1. When and how to achieve urgent weight loss
The need for urgent weight loss can be defined as an imperative requirement of achieving a rapid and significant body weight reduction to get a prompt clinical improvement. Urgent body weight reduction may be required when obesity is associated with severe complications that can be attenuated by rapid and significant body weight reduction. This is the case for respiratory complications such as sleep apnea and hypoventilation syndrome, uncontrolled hypertension, hyperglycemia or dyslipidemia resistant to conventional therapy, incapacitating joint disease, presurgical weight reduction to diminish operative risks, including preparation for bariatric surgery, and treatment of severe weight regain. The question posed in the title refers to severe obesity that, as defined by a BMI> 40 kg/m2, has not been widely tested in studies involving pharmacological obesity therapy. In those cases attention should be especially paid to obesity-related comorbidities as well as to the impact of weight excess on quality of life, which may also be severely affected in patients with lower BMI values.
Significant caloric restriction represents a key factor to achieve a negative energy balance and hence, successful weight loss. In most cases the prescription of a very low-calorie diet (VLCD) is an effective option to achieve a rapid improvement in sleep apnea, hypertension or type 2 diabetes control as well as a substantial preoperative weight loss to ensure an affordable operative risk [Citation1]. In all these situations a 10% weight loss is usually followed by a significant clinical improvement. VLCD treatment for 6 weeks is associated with rapid weight loss, significant subcutaneous and visceral adipose tissue reduction as well as an improvement in liver fat content and insulin sensitivity [Citation2]. As a consequence, glucose values improve as well as other complications such as hyperchylomicronemia, sleep apnea, hypertension or pulmonary insufficiency. In a presurgical scenario, a 4–13% BMI reduction can be achieved by VLCD treatment over 2–12 weeks [Citation3], leading to a reduction of preoperative surgical risks. In general, VLCDs induce higher weight loss than low-calorie diets (LCDs) over three months of treatment [Citation4], reaching up to 15–25% weight reduction [Citation5]. However, therapy with a VLCD may be associated with some adverse effects such as alopecia, cold intolerance, constipation, electrolyte disorders, hypotension, or gallstone formation [Citation5]. Moreover, VLCD use is contraindicated in the presence of cardiac arrhythmias, acute heart failure, psychiatric disorders, type 1 diabetes, liver and renal failure [Citation1]. Although classically VLCDs are contraindicated in elderly patients, a recently published study shows that older obese adults treated with Optifast, a VLCD program, over three months experienced 11% weight loss with only 5% fat-free mass reduction [Citation6]. Moreover, the adherence to a VLCD may be difficult to maintain, attrition reaching up to 22% [Citation5]. Another concern regarding VLCDs is weight regain, which seems to be related to fat-free mass loss [Citation7]. A complementary option to facilitate caloric restriction is represented by the use of endoscopic devices such as intragastric balloons (IGB) or duodenum-jejunal liners [Citation3]. These devices are effective in the short-term to induce rapid weight loss but may also be associated with adverse effects that can lead to early removal, which in many cases is followed by significant weight regain.
2. New antiobesity medications
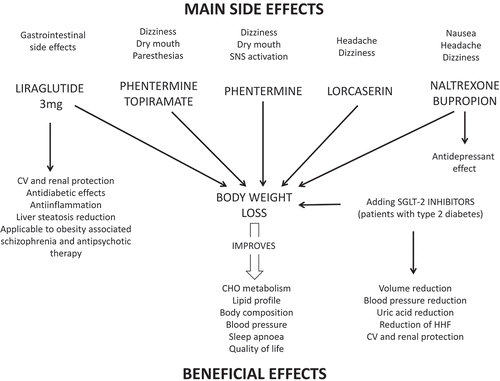
In the last years, new antiobesity medications (AOMs) have been marketed for obesity therapy. The combinations of phentermine-topiramate and naltrexone-bupropion, the GLP-1 receptor agonist (GLP-1 RA) liraglutide and lorcaserin, a serotonin receptor-2C agonist, have been approved in the US for obesity treatment, whereas only the naltrexone-bupropion combination and liraglutide have been authorized in the EU [Citation8]. Recently, semaglutide, a powerful GLP-1 RA, has also been launched in different countries for the treatment of type 2 diabetes. All these drugs join orlistat, a well-known intestinal fat absorption blocker, and phentermine, a sympathomimetic drug, in the pharmacological armamentarium available for obesity management [Citation8].
In association with LCDs, physical activity and behavior modification, the administration of AOMs is associated with 5–14% weight loss, which is accompanied by a simultaneous improvement in body composition, carbohydrate metabolism, lipid profile, blood pressure and sleep apnea [Citation8,Citation9]. At present, semaglutide exhibits more effectiveness in losing body weight when compared to liraglutide 3 mg, reaching 13.4% of mean weight reduction after 0.4 mg subcutaneous daily administration for one year, which translates into a 65%, 41% and 27% of patients reaching more than 10%, 15% and 20% weight loss, respectively [Citation9]. Although other AOMs have been studied in different patients, including some of them with impaired glucose tolerance or type 2 diabetes, 10% body weight reduction was observed in 48%, 42%, 37% and 23% for phentermine-topiramate, naltrexone-bupropion, liraglutide 3 mg and lorcaserin, respectively [Citation10–Citation13]. Also, the weight loss effect of semaglutide has shown to be superior to that seen following dulaglutide administration, another GLP-1 RA, when given to patients with type 2 diabetes [Citation14]. All these effects exerted by AOMs are obtained by association with standard caloric restriction, which along with regular physical activity and behavior modification are essential to achieve a significant weight loss.
Besides the differences in weight loss potency observed between different AOMs, the characteristics of their mechanisms of action are responsible for distinct effects that may be important to select a particular drug for an individual patient (). For example, GLP-1 RAs exhibit a glucose-dependent stimulatory effect on insulin secretion that may contribute to improve glucose control in patients with type 2 diabetes. Contraindications also are a key factor for selecting patients for AOMs therapy. Thus, phentermine, phentermine-topiramate and naltrexone-bupropion are contraindicated in uncontrolled hypertension and in patients treated with monoamine oxidase inhibitors, making those AOMs inadequate for use in those situations. Other diseases, such as bipolar disorder or epilepsy also represent formal contraindications for naltrexone-bupropion administration [Citation8].
3. Advantages and disadvantages of AOMs for urgent weight loss
Most studies have tested the effects of AOMs administration in class 1 and 2 obesity when associated with standard LCDs. However, combining AOMs with severe caloric restriction can induce a significant and rapid weight loss over 12 weeks, as is the case for phentermine administration that, in association with a VLCD, leads to 7.8 % weight loss [Citation15]. Nevertheless, there are no solid evidences in favor of a potentiating effect for the new AOMs on VLCD-induced weight loss. Orlistat will not be effective in that setting due to the low amount of fat of VLCDs.
Provided that, except orlistat, AOMs work by modulating appetite and satiety leading to a reduction of food intake, these drugs contribute to increase adherence to caloric restriction preventing attrition and weight regain. In fact, liraglutide 3 mg has been effective to induce weight loss following previous 6% weight reduction induced by treatment with a VLCD [Citation16], indicating that this AOM, in contrast with placebo, is able to prolong weight loss after intense caloric deprivation.
On the other hand, fat-free mass loss represents an adverse effect of severe caloric restriction that may be pathophysiologically linked to ulterior weight regain [Citation7]. Treatment with AOMs leads to beneficial changes in body composition with a clear dominance in the reduction of fat mass over fat-free mass loss. In a recent study, liraglutide 3 mg associated with intensive lifestyle modification (ILM) achieved a significant 24% BMI reduction after one year of therapy, which doubled that seen after ILM alone. Although the weight loss induced by liraglutide was inferior to that seen following sleeve gastrectomy, lean body mass reduction was less pronounced after liraglutide administration than following bariatric surgery [Citation17].
In cases of VLCD contraindication the administration of AOMs on top of a LCD can reach weight losses up to 13%, which may help to control obesity-related comorbidities, including diabetes, sleep apnea and high blood pressure. In that situation, 5–10% weight reduction can be usually seen after 8 weeks of therapy despite that in most cases patients are submitted transiently to lower drug exposure due to the progressive dose escalation needed when starting AOM therapy before achieving the full therapeutic dose [Citation9–Citation13]. These results suggest that AOMs use may be beneficial for preoperative preparation.
Weight regain of 15% referred to postoperative weight nadir may affect up to 50% of patients 5 years after gastric by-pass, and can be associated with impairment of obesity comorbidities and quality of life [Citation18]. Recently published data show that adjuvant AOMs are able to stop weight regain reaching an additional 5% weight loss in 37–54% of patients, more than 10% body weight reduction in 19–34% and 15% weight loss in up to 23% of operated patients [Citation19,Citation20]. Therefore, AOMs may represent a therapeutic alternative to revisional surgery for weight regain after bariatric surgery. Nevertheless, more studies are needed to establish the best moment to start AOMs administration in post-bariatric weight regain.
Patients with severe psychiatric diseases frequently develop obesity, hyperglycemia, metabolic syndrome and high cardiometabolic risk making weight loss a clear priority in those cases. Compatibility of AOMs and antipsychotic drugs represents an issue in that particular group of patients, especially in those who do not fulfill criteria for bariatric surgery programs. In this context, liraglutide administration at a daily dose of 1.8 mg for 16 weeks has been reported to be effective and safe in patients with schizophrenia and obesity treated with clozapine or olanzapine leading to significant body weight reduction as well as improvement in glucose tolerance, systolic blood pressure and plasma LDL-cholesterol concentrations [Citation21].
Some AOMs, such as liraglutide, semaglutide and the SGLT-2 inhibitors empagliflozin and canagliflozin, have demonstrated to have beneficial effects on cardiovascular and renal function in patients with type 2 diabetes [Citation22–Citation25], creating good expectations for patients with obesity and normal glucose tolerance, although no specific studies have been carried out yet in that population.
The different mechanisms of action of AOMs may explain why some patients respond to one AOM but not to another. In the same way, one patient may have alternative pharmacological therapeutic options in case of developing side effects with a particular AOM.
One of the main disadvantages of AOM therapy is related to the inter-individual heterogeneity of weight loss responses. On the other hand, AOM withdrawal is followed by weight regain. Therefore, when urgent weight loss is the main goal, its administration should be maintained as long as weight loss reduction or maintenance are needed.
Side effects such as gastrointestinal intolerance in the case of GLP-1 RAs, and adrenergic activation, dry mouth, constipation, insomnia, nausea or headache may appear when using phentermine, phentermine-topiramate or naltrexone-bupropion, complicating drug therapy adherence. Moreover, topiramate and bupropion may increase the risk of suicidal thoughts and the association phentermine-topiramate may induce cognitive dysfunction. On the other hand, rapid weight loss increases the risk of gallbladder disease, which may be potentiated by the use of GLP-1 RAs [Citation8].
The elevated price of these drugs represents another drawback since they are not reimbursed by national health systems requiring a significant economic effort by treated patients.
4. Conclusions
The new AOMs offer novel perspectives in obesity management. When urgent weight loss is needed, its role is related to an increase of adherence to caloric restriction leading to rapid and significant body weight reduction. Moreover, AOMs may provide additional weight loss-independent specific benefits to control obesity-related comorbidities. To achieve a rapid and safe weight loss AOMs need to be integrated in a holistic therapeutic program including caloric restriction, regular physical activity and behavior modification. Although more studies are needed to test its value in severe obesity and in association with severe caloric restriction, rapid and effective weight loss can also be achieved by its use with less restrictive hypocaloric diets. The spectrum and severity of obesity comorbidities will be crucial for individualization and success of AOMs treatment when urgent weight loss is indicated in a particular patient.
5. Expert opinion
When urgent weight loss is the objective, a rapid, patient-adapted, effective and safe body weight reduction is needed to improve the patient clinical condition. Achieving a significant negative energy balance is essential to get enough body weight reduction in a short period of time.
Depending on the situation and when no contraindication is present, VLCD treatment with or without the use of endoscopic devices can be recommended to reduce caloric intake, facilitate intensive weight loss and control obesity-related comorbidities. However, adherence to severe caloric deprivation can be difficult to maintain. On the other hand, when VLCD use is contraindicated, as is the case of heart failure or severe psychiatric disease, treatment goals may be accomplished with standard caloric restriction in the context of an integrated obesity therapeutic program.
Adherence to hypocaloric diets represents a significant problem that may be more difficult to overcome when intensive caloric deprivation is prescribed. Independently that severe obesity will need long-term treatment for sustained weight reduction, it is well established that 5–10% weight loss is associated with significant improvement in obesity burden.
New marketed AOMs act by modulating appetite and satiety, leading to food intake reduction and significant weight loss when compared to placebo administration. Previously reported randomized clinical trials reveal that these drugs are effective and safe to promote weight loss in different clinical conditions, and to improve diverse comorbidities commonly found in severe obesity, such as type 2 diabetes, hypertension, dyslipidemia and sleep apnea. Although some trials have included patients with severe obesity, the majority of studied individuals had a BMI less than 40 kg/m2. The performance of AOMs may not be significantly different in patients with high BMI values when compared with subjects with lower obesity degrees, as has been shown by Le Roux et al [Citation26]. Nevertheless, more specific studies in patients with severe obesity will help to clarify this point.
Provided that AOMs promote weight loss and improve obesity-related comorbidities, and considering that body weight effects are evident from the first weeks of AOMs administration, its use should be contemplated as beneficial for urgent weight loss. Moreover, some AOMs offer important added value to weight loss. Thus, the combination naltrexone-bupropion displays an antidepressant action, whereas GLP-1 RAs such as liraglutide 3 mg have some weight-independent advantages on carbohydrate metabolism, blood pressure and lipid profile [Citation27]. These metabolic weight-independent effects place GLP-1 RAs in a preferential position to be used in patients with type 2 diabetes, dyslipidemia or hypertension for urgent weight loss.
In favor of GLP-1 RAs use, liraglutide has also shown to be effective and safe in patients with severe psychiatric diseases treated with antipsychotic therapy, suggesting that there are no compatibility issues of GLP-1 RAs with these central acting psychotropic drugs, which may also be taken by patients with severe obesity.
Side effects have to be taken into account, since they can limit AOMs administration, especially during the beginning of the treatment period. In the context of urgent weight loss, a rapid escalation of AOMs dose is not recommended since may result in intolerance and early withdrawal of AOM treatment.
On the other hand, contraindications for the use of AOMs should also be considered. Uncontrolled hypertension represents a contraindication for the use of phentermine and naltrexone-bupropion combination. In those cases, GLP-1 RAs are a preferred option. In contrast, on the basis of experimental studies, patients with gastroparesia, pancreatitis or medullary thyroid carcinoma should not be treated with GLP-1 RAs.
As in other chronic diseases, such as hypertension or type 2 diabetes, combinations of drugs with endoscopic devices or between medications displaying different mechanisms of action widen the spectrum of therapeutic effects contributing to potentiate weight loss and comorbidities control. This is the case of the association between GLP-1 RAs and SGLT-2 inhibitors, which has shown to increase weight loss, also improving glucose and blood pressure control when compared to any of the drugs in monotherapy [Citation28]. This strategy may be useful to increase the probability to achieve rapid weight loss in patients with type 2 diabetes and hypertension.
Finally, up to 15–20% of patients are not responders to liraglutide 3 mg or naltrexone-bupropion combination [Citation29], showing a weight loss lower than 5% following one-year therapy. Up to now, the only way to predict this behavior is to assess early weight loss after 16 weeks of therapy, which may be a too long period of time when urgent weight loss is planned. We do not have yet any other reliable predictive markers of long-term weight loss success and this aspect represents a limitation to ensure AOM performance when the goal is rapid body weight reduction.
Both, advantages, side effects and contraindications of AOMs, along with patients clinical characteristics, form part of the individualization of obesity management, which should also apply to urgent weight loss programs in order to reach maximal efficacy and safety.
According to these data and answering the question posed in the title, all data point to the fact that individualized AOM therapy is useful in most patients with severe obesity requiring urgent weight loss. Nevertheless, AOM administration by its own may not be enough to reach the objectives expected when rapid weight loss is needed in patients with complicated obesity. The achievement of an effective caloric restriction in the context of an integrated obesity treatment program including physical activity, conductive-cognitive treatment and therapeutic education is essential for AOM treatment to be maximally effective. On the other hand, the heterogeneity of short- and long-term weight loss responses does not guarantee their efficacy in all cases. Moreover, a specific treatment of obesity comorbidities will also be required in most situations to achieve an adequate clinical control. New progress in the near future should contemplate the individualization of obesity therapy referred to prediction of efficacy and safety of AOMs for weight loss and control of comorbidities. We hope that precision medicine may contribute to fulfill these needs as soon as possible [Citation30].
Declaration of interest
J Salvador has participated on advisory boards for Novo Nordisk and Eli Lilly and Company while G Frühbeck has received lecturing fees from Merck, Sharp and Dohme. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee is an employee of Sierra Scientific Solutions while another has consulted and received research funding from Novo Nordisk, Eisai, Janssen Pharmaceuticals, Allurion, Zafgen and Gelesis. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) is an initiative of the ISCIII, Spain.
Additional information
Funding
References
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care. 1991;14:802–23.
- Wahlroos S, Phillips ML, Lewis MC, et al. Rapid significant weight loss and regional lipid deposition: implications for insulin sensitivity. Obes Res Clin Pract. 2007;1:7–16. PubMed PMID: 24351427.
- van Wissen J, Bakker N, Doodeman HJ, et al. Preoperative methods to reduce liver volume in bariatric surgery: a systematic review. Obes Surg. 2016;26:251–256. PubMed PMID: 26123526.
- Hemmingsson E, Johansson K, Eriksson J, et al. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr. 2012;96:953–961. PubMed PMID: 22990030.
- Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring). 2006;14:1283–1293. PubMed PMID: 16988070.
- Haywood CJ, Prendergast LA, Purcell K, et al. Very low calorie diets for weight loss in obese older adults-a randomized trial. J Gerontol A Biol Sci Med Sci. 2017;73:59–65. PubMed PMID: 28329121.
- Vink RG, Roumans NJ, Arkenbosch LA, et al. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity (Silver Spring). 2016;24:321–327. PubMed PMID: 26813524.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. PubMed PMID: 25590212.
- O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. PubMed PMID: 30122305.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. PubMed PMID: 21481449.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110–120. PubMed PMID: 20559296.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. SCALE obesity and prediabetes NN8022–1839 study group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. PubMed PMID: 26132939.
- Fidler MC, Sanchez M, Raether B, et al. BLOSSOM clinical trial group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067–3077. PubMed PMID: 21795446.
- Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275–286. PubMed PMID: 29397376.
- Li Z, Hong K, Yip I, et al. Body weight loss with phentermine alone versus phentermine and fenfluramine with very-low-calorie diet in an outpatient obesity management program: a retrospective study. Curr Ther Res Clin Exp. 2003;64:447–460. PubMed PMID: 24944395.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond). 2015;39:187. PubMed PMID: 25311036.
- Capristo E, Panunzi S, De Gaetano A, et al. Intensive lifestyle modifications with or without liraglutide 3 mg vs. sleeve gastrectomy: a three-arm non-randomised, controlled, pilot study. Diabetes Metab. 2018;44:235–242. PMID:29398254.
- King WC, Hinerman AS, Belle SH, et al. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320:1560–1569. PubMed PMID: 30326125
- Hanipah ZN, Nasr EC, Bucak E, et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Rel Dis.2018;14:93–98.
- Toth AT, Gomez G, Shukla AP, et al. Weight loss medications in young adults after bariatric surgery for weight regain or inadequate weight loss: a multi-center study. Children (Basel). 2018;5:116. PubMed PMID: 30158481.
- Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder. A radomized clinical trial. JAMA Psychiatry. 2017;74:719–728. PubMed PMID:28601891.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. PubMed PMID: 27295427.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. PubMed PMID: 27633186.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. PubMed PMID: 26378978.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. PubMed PMID: 28605608.
- le Roux C, Aroda V, Hemmingsson J, et al. Comparison of efficacy and safety of liraglutide 3.0 mg in individuals with BMI above and below 35 kg/m2: a post-hoc analysis. Obes Facts. 2017;10:531–544. PubMed PMID: 29145215.
- Bays H, Pi-Sunyer X, Hemmingsson JU, et al. Liraglutide 3.0 mg for weight management: weight-loss dependent and independent effects. Curr Med Res Opin. 2017;33:225–229. PubMed PMID: 27817208.
- Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. PubMed PMID:29483060.
- Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278–2288. PubMed PMID: 27804269.
- Frühbeck G, Kiortsis DN, Catalán V. Precision medicine: diagnosis and management of obesity. Lancet Diabetes Endocrinol. 2018;6:164–166. PubMed PMID: 28919063.