to show that a significant number of ischemic events were prevented when administering prasugrel, when compared with clopidogrel at a dose of 75 mg; thus, after consultation with the regulatory authorities, and taking into account that the trial was to be conducted only in Japan, this study was designed to verify the non-inferiority of prasugrel to clopidogrel. The purpose of the present article is to present the rationale and study design for this trial. The safety and efficacy results are forthcoming and will be published separately.
2. Methods
2.1. Study design
This was a randomized, double-blind, active-controlled, parallel group, multicenter (224 institutions) trial. Non-cardioembolic stroke patients were randomly assigned to the prasugrel group (3.75 mg) or clopidogrel group (75 mg). All of the patients were enrolled and observed based on the diagnosis of stroke experts. The observation period was 96–104 weeks, and the study was conducted from July 2011 to March 2016 ().
The trial was approved by the institutional review boards of the respective institutions, and all patients gave written informed consent. The trial adheres fully to the ethical principles of the Declaration of Helsinki as well as Good Clinical Practice (GCP) guidelines. The trial was registered with the Japan Pharmaceutical Information Center (JapicCTI-111,582). This trial was sponsored by Daiichi Sankyo Co., Ltd.
2.2. Patients
The target sample size was 3600 (1800 in each group) patients with non-cardioembolic stroke. Patients were eligible if they fulfilled the following inclusion criteria: age 20–74 years at the time of giving consent; body weight >50 kg; interval from last stroke attack to time of consent of 1–26 weeks; and infarct area confirmed by magnetic resonance imaging (MRI).
Patients were excluded if they met any of the following criteria: presence of cardioembolic stroke, paradoxical cerebral embolism, and asymptomatic ischemic stroke; presence of atrial fibrillation or other cardiovascular disease causing cardioembolic stroke; those requiring coadministration of other antiplatelet agents; current evidence or previous history of intracerebral hemorrhage; current evidence or high risk of subarachnoid hemorrhage; poorly controlled hypertension; and resting seated systolic or diastolic blood pressure of ≥180 mmHg or ≥110 mmHg, respectively, for patients within 4 weeks after the last ischemic attack, and ≥160 mmHg or ≥100 mmHg, respectively, for patients over 4 weeks after the last ischemic stroke event.
2.3. Intervention
The study drug assignment table was formulated by the responsible person as per the protocol. Randomization was performed using a uniform random numbers list generated using SAS Release 9.1.3 (SAS Institute, Cary, NC, USA). Patients were stratified by stroke subtype using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification [Citation20], and the subject registration center then randomly assigned eligible patients at a 1:1 ratio to prasugrel or clopidogrel groups such that the stroke subtypes of large-artery atherosclerosis and small-artery occlusion (lacune) were equally distributed between the two groups. The patients in each group received 3.75 mg prasugrel or 75 mg clopidogrel orally once daily after breakfast according to a double-blind and double-dummy design.
To achieve blinding, the responsible person sealed and stored the assignment table until unblinding. Two sets (one for use in Japan and the other for use in reporting to the regulatory authorities in Europe) of emergency key codes for each drug were created and sealed so that drugs could be unblinded in emergency situations to identify without delay the treatment assigned to the subject in danger. When a serious and unexpected adverse event (AE) occurred during the study and required reporting to the regulatory authorities in Europe, the safety manager at Eli Lilly & Co. could break the emergency key code according to predefined procedures. Specifically, before unblinding, the sponsor confirmed and recorded that the case report form database had been locked; the person responsible for study drug assignment would then verify and record that the emergency key code was appropriately managed. The person responsible for study drug assignment could then break the seal of the assignment table envelope after judging that these steps had been taken.
During the study period, blood pressure was strictly controlled to achieve a target level of <140/90 mmHg. Concomitant use of antiplatelet agents, anticoagulants, thrombolytic agents, acidic nonsteroidal anti-inflammatory drugs, and other study drugs was prohibited throughout the study period.
2.4. Data monitoring
Meetings of an independent (and unblinded) data monitoring committee were held once every 6 months. The committee meetings were held independently of the trial sponsor and trial investigators. The role of this committee was to monitor the safety and efficacy data to determine the feasibility of continuing the study under unblinded conditions.
2.5. Evaluation of efficacy and safety end points
The Efficacy Evaluation Committee and the Bleeding Evaluation Committee, independent of the sponsor, evaluated the efficacy events and bleeding events, respectively, under blinded conditions. The primary efficacy end point was the incidence of composite events comprising ischemic stroke, myocardial infarction, and death from other vascular causes observed from the start of the study drug administration to one day following study drug completion or discontinuation. The secondary end points were the incidences of stroke, TIA, myocardial infarction, unstable angina, peripheral arterial disease, cardiovascular death, and death from other vascular causes. The primary safety event was the incidence of bleeding events, namely, life-threatening bleeding, major bleeding, and clinically relevant bleeding. The definitions of all these efficacy and bleeding events are shown in the Supplemental Materials.
2.6. Intracranial and extracranial diagnostic imaging
Infarct size and location were determined using intracranial magnetic resonance angiography, and diffusion-weighted and fluid-attenuated inversion recovery (or T1-weighted) MRI. These were performed on all subjects during the pretreatment period (week 0), at week 48, and at completion. In addition, the pretreatment examination included a T2*-weighted MRI or computed tomography (CT) image that determined the presence or absence of intracranial hemorrhage. Magnetic resonance angiography, CT angiography, or ultrasound was performed during the pretreatment period, at week 48, and at completion to determine carotid artery stenosis.
2.7. Pharmacodynamic measurements
Platelet vasodilator stimulated phosphoprotein (VASP) phosphorylation was determined using enzyme-linked immunosorbent assay at an optical density (OD) of 450 nm. In brief, 2.0 ml of venous blood was collected during the pretreatment period and 8 h after study treatment at weeks 4, 24, 48, and at the final week of the study from patients who had received the study drug for at least 5 consecutive days (including the visit day).
The platelet reactivity index (PRI) was determined for specimens labeled with antiphosphorylated VASP antibody [Citation21] after incubation with either prostaglandin E1 (PGE1) or with PGE1 and ADP. VASP measurements results were not disclosed to investigators before data lock.
Platelet aggregation was determined using the VerifyNow® P2Y12 assay (Accriva Diagnostics, San Diego, CA, USA), a turbidimetric-based optical detection system that measures platelet aggregation. In brief, 2.0 ml of venous blood were collected at the same time points as the blood collection for the VASP measurement during the pretreatment period, and 8 h after study treatment at weeks 4, 24, 48, and at the final week of the study from patients who had received the study drug for at least 5 consecutive days (including the visit day). The VerifyNow® P2Y12 assay was performed within 4 h of blood collection. VerifyNow® P2Y12 assay results were not disclosed to investigators before data lock.
2.8. Pharmacokinetic measurements
Inactive drug metabolite concentrations were quantitatively analyzed by collecting 7.0 ml of venous blood at weeks 2 and 4 from patients who had received the study drug for at least 5 consecutive days (including the visit day). Furthermore, blood specimens were collected at 0.5 and 4 h after drug intake on the visit day. Blood samples were centrifuged at 3000 rpm for 10 min at room temperature to separate the plasma fraction. Plasma samples were stored at −20°C until shipment to the LSI Medience Corporation laboratory (Tokyo, Japan) for analysis of target inactive metabolites R-95913, R-119251, and R-106583 by liquid chromatography-tandem mass spectrometry. The pharmacokinetic profile of prasugrel was estimated from the pharmacokinetics of these inactive metabolites by model-based analysis. Pharmacokinetic profiles were not disclosed to investigators before data lock.
2.9. Genetic analysis
Of 3747 randomized patients, 3461 patients underwent genetic analysis (prasugrel group: n = 1742, clopidogrel group: n = 1719). To determine the relationship between CYP genetic polymorphism and drug inhibition of platelet aggregation, the frequencies of genetic polymorphisms in CYP2B6, CYP2C9, and CYP2C19 were analyzed. In addition, polymorphisms of the ATP-binding cassette subfamily B member 1 (ABCB1) gene, which regulates prasugrel and clopidogrel absorption, were also analyzed. Patients enrolled in this part of the study signed an additional informed consent form for the genetic analysis. DNA was extracted from the leukocytes in the 2.0-ml blood samples that were collected once consent was confirmed and before the end of week 8. Samples were genotyped at an external gene analysis laboratory (LSI Medience Corporation). The target gene polymorphisms are shown in the Supplemental Materials. The results of genetic analyses were not disclosed to investigators before data lock.
2.10. Other measurements
Patients’ vital sign measurements included blood pressure (systolic and diastolic) and pulse rate in a sitting position, which were measured at every visit from pretreatment to completion of the study. A standard 12-lead electrocardiogram (ECG) measurement was also taken at week 0, week 48, and at completion. ECG readings were classified either as normal, absence of a clinically significant abnormality, or the presence of a clinically significant abnormality.
2.11. Statistical analysis
The details of the statistical analysis used for efficacy and safety results will be published together with the efficacy and safety results. However, the rationale for the statistical analysis plan is presented here. The primary efficacy end point was assessed using a non-inferiority margin of 1.35. That is, non-inferiority was considered established if the upper limit of the 95% confidence interval for the risk ratio (RR) for the primary event in the prasugrel group against the clopidogrel group did not exceed the non-inferiority margin of 1.35. Because no previous study used end points that were similar to those in this trial, a non-inferiority margin was determined by predicting the incidence of ischemic stroke, myocardial infarction, and death from other vascular causes. This non-inferiority margin is in reference to the incidence of ischemic stroke plus vascular death in the Sarpogrelate-Aspirin Comparative Clinical Study for Efficacy and Safety in Secondary Prevention of Cerebral Infarction (S-ACCESS) trial [Citation22]. The reason for referring to the results of this trial to determine the non-inferiority margin is that, when the present trial protocol was finalized in 2011, FDA guidance for the formulation of a non-inferiority margin (two-sided 95% confidence interval method) had not yet been published. When formulating a non-inferiority margin, we needed to utilize results from clinical studies of clopidogrel versus placebo. However, a systematic review revealed no previously published placebo-controlled study. Therefore, taking into account that the study would only be conducted in Japan, we determined that the non-inferiority margin was to be based on information from the S-ACCESS trial [Citation22] as well as results from a randomized, double-blind study comparing clopidogrel with ticlopidine in Japanese patients with noncardioembolic cerebral infarction [Citation23].
The rationale for determination of the non-inferiority margin was as follows. Based on the annual event rates (5.35%) of ischemic stroke and vascular event-related death in the aspirin group reported in the S-ACCESS trial, the annual incidence of primary end point in patients taking aspirin was estimated as 5.4% [Citation22]. Assuming the annual incidence of primary end points in patients taking clopidogrel is 4% [Citation23], the RR in patients taking aspirin relative to those taking clopidogrel was estimated as 1.35 (5.4/4). Consequently, the non-inferiority margin for the present trial was determined as 1.35.
Efficacy analysis was performed on the full analysis set (FAS) defined as the entire population, excluding study participants who had violated the GCP guidelines, those who had not taken the study drug even once, and those for whom there were no data after taking the study drug. Safety analysis was performed on the population, excluding study participants with serious GCP violations, and those who had not taken the study drug.
Efficacy analyses was conducted in the per protocol set (PPS) to investigate the robustness of the results. The PPS was defined as comprising subjects in the FAS, excluding those who did not satisfy all of the inclusion criteria or who met one or more of the exclusion criteria, those whose compliance was <75% during study treatment, those who took a prohibited concomitant drug, and subjects with a major protocol violation.
For sample size estimation, we assumed that the incidence of primary end points was 7% during 2 years in the clopidogrel group based on the results of a trial of clopidogrel conducted in Japan [Citation23]. In the TRITON-TIMI 38 trial, prasugrel showed approximately 18% risk reduction in cardiovascular patients [Citation16]. In addition, the frequencies of CYP2C19 PMs and IMs are higher in Japanese patients than those in Caucasians [Citation14,Citation15]. Therefore, we assumed a relative risk reduction of 15% for prasugrel versus clopidogrel. Using the non-inferiority margin (1.35), we calculated the number of patients for the conditions α = 2.5% (one-sided) and statistical power of 80%, and determined the target number of patients as 2200. However, during the study (as of April 2014), the incidence of the primary end point under blinded conditions was found to be lower than initially assumed, and a final incidence of around 4% in 2 years was expected. Therefore, without changing any condition other than event incidence, the number of patients was recalculated, and the target number of patients was reset to 3600. Based on these hypotheses, the target number of primary end points was assumed to be 143 events.
3. Results
3.1. Patients
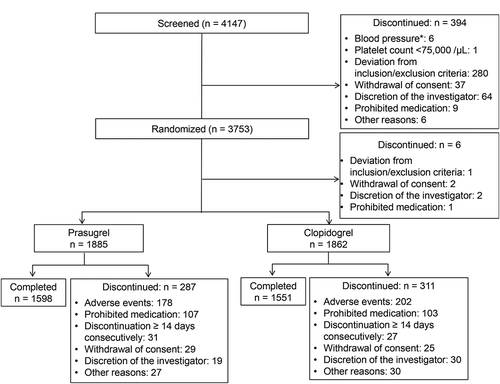
Of the 4147 patients who consented to participate in the study, 3753 were eligible and included in the random assignment for pretreatment (≥3 days to ≤2 weeks) (). Of those, 1885 patients in the prasugrel group and 1862 patients in the clopidogrel group were confirmed to have taken the study drug at least once, and six patients dropped out before administration of the study drug. Thus, a total of 3747 patients were included in the FAS.
The number of patients completing the study treatment was 1598 in the prasugrel group and 1551 in the clopidogrel group. There was no difference in the median dosing period between the groups (673.0 days in the prasugrel group and 672.0 days in the clopidogrel group).
During the study period, 287 (15.2%) patients in the prasugrel group and 311 (16.7%) in the clopidogrel group discontinued the treatment. The major reason for discontinuation for both groups was AEs, followed by concomitant use of prohibited drugs.
Baseline characteristics and efficacy and safety results will be published separately and will conform to the CONSORT criteria for non-inferiority trials.
4. Discussion
The risk of vascular events after ischemic stroke has been shown to be reduced through the inhibition of platelet aggregation with aspirin or clopidogrel [Citation6–Citation12]. However, the decrease in the rate of ischemic events by antiplatelet agents is accompanied by an increase in bleeding complications [Citation9,Citation10]. Regarding clopidogrel, there has been a need to develop new suppressors of platelet aggregation that are effective in poor responders to standard antiplatelet therapies, particularly those with reduced CYP2C19 function.
In the TRITON-TIMI 38 trial, prasugrel and clopidogrel were administered with aspirin in ACS patients scheduled for PCI treatment, using a regimen consisting of an initial loading dose (60 and 300 mg, respectively) followed by 6–15-month maintenance dose administration (10 mg and 75 mg, respectively) [Citation16]. The outcomes showed that prasugrel was statistically superior to clopidogrel in terms of reducing the risk of ischemic events (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke). However, the risk of fatal and other serious bleeding events unrelated to coronary artery bypass grafting was higher for prasugrel than clopidogrel. Based on analyses of prespecified clinical features in TRITON-TIMI 38, age ≥75 years, body weight <60 kg, and a history of stroke or TIA were identified risk factors of bleeding [Citation16]. Therefore, we considered that the lower dose of prasugrel could diminish the risk of bleeding in Japanese patients. In the PRASugrel compared with clopidogrel For Japanese patIenTs with ACS undergoing PCI (PRASFIT-ACS) trial, the prasugrel dosing regimen (loading/maintenance doses: 20/3.75 mg) resulted in a low incidence of ischemic events, similar to the results of TRITON-TIMI 38, and a low risk of bleeding in Japanese ACS patients [Citation24]. In the PRASugrel compared with clopidogrel For Japanese patIenTs undergoing Elective PCI (PRASFIT-Elective) trial, prasugrel (loading/maintenance doses: 20/3.75 mg) showed efficacy and safety consistent with that seen in the PRASFIT-ACS trial in patients undergoing elective PCI [Citation25].
As a rationale for the dose regimen for Japanese stroke patients, trials of prasugrel in healthy patients evaluated the administration of prasugrel as a single dose (2, 5, 10, 20, and 30 mg) and in multiple doses for 7 days (2.5, 5, 7.5, and 10 mg) [Citation26]. In the single- and multiple-dose trials of prasugrel in Japan, bleeding AEs, including epistaxis and positive fecal occult blood, were reported. All events were mild and resolved without intervention, although several other AEs were also reported. However, there were no clinically relevant AEs noted for prasugrel, which suggested that the drug was safe and well tolerated.
To further evaluate the safety, efficacy, and pharmacodynamics of prasugrel, a trial conducted in Japan enrolled 129 patients with non-cardioembolic stroke (age ≤75 years; no lower body weight limit). In this randomized double-blind crossover trial, patients received clopidogrel 75 mg/day for >4 weeks before commencing prasugrel treatment. The patients were divided into groups A (n = 64) and B (n = 65). They then received 4-week treatment with prasugrel 3.75 mg/day or 2.5 mg/day followed by a 4-week switched-dose regimen. A significant reduction in platelet reactivity units (PRU) was noted after treatment with prasugrel 3.75 mg compared with the pre-dose value (after treatment with clopidogrel) (P < 0.0001). PRU, however, was similar to the pre-dose value after treatment with prasugrel 2.5 mg. When stratified by CYP2C19 phenotype, a significant reduction in PRU was noted in IMs and PMs after treatment with prasugrel 3.75 mg and PMs after treatment with prasugrel 2.5 mg, compared with the pre-dose value (P < 0.0001). No significant differences were found between the two treatment groups in terms of the incidence of AEs (unpublished data on file; Daiichi Sankyo Co., Ltd., Tokyo, Japan). Based on these results, we selected prasugrel 3.75 mg as the dose for the PRASTRO-I trial because it was tolerated and had more potent platelet inhibition compared with clopidogrel 75 mg, irrespective of CYP2C19 genotype.
This study has some limitations. Elderly patients aged ≥75 years, as well as patients with documented atrial fibrillation and high risk of cardioembolism, were excluded. Moreover, blood pressure was well controlled throughout the trial period. These factors may have reduced the incidence of primary events (ischemic stroke, myocardial infarction, or death from other vascular causes) or minimized the risk of bleeding events in patients treated with prasugrel when compared to clopidogrel, and may mean that out study population is not representative of a more clinically relevant real-world population.
The current PRASTRO-I trial is investigating the prevention and recurrence of ischemic stroke and cardiovascular events in non-cardioembolic stroke patients. It is a multicenter, active-controlled, randomized, double-blind, double-dummy, parallel-group study involving patients receiving 3.75 mg prasugrel or 75 mg clopidogrel orally once daily for 96–104 weeks. This article has presented the study design and rationale for this trial and detailed the demographic measures. Baseline characteristics, efficacy, and safety results from this trial will be presented in a follow-up publication.
5. Conclusions
The design of and rationale for a clinical trial assessing the non-inferiority of prasugrel 3.75 mg to clopidogrel 75 mg for prevention of primary events (ischemic stroke, myocardial infarction, and death from other vascular causes) in Japanese patients with non-cardioembolic stroke are presented. The PRASTRO-I study will contribute to defining the efficacy and safety of prasugrel 3.75 mg in Japanese patients with non-cardioembolic stroke.
Health and safety
All mandatory laboratory health and safety procedures have been complied with in the course of conducting the work reported in this paper.
Declaration of interest
K Abe is an employee of Daiichi Sankyo Co, Ltd. T. Nagao has received personal fees from Daiichi Sankyo. K. Kitagawa has received grants and personal fees from Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Astellas Pharma Inc., Kyowa Hakko Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Byer Inc., and Sanofi K.K.; personal fees from Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd., Shionogi Inc., Pfizer Inc., and Bristol-Myers Squibb Co.; and grants from AstraZeneca K. K. and Eisai Co., Ltd. T. Kitazono has received grants and personal fees from Daiichi Sankyo Co., Ltd., and Bayer Yakuhin, Ltd., as well as grants from Sanofi K.K. H. Yamagami has received personal fees from Bayer, Daiichi Sankyo, Boehringer Ingelheim, and Stryker Japan K. K., as well as grants and personal fees from Bristol-Myers Squibb Co. S. Uchiyama has received personal fees from Daiichi Sankyo Co., Ltd., Bayer, Boehringer Ingelheim, Sanofi K. K., Takeda Pharmaceutical Co., Ltd., AstraZeneka K. K., Bristol Myers-Squibb Co., and Pfizer Inc. M. Matsumoto has received grants and personal fees from Sanofi K. K., Bayer Yakuhin, Ltd., Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., and Bristol-Myers Squibb Co., as well as grants from Mochida Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Nihon Medi-Physics Co., Ltd., MSD K. K., Pfizer Japan Inc., grants from Shionogi & Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Eisai Co., Ltd.. K. Minematsu has received personal fees from Bayer Healthcare, personal fees from Otsuka Pharmaceuticals, personal fees from Boehringer Ingelheim, AstraZeneca, Pfizer Japan Inc., Mitsubishi Tanabe Pharma Corporation, Stryker Japan K. K., Kowa Co., Ltd., Nihon Medi-Physics Co., Bristol-Myers Squibb Co., Sawai Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Medico’s Hirata, Kyowa Hakko Kirin Co. Ltd., Sanofi K. K., Merck Sharp Dohme K. K., Eisai Co. Ltd., Towa Pharmaceutical Co., Ltd., and Nippon Chemiphar. M. Nishikawa has received grants and personal fees from Daiichi Sanyko Co. Ltd., and personal fees from Sanofi K. K. S. Nanto has received personal fees from Daiichi Sanyko Co. Ltd. K. Abe has received personal fees from Daiichi Sanyko Co. Ltd. Y. Ikeda has received grants from Daiichi Sanyko Co. Ltd. A. Ogawa has received personal fees from Daiichi Sanyko Co. Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Writing assistance was utilized in the production of this manuscript and funded by Daiichi Sankyo Co. Ltd. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Suppl_ieop_a_1444029_sm0220.docx
Download MS Word (17.9 KB)Acknowledgments
The authors deeply appreciate the contributions of all the investigators, other clinical/research staff, and the patients and their families involved in the present trial. The authors also thank James Graham, PhD, of Edanz Medical Writing for their medical writing assistance.
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy—Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308:81–106.
- Antiplatelet Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86.
- Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–341.
- Kernan WH, Ovbiagele B, Black HR, et al. AHA/ASA guideline: guidelines for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2014;45:2160–2236.
- Ogawa A, III-2. The Japan Stroke Society. Committee on Guidelines for the Management of Stroke. Japanese guidelines for the management of stroke 2015. Tokyo: Kyowa Kikaku; 2015. p. 101–112.
- Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81–106.
- Fiolaki A, Katsanos AH, Kyritsis AP, et al. High on treatment platelet reactivity to aspirin and clopidogrel in ischemic stroke: a systematic review and meta-analysis. J Neurol Sci. 2017;376:112–116.
- Yi X, Lin J, Zhou Q, et al. Clopidogrel resistance increases rate of recurrent stroke and other vascular events in Chinese population. J Stroke Cerebrovasc Dis. 2016;25(5):1222–1228.
- Gengo FM, Rainka M, Robson M, et al. Prevalence of platelet nonresponsiveness to aspirin in patients treated for secondary stroke prophylaxis and in patients with recurrent ischemic events. J Clin Pharmacol. 2008;48(3):335–343.
- Krasopoulos G, Brister SJ, Beattie WS, et al. Aspirin “resistance” and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336(7637):195–198.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54(8):2430–2435.
- Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5(12):2429–2436.
- Kim KA, Park PW, Hong SJ, et al. The effect of CYP2C19 polymorphism on the pharmacokinetics and pharmacodynamics of clopidogrel: a possible mechanism for clopidogrel resistance. Clin Pharmacol Ther. 2008;84(2):236–242.
- Nakamura K, Goto F, Ray WA, et al. Interethnic differences in genetic polymorphism of debrisoquin and mephenytoin hydroxylation between Japanese and Caucasian populations. Clin Pharmacol Ther. 1985;38(4):402–408.
- Myrand SP, Sekiguchi K, Man MZ, et al. Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther. 2008;84(3):347–361.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in myocardial infarction 38. Circulation. 2008;118(16):1626–1636.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560.
- Stroke Guideline Committee of the Japan Stroke Society. Japanese guidelines for the management of stroke. Tokyo: Kyowa Kikaku; 2015.
- Adams HP Jr., Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41.
- Jakubowski JA, Bourguet N, Boulay-Moine D, et al. Comparison of a new ELISA assay with the flow cytometric assay for platelet vasodilator-associated stimulated phosphoprotein (VASP) phosphorylation in whole blood to assess P2Y(12) inhibition. Thromb Haemost. 2012;107(2):388–395.
- Shinohara Y, Nishimaru K, Sawada T, et al. Sarpogrelate-Aspirin Comparative Clinical Study for Efficacy and Safety in Secondary Prevention of Cerebral Infarction (S-ACCESS): A randomized, double-blind, aspirin-controlled trial. Stroke. 2008;39(6):1827–1833.
- Fukuuchi Y, Tohgi H, Okudera T, et al. A randomized, double-blind study comparing the safety and efficacy of clopidogrel versus ticlopidine in Japanese patients with noncardioembolic cerebral infarction. Cerebrovasc Dis. 2008;25(1–2):40–49.
- Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78(7):1684–1692.
- Isshiki T, Kimura T, Ogawa H, et al. Prasugrel, a third-generation P2Y12 receptor antagonist, in patients with coronary artery disease undergoing elective percutaneous coronary intervention. Circ J. 2014;78(12):2926–2934.
- Umemura K, Ikeda Y, Kondo K. Pharmacokinetics and pharmacodynamics of prasugrel in healthy Japanese subjects. Drug Metab Pharmacokinet. 2016;31(4):285–291.