ABSTRACT
Background: Information on prescriptions of oral analgesics for the treatment of pain is beneficial. However, there have been few reports on the prescription status of oral analgesics from a nation-wide, large-scale prescription database in Japan.
Research design and methods: The authors analyzed the prescription data of 2,042,302 patients prescribed oral analgesics in 2017. The numbers/proportions of patients prescribed oral analgesics, adherence with approved doses, co-prescription patterns, dose changes, drug adherence, and treatment-discontinuation rates were evaluated.
Results: Loxoprofen was prescribed to 32.5% of the patients, followed by celecoxib, prescribed to 16.0% of patients. Acetaminophen and pregabalin were prescribed to 10.5% and 9.4% of patients, respectively. Many analgesics were prescribed at lower doses than the approved doses. The most frequently used concomitant medication was pregabalin. For duloxetine and pregabalin, high proportions of patients were prescribed these drugs for > 90 days.
Conclusions: Loxoprofen was the most prescribed of the non-steroidal anti-inflammatory drugs in Japan. The information obtained provides an overview of prescribed oral analgesics in Japan and could be useful for potential research into prescribed oral analgesics in the future.
1. Introduction
In Japan, non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, opioids, pregabalin, and duloxetine are commonly used to treat pain associated with various acute and chronic diseases [Citation1]. In Japan, acetaminophen has been prescribed less commonly than NSAIDs because of its weak analgesic effects associated with the lower prescribed doses than those used in other countries [Citation2]. In 2011, a higher adult dose of acetaminophen (up to the same maximum daily dose of 4,000 mg as that used overseas) was approved in Japan, thereby allowing more effective pain relief, and an increase in the number of patients who were prescribed this medication was expected [Citation3]. To reduce adverse drug reactions (ADRs) such as cardiac and gastrointestinal disorders of conventional NSAIDs, prodrugs of NSAIDs such as loxoprofen, which have a short elimination half-life and no direct effects on the gastrointestinal tract, and cyclooxygenase 2 (COX-2) inhibitors such as celecoxib were developed [Citation4,Citation5]. Both NSAIDs and COX-2 selective NSAIDs are now widely used in daily practice in Japan. In addition, pregabalin and duloxetine, which have mechanisms of action different from those of conventional oral analgesics, are frequently used for neuropathic pain and fibromyalgia that are inadequately treated by other therapies [Citation6,Citation7]. Information on recent prescriptions of these oral analgesics is not only beneficial for clinicians who treat pain in daily practice, but also important from a viewpoint of medical economics because of the large number of prescriptions issued. Although several studies on prescription practices regarding oral analgesics have been conducted overseas [Citation8–Citation10], the prescription status of oral analgesics based on a recent large-scale prescription database in Japan has not been reported. Therefore, it is important to clarify recent prescription information including the number of patients who are prescribed these medications, adherence with the dosage and administration defined in Japanese package inserts in daily practice, co-prescription patterns, dose changes, drug adherence, and treatment discontinuation.
The objective of this study was to provide an overview of the recent prescription status of patients who were newly prescribed oral analgesics for pain in real-world clinical settings, using a large-scale prescription database in Japan.
2. Patients and methods
2.1. Patients
Patients who were newly prescribed oral analgesics from January 1 to 31 December 2017 were targeted, and those who had been prescribed any oral analgesic within 6 months before the first prescription date were excluded.
2.2. Ethics
This study (clinical trial registration number: UMIN000032810) was conducted in adherence with the Declaration of Helsinki [Citation11] and the ‘Act on the Protection of Personal Information’ [Citation12]. We also referred to the ‘Guideline on implementation of pharmacoepidemiology studies in safety assessment of pharmaceuticals using database of medical information’ [Citation13]. This was a retrospective observational study using prescription data stored in the database of the Japan Medical Information Research Institute Inc. (JMIRI, Tokyo, Japan), and the data were not obtained newly for this study. Since the prescription data provided by JMIRI were classified as anonymously processed information or only statistical information was specified, patient consent was not required for this study. In addition, no ethics committee reviews were required because the data used were anonymous.
2.3. Prescription data used for the study
JMIRI stored data on approximately 30 million prescriptions (prescription information database version G8) annually collected from approximately 1,450 dispensing pharmacies nationwide, selected evenly across regions in Japan. Database studies on various types of drugs using these prescription data have been reported [Citation14–Citation18]. The information available for this study included the following prescription data: sex, age, clinical departments, pharmacy locations, and prescription information (e.g. drug name, doses, and number of prescription days). The collected data contained no information that could identify individual patients because these data were irreversibly encrypted using a hash function at the dispensing pharmacies at the time of collection.
2.4. Study design
This was a retrospective observational study using a large-scale prescription database. The study design is shown in . The screening period was established to exclude patients prescribed any oral analgesic and/or oral liquid formulation of analgesic up to 6 months before the first prescription date. The period for the collection of prescription data for patients newly prescribed oral analgesics was January 1 to 31 December 2017. For any patient initially prescribed oral analgesics between October 1 and 31 December 2017, there was an additional follow-up period of at least 3 months for collecting prescription data. Therefore, the total data analysis period was between 1 July 2016 and 31 March 2018.
2.5. Endpoints
2.5.1. Numbers/proportions of patients prescribed
The numbers/proportions of patients prescribed each drug and the mean prescription days (total number of prescription days per patient divided by number of patients) were tabulated.
2.5.2. Median daily prescription doses
The total dose was calculated as the total amount of each drug prescribed during the study period. The median daily prescription dose (median, first quartile, and third quartile) for continued prescriptions was calculated with respect to each drug.
2.5.3. Co-prescription patterns
The co-prescription patterns of oral analgesics were classified by single drug (one drug) and any combination of 2 drugs and 3 or more drugs recorded on the same prescription form, and the numbers/proportions of patients prescribed these drugs as a first prescription, continued prescription, and pro re nata (PRN, as needed) prescription were calculated.
2.5.4. Distribution of initial doses and changes from the initial doses
The distribution of initial doses and the changes from the initial doses in continued prescriptions were indicated as the distribution of daily prescription doses and the change in dose at various time points (14, 30, 60, and 90 days after the first prescription date), respectively. The maintenance duration of the initial dose was defined as the period during which the initial dose was maintained or achieved without any dose change. The maintenance dose was defined as the daily prescribed dose maintained for the longest duration during the study period. The achieved dose was defined as the daily prescribed dose on the date of the final prescription during the study period.
2.5.5. Duration of continued prescription and medication possession ratio (MPR)
The duration of continued prescription was defined as the period between the first prescription date and the final prescription date (including any gap period. i.e. a period between prescription phases in which the prescription was regarded as continued. See Supplemental materials for detailed description of gap period). Continued prescription data were collected at various time points (8 to 14 days, 15 to 30 days, 31 to 60 days, 61 to 90 days, and > 90 days). MPR (< 80%, 80% to ≤ 110%, and > 110%) was calculated by dividing the total number of prescription days by the number of days during the study period.
2.5.6. Treatment-discontinuation rate
Treatment discontinuation for a continued prescription was defined as a case in which the next prescription could not be confirmed during any gap period after the date of the final prescription. The date of treatment discontinuation was defined as the date of the final prescription. Treatment-discontinuation rates with respect to duration (8 to 14 days, 15 to 30 days, 31 to 60 days, and 61 to 90 days) were calculated by dividing the number of patients with treatment discontinuation by the number of patients prescribed the drug.
2.6. Subgroup analyses
Subgroup analyses were performed to assess the effects of sex, age, and clinical departments on the endpoints. The subgroup analysis data are described in the Supplemental materials: subgroup analysis.
2.7. Statistical analysis
The numbers/proportions of patients prescribed drugs and the mean prescription days were tabulated with respect to each drug. The numbers/proportions of patients prescribed drugs were tabulated for categorical variables and descriptive statistics were calculated for continuous variables. In subgroup analyses of the endpoints, the number of patients prescribed each drug and the daily prescription doses were tabulated for each patient characteristic.
No statistical hypothesis test was performed.
3. Results
3.1. Patient composition
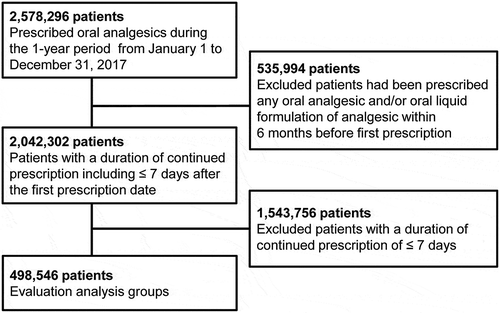
shows the composition of patients who were prescribed drugs. Oral analgesics were prescribed to 2,578,296 patients (2.07% of prescribed patients per total Japanese population [124,763,000 people]) during the one-year period from January 1 to 31 December 2017. Of these, 2,042,302 patients (1.64%, excluding those prescribed any oral analgesic and/or oral liquid analgesic formulation within 6 months before the first prescription date) were included in the patient groups prescribed analgesics during the study period, including ≤ 7 days after the first prescription date. Of these, 498,546 patients (0.40%, excluding those with a continued prescription duration of ≤ 7 days) were included in the evaluation analysis groups.
3.2. Patient characteristics
The patient characteristics are shown in . The proportion of female patients (56.4%) was higher than that of male patients (43.6%). The mean age was higher for female (59.1 years) than for male patients (56.8 years). Elderly patients (age ≥ 65 years) accounted for 43.5% of the patients and 23.3% were aged ≥ 75 years. In contrast, there were few pediatric patients aged ≤ 14 years (only 1.6%). Regarding clinical departments, the proportions of patients prescribed drugs for orthopedic surgery and general internal medicine were the highest (45.2% and 17.6%, respectively).
Table 1. Patient characteristics.
3.3. Endpoints
The number of patients prescribed the top 20 drugs during the continued prescription and PRN prescription periods, including ≤ 7 days after the first prescription date, is shown in Table S1 in the Supplemental materials. Drugs widely prescribed for the common cold (acetaminophen, acetaminophen combinations, Kakkon-to [a traditional Japanese herbal medicine], ibuprofen, and codeine) were included in the top 10 drugs prescribed in this study, and were prescribed for all continued prescription durations, including ≤ 7 days after the first prescription date (Table S1 in the Supplemental materials). Since common cold symptoms usually resolve in approximately 1 week [Citation19–Citation21], patients prescribed drugs for ≤ 7 days were excluded from the analysis, and those prescribed drugs for ≥ 8 days were tallied. Consequently, acetaminophen and acetaminophen combinations still remained in the top 10 drugs, but the number of patients prescribed acetaminophen and acetaminophen combinations markedly decreased when excluding those with prescriptions for ≤ 7 days (acetaminophen [including ≤ 7 days: 753,426 patients, ≥ 8 days: 52,350 patients] and acetaminophen combinations [228,858 patients and 20,269 patients, respectively]). Similarly, the numbers of patients prescribed Kakkon-to, ibuprofen, and codeine clearly decreased. In contrast, the numbers of patients prescribed pregabalin and duloxetine, which were not prescribed for the common cold, were almost the same in the data for ≤ 7 days and ≥ 8 days. Therefore, most patients prescribed drugs for the common cold could reasonably be excluded from this analysis.
3.3.1. Numbers/proportions of patients prescribed
shows the numbers/proportions of patients with continued prescriptions or PRN prescriptions and the mean prescription days. Loxoprofen was prescribed to the highest proportion of patients (32.5%), followed by celecoxib (16.0%), acetaminophen (10.5%), and pregabalin (9.4%); the total proportion of patients prescribed these drugs was approximately 70%. The proportions of patients who were prescribed loxoprofen, celecoxib, acetaminophen, and pregabalin as continued prescriptions were almost the same as those of the total patients prescribed oral analgesics. The mean prescription days were greater for patients prescribed duloxetine (58.2 days) and pregabalin (52.2 days) than for those prescribed other drugs. Regarding PRN prescriptions, the proportions of patients prescribed loxoprofen (44.0%) and acetaminophen (34.9%) were high, while those of patients prescribed celecoxib and pregabalin were small. There was a difference in the patient proportions of oral analgesics used for continued and PRN prescriptions.
Table 2. Number of patients prescribed drugs and mean prescription days for each drug.
3.3.2. Median daily prescription doses
shows the median daily prescription doses for continued prescriptions for each drug. Among the top 10 drugs, the median daily prescription doses of acetaminophen, acetaminophen combination, pregabalin, Shakuyaku-kanzo-to (a traditional Japanese herbal medicine), tramadol & acetaminophen combination, and duloxetine in patients were lower than the approved doses described in their corresponding package inserts.
Table 3. Median daily prescription doses for each drug.
3.3.3. Co-prescription patterns
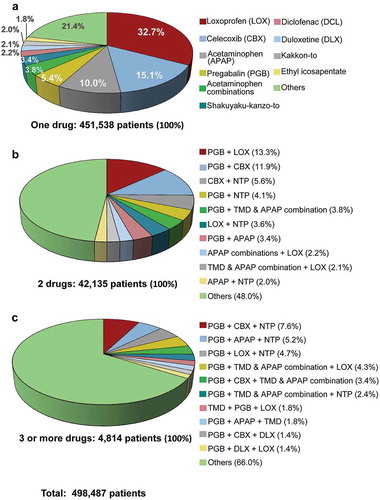
The proportion of patients prescribed one drug was 90.6% of the 498,487 patients with continued and PRN prescriptions. The remaining patients were prescribed combinations of 2 drugs (8.5%) and 3 or more drugs (1.0%). The most commonly prescribed one drug was loxoprofen (32.7% of 451,538 patients), followed by celecoxib, acetaminophen, and pregabalin (). The most common combinations of 2 drugs among the top 10 drugs were pregabalin + loxoprofen (13.3% of 42,135 patients) and pregabalin + celecoxib (11.9%); pregabalin was contained in all 3 drug combinations (each drug combination accounted for ≤ 7.6% of 4,814 patients).
3.3.4. Distribution of initial doses and changes from the initial doses
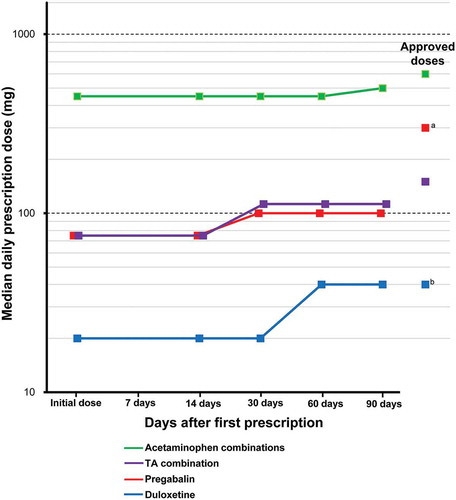
The changes from the initial doses during the study period for continued prescriptions of the drugs are shown in . The doses of acetaminophen combinations (doses as acetaminophen), tramadol & acetaminophen combination, pregabalin, and duloxetine increased during the study period. The median daily doses of acetaminophen combinations, pregabalin, and tramadol & acetaminophen combination during the study period were lower than their approved doses, whereas the daily doses of duloxetine at 60 and 90 days after first prescription were the same as its approved dose. The doses of other drugs did not increase during the study period.
Figure 4. Dose changes during the study period for the drugs.a The approved dose of pregabalin: the usual adult dosage is 150 mg/day of pregabalin as the initial dose, in 2 divided doses daily, administered orally. Then, the dose can be increased gradually up to 300 mg/day over at least 1 week.b The approved dose of duloxetine: for adults, after breakfast once a day, 40 mg (diabetic peripheral neuropathy) or 60 mg (chronic low back pain, osteoarthritis, and fibromyalgia) is administered. Administration starts at 20 mg daily and increases by 20 mg as a daily dose at intervals of 1 week or more.
TA combination: tramadol & acetaminophen combination.
3.3.5. Duration of continued prescription and MPR
shows the proportions of patients with a continued prescription period and MPR for each drug. The patient proportions for a continued prescription period exceeding 90 days after the first prescription date were the highest for duloxetine (36.0%), followed by pregabalin (29.2%), neurotropin (26.3%), and tramadol & acetaminophen combination (26.2%). Short continued prescription periods of 8 to 14 days were commonly observed for NSAIDs (loxoprofen, celecoxib, and diclofenac), acetaminophen, and acetaminophen combinations.
Table 4. Number of patients during continued periods and MPR for each drug.
The MPR for pregabalin and duloxetine (in the range of 80% to <110%) was 82.3% and 90.0% of patients, respectively, while that for acetaminophen combination was 45.2%.
3.3.6. Treatment-discontinuation rate
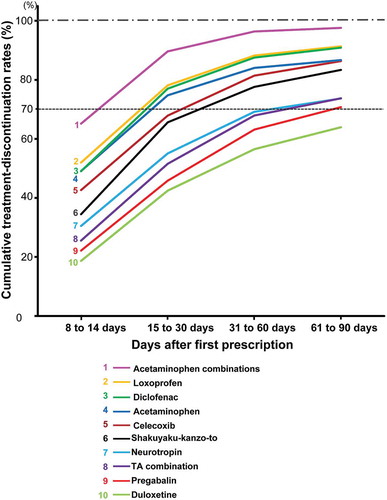
The cumulative treatment-discontinuation (CTD) rates at mean treatment-discontinuation periods for the top 10 drugs in the continued prescriptions were approximately 70%. To evaluate the period in which more patients discontinued treatment, the periods in which the CTD rates exceeded 70% were investigated. Loxoprofen, acetaminophen, acetaminophen combinations, and diclofenac exceeded 70% within 30 days, whereas duloxetine was the only drug with a < 70% CTD rate within 90 days (). Pregabalin, tramadol & acetaminophen combination, and neurotropin initially reached rates of > 70% of patients during the period from 61 to ≤ 90 days.
4. Discussion
This study was designed to investigate the recent prescription status of oral analgesics for pain in real-world clinical settings using a large-scale prescription database in Japan. The results of this study revealed the numbers of patients prescribed oral analgesics, adherence with approved doses, co-prescription patterns, dose changes, drug adherence, treatment-discontinuation rates, and differences in prescription practices by sex, age, and clinical departments.
In this study, to minimize the influence of drugs prescribed for the common cold on the data, patients prescribed drugs for ≤ 7 days were excluded from the analysis, and those prescribed drugs for ≥ 8 days were tallied. Consequently, acetaminophen and acetaminophen combinations still remained in the top 10 drugs, but the number of patients prescribed acetaminophen and acetaminophen combinations markedly decreased when excluding those with prescriptions for ≤ 7 days (acetaminophen [including ≤ 7 days: 753,426 patients, ≥ 8 days: 52,350 patients] and acetaminophen combinations [228,858 patients and 20,269 patients, respectively]). Similarly, the numbers of patients prescribed Kakkon-to, ibuprofen, and codeine clearly decreased. In contrast, the numbers of patients prescribed pregabalin and duloxetine, which were not prescribed for the common cold, were almost the same in the data for ≤ 7 days and ≥ 8 days. Therefore, most patients prescribed drugs for the common cold could reasonably be excluded from this analysis.
4.1. Numbers/proportions of patients prescribed
In this study, NSAIDs, acetaminophen/acetaminophen combinations, pregabalin, and duloxetine were in the top 10 drugs prescribed. Notably, NSAIDs and acetaminophen are also highly prescribed in the EU countries and the USA [Citation8,Citation9,Citation25,Citation26]. The high prescription rates of NSAIDs and acetaminophen obtained in our study were generally similar to those in these previously reported studies. However, loxoprofen was the most prescribed among NSAIDs in Japan, whereas the most frequently prescribed drugs were ibuprofen in the USA [Citation26], Germany and France [Citation8], and naproxen in the UK [Citation8].
Loxoprofen is an NSAID developed in Japan and is widely used in at least 28 countries worldwide [Citation27]. The high proportions of patients prescribed loxoprofen in this study could be explained by it being a prodrug with a short plasma half-life and having no direct effects on the gastrointestinal tract, thereby reducing gastrointestinal disorders. ADRs of loxoprofen were reported in 409 of 13,486 patients (3.03%), with low incidence rates of gastrointestinal ADRs (total of 2.25%) and cardiac ADRs (0.03% such as palpitation (2 patients), hypertension, and increased blood pressure (each 1 patient) [Citation28]. A COX-2-selective inhibitor, celecoxib, has also been reported to be associated with the development of gastrointestinal disorders, although less frequently [Citation29,Citation30]. The proportions of patients prescribed loxoprofen and celecoxib were 32.5% and 16.0%, respectively, but the mean prescription days were greater for celecoxib (34.6 days) than for loxoprofen (25.7 days). Although clinical study conducted overseas has shown that rofecoxib, a COX-2-selective inhibitor could increase the risk of serious and fatal cardiovascular disorders such as myocardial infarction and stroke [Citation31], a recent large-scale, long-term study in patients with chronic pain (PRECISION trial) showed that the cardiovascular risk associated with celecoxib (2.3%) does not exceed that for ibuprofen (2.7%) and naproxen (2.5%) [Citation32]. Therefore, loxoprofen and celecoxib can continue to be used frequently for pain relief. Acetaminophen reportedly causes gastrointestinal disorders less frequently than NSAIDs [Citation33]. The prescription dose of acetaminophen in Japan was lower than that overseas (maximum daily dose, 4,000 mg) until 2010, which resulted in a weak analgesic effect, and acetaminophen was, therefore, prescribed less frequently than NSAIDs in Japan [Citation3]. In 2011, an increased maximum daily dose of acetaminophen, which was equivalent to that prescribed overseas, was approved in Japan [Citation3], thereby allowing more potent analgesic effects to be achieved. Accordingly, the number of patients prescribed acetaminophen was expected to increase. However, the present study, which was conducted in 2017, shows that acetaminophen was still prescribed less frequently (10.5%) than loxoprofen (32.5%), and its median daily prescription dose (1,200 mg) was lower than that of the approved dose (4,000 mg), although the reasons were not clear.
Pregabalin is widely used to treat patients with postherpetic neuralgia, neuropathic pain associated with diabetic peripheral neuropathy, fibromyalgia, neuropathic pain associated with spinal cord injury, and epilepsy in the USA [Citation34]. In Japan, pregabalin is approved for neuropathic pain [Citation6]. A prescription database study of patients with pain in Japan demonstrated a marked increase in the number of patients prescribed pregabalin from its market release in 2010 until 2013 [Citation35]. In this previous study, the proportion of patients prescribed pregabalin was approximately 0.13% during the 6 months between July 2013 and December 2013 [Citation35]. Our study revealed that the proportion of patients prescribed pregabalin was 9.4% in 2017, which is a marked increase compared to that in the previous study conducted in 2013. Pregabalin was shown to be effective for neuropathic pain and fibromyalgia [Citation6], for which NSAIDs and acetaminophen were minimally effective, and gastrointestinal disorders were reported less frequently than with other analgesics. Therefore, pregabalin could be a clinically important oral analgesic. Duloxetine was approved as an antidepressant in Japan in 2010 and was additionally approved for neuropathic pain associated with diabetic peripheral neuropathy, fibromyalgia, chronic lumbago, and osteoarthritis [Citation7]. In this study, the number of patients prescribed duloxetine was the tenth highest among the drugs studied, but duloxetine had the highest number of mean prescription days, duration of continued prescription, proportion of patients with MPR, and the lowest treatment-discontinuation rate. Therefore, duloxetine is expected to be prescribed more frequently as its indications are further expanded.
The prescription rates of oral strong opioids, oxycodone and morphine, were lower than those of acetaminophen, NSAIDs, and Japanese herbal medicines (oxycodone ranked 32nd [1525 patients] and morphine 72nd [85 patients]), and the reason for the lower ranks of strong opioids was unclear. One possible reason was that the proportions of Japanese patients with severe pain from the limited diseases, such as cancer, prescribed strong opioids may be lower than those of most cancer and non-cancer patients prescribed NSAIDs, neuropathic pain drugs and Japanese herbal medicines.
4.2. Median daily prescription doses
The mean daily doses of pregabalin in this study were lower than the FDA and Japanese approved doses, but the reason for the lower doses of pregabalin used is unclear. One possible reason for the low doses could be the reported ADRs induced by pregabalin such as dizziness, somnolence, or loss of consciousness, leading to falls and fractures in elderly patients [Citation6] and therefore, Japanese medical practitioners may carefully prescribe pregabalin with lower doses than approved dose, paying attention to these side effects. In elderly patients, the daily prescription dose of pregabalin was lower than the approved dose (300 mg) in Japan (daily prescription dose in this study [15 to 64 years: 100 mg, ≥ 65 years: 75 mg], Table S2 in the Supplemental materials). These dose differences among the age groups are generally consistent with the results of a large-scale prescription database reported by Hiraoka et al. (males aged < 65 years: 110.6 mg, ≥ 65 years: 98.0 mg, females aged < 65 years: 101.9 mg, ≥ 65 years: 86.6 mg) [Citation35]. The median daily prescription doses of Shakuyaku-kanzo-to and tramadol & acetaminophen combination were also lower in elderly patients aged ≥ 65 years than in patients aged 15 to ≤ 64 years (the Supplemental materials: subgroup analysis Table S2). This lower dose might be attributable to the decrease in daily prescription dose in response to the caution in the package inserts of these drugs stating that, ‘The product should be administered to elderly patients at a reduced dose or with care’ [Citation36,Citation37].
4.3. Co-prescription patterns
In this study, pregabalin was co-prescribed with loxoprofen, celecoxib, neurotropin, tramadol & acetaminophen combination, and acetaminophen as the first prescription. The reasons for co-prescription of pregabalin are considered to be the additive and synergistic analgesic effects due to its mechanisms of action, which differ from those of other analgesics, and its ease of use in combination with other drugs because there is no induction/inhibition of hepatic cytochrome P450 [Citation6]. With respect to age, pregabalin was mainly co-prescribed with loxoprofen or celecoxib in patients aged ≥ 15 years. Regarding clinical departments, pregabalin + loxoprofen and pregabalin + celecoxib were co-prescribed in many clinical departments. Therefore, pregabalin may be co-prescribed with other drugs to treat neuropathic pain and fibromyalgia.
4.4. Distribution of initial doses and changes from the initial doses
The doses of pregabalin, acetaminophen combinations, tramadol & acetaminophen combination, and duloxetine increased during the study period to 90 days. The increase in the daily prescription doses of pregabalin and duloxetine may be attributable to the instructions on their package inserts: ‘After the administration of the initial dose of the drug, the dose should be gradually increased over at least 1 week’ [Citation6,Citation7]. Although the precise reasons for the increases in the doses of acetaminophen combinations and tramadol & acetaminophen combination are unclear, the drug doses, which should be adjusted according to patient symptoms, are suggested in their package inserts [Citation37,Citation38].
4.5. Duration of continued prescription and MPR
Pregabalin and duloxetine could be considered to be suitable for patients requiring long-term continued prescription due to their efficacy and low incidences of gastrointestinal diseases. For duloxetine and pregabalin, high proportions of patients were prescribed these drugs for > 90 days and their MPRs were also high (> 80% of patients in the range of 80% to ≤ 110%). Since pregabalin and duloxetine are indicated for neuropathic pain and fibromyalgia with severe pain, these patients are considered to have been compliant with continuation of treatment.
4.6. Treatment-discontinuation rate
CTD rates for loxoprofen, diclofenac, acetaminophen, and acetaminophen combinations (49.0% to 65.2%) were observed during the short period of 8 to 14 days after the first prescription date. In contrast, for pregabalin and duloxetine, their CTD rates were approximately 20% from 8 to 14 days and approximately 60% from 31 to 60 days. These results suggest that NSAIDs and acetaminophen are suitable for short-term treatment, and that pregabalin and duloxetine can be used for both short- and long-term treatments.
Regarding study limitations, since the diseases treated were not included in the database, prescriptions for the common cold and depression could not be completely excluded. The prescription data included information on drug names, prescription doses, prescription days, sex, age, clinical departments, and pharmacy locations, but they did not include other patient information (disease names, drug efficacy and safety) or information on drugs prescribed in hospitals. Therefore, the condition/progression/resolution of the treated diseases and the efficacy/safety of the drugs could not be analyzed. In addition, although the data of patients who were prescribed oral analgesics for ≤ 7 days after initial treatment were excluded in this study, the numbers of patients prescribed these drugs during this period were high, and were not considered to be negligible.
5. Conclusions
We investigated the prescription status of oral analgesics in Japan in real-world clinical settings using a large-scale database. This study, based on a recent large-scale prescription database, showed that loxoprofen, celecoxib, acetaminophen, and pregabalin were prescribed at high frequency to patients in Japan. Loxoprofen was the most prescribed among NSAIDs in Japan. The study revealed the recent status of prescriptions with respect to adherence with approved doses of oral analgesics, dose changes, co-prescription patterns, drug adherence, treatment-discontinuation rates, and differences in prescription practices by sex, age, and clinical departments. In particular, many oral analgesics were prescribed at lower than the approved doses described in their package inserts. The results suggest that these drugs had been carefully prescribed in accordance with their package inserts. Further study will be required to clarify why these drugs were prescribed at doses lower than those described in the package inserts and to overcome the above-mentioned study limitations.
These results are considered useful not only as an overview of prescribed oral analgesics in Japan, but also for potential research of prescribed oral analgesics in the future.
Author Contributions
T Ushida contributed to the study design and writing of the manuscript.
D Matsui, T Inoue, M Yokoyama, T Kurusu, and K Okuizumi contributed to the study design, conducted and performed the data collection and writing of the manuscript.
T Matsumoto and A Takita were involved in data analysis.
H Takatsuna and H Sakoda contributed to the writing of the manuscript.
All authors contributed to interpretation of data and reviewing the manuscript, and approved this manuscript for submission.
Declaration of interest
T Ushida has received consulting fees for the performance of the study from Daiichi Sankyo Co., Ltd. D Matsui, T Inoue, M Yokoyama, H Takatsuna, T Matsumoto, A Takita, H Sakoda and K Okuizumi are full-time employees of Daiichi Sankyo Co., Ltd. T Kurusu is a full-time employee of Japan Medical Information Research Institute, Inc. Daiichi Sankyo Co., Ltd. participated in study design, decision to publish, and preparation of the manuscript. The Japan Medical Information Research Institute, Inc. has participated in data collection and analysis, preparation of the manuscript, study design, and decision to publish. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee discloses that they have served as Consultant/Speaker and Researcher for Daiichi Sankyo USA, US World Meds, BDSI, Salix, Enalare, and Neumentum. They however have no relationship with this specific research. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (182.4 KB)Acknowledgments
Medical writing assistance was provided by Honyaku Center Inc. during the preparation of this manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Supplemental materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Committee on the Guidelines for Pain Treatment, the Japanese Society of Pain Clinicians. The guidelines for pain treatment (version 5). Tokyo: Shinko Trading Company Ltd.; 2016. Japanese.
- Kai K, Ikeda S, Muto M. The difference in analgesic use of acetaminophen between in Japan and other countries, and possible drug cost reduction caused by the acetaminophen prevalence in Japan. Jpn J Pharmacoepidemiol. 2013;17:75–86. Japanese.
- Nishimoto N, Tsujimoto T. A survey of pain treatment with acetaminophen- Questionnaire study for hospital pharmacists. Jpn J Pharm Health Care Sci. 2014;40:124–134. Japanese.
- Sakamoto C, Soen S. Efficacy and safety of the selective cyclooxygenase-2 inhibitor celecoxib in the treatment of rheumatoid arthritis and osteoarthritis in Japan. Digestion. 2011;83:108–123.
- Greig SL, Garnock-Jones KP. Loxoprofen: a review in pain and inflammation. Clin Drug Investig. 2016;36:771–781.
- Pfizer Japan Inc. Lyrica (pregabalin) OD Tablets 25mg, 75mg, 150mg, package insert. Japanese. [cited 2019 Feb 12]. Available from: http://www.info.pmda.go.jp/downfiles/ph/PDF/671450_1190017F1029_1_04.pdf
- Eli Lilly Japan K.K. Cymbalta (duloxetine) Capsules, package insert. Japanese. [cited 2019 May 24]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/340018_1179052M1022_2_21
- Jacob L, Kostev K. Prevalence of pain medication prescriptions in France, Germany, and the UK - a cross-sectional study including 4,270,142 patients. Postgrad Med. 2018;130:32–36.
- Wertli MM, Reich O, Signorell A, et al. Changes over time in prescription practices of pain medications in Switzerland between 2006 and 2013: an analysis of insurance claims. BMC Health Serv Res. 2017;17:167:[11p.].
- Shmagel A, Ngo L, Ensrud K, et al. Prescription medication use among community-based U.S. adults with chronic low back pain: a cross-sectional population based study. J Pain. 2018;19:1104–1112.
- World Medical Association (WMA). WMA declaration of Helsinki - ethical principles for medical research involving human subjects. [cited 2019 Feb 12]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Act on the protection of personal information (Amendment of Act No. 65 of 2015). Japanese. [cited 2019 Feb 12]. Available from: https://www.ppc.go.jp/files/pdf/151112_kaiseian.pdf
- Guideline on implementation of pharmacoepidemiology studies in safety assessment of pharmaceuticals using database of medical information (2014). Japanese. [cited 2019 Feb 12]. Available from: https://www.pmda.go.jp/files/000147250.pdf
- Sato D, Sato Y, Masuda S, et al. Effects of a sitagliptin safety alert on prescription behaviour for oral antihyperglycaemic drugs: a propensity score-matched cohort study of prescription receipt data in Japan. Drug Saf. 2013;36:605–615.
- Sato D, Sato Y, Masuda S, et al. Impact of the sitagliptin alert on prescription of oral antihyperglycemic drugs in Japan. Int J Clin Pharm. 2012;34:917–924.
- Takada M, Fujimoto M, Hosomi K. Association between benzodiazepine use and dementia: data mining of different medical databases. Int J Med Sci. 2016;13:825–834.
- Takada M, Fujimoto M, Yamazaki K, et al. Association of statin use with sleep disturbances: data mining of a spontaneous reporting database and a prescription database. Drug Saf. 2014;37:421–431.
- Tanaka I, Sato M, Sugihara T, et al. Adherence and persistence with once-daily teriparatide in Japan: a retrospective, prescription database, cohort study. J Osteoporos. 2013;654218:[8 p.].
- Institute for Quality and Efficiency in Health Care (IQWiG). Common colds: overview, informed health online [Internet]. Version: November 15, 2018. [cited 2019 Feb 12]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279543/
- Gwaltney JM Jr. The Common Cold. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practices of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. p. 651–656.
- Tomii K, Matsumura Y, Maeda K, et al. Minimal use of antibiotics for acute respiratory tract infections: validity and patient satisfaction. Intern Med. 2007;46:267–272.
- AYUMI Pharmaceutical Co. CALONAL (acetaminophen), package insert. Japanese. [cited 2019 Feb 12]. Available from: http://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/112429_1141001X1100_3_01
- NIPPON ZOKI. Neurotropin tab. 4 N.U., package insert. Japanese. [cited 2019 Feb 12]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530288_1149023F1036_1_05
- Novartis Pharma K.K. Voltaren (diclofenac sodium) Tablets 25mg, package insert. Japanese. [cited 2019 Feb 12]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/300242_1147002F1560_1_09
- Milani GP, Benini F, Dell’Era L, et al. Acute pain management: acetaminophen and ibuprofen are often under-dosed. Eur J Pediatr. 2017;176:979–982.
- Kane SP. The Top 200 of 2019. ClinCalc DrugStats Database, Version 19.1. ClinCalc [Internet]. Updated 2018 Nov 24 [cited 2019 Feb 26]. Available from: https://clincalc.com/DrugStats/Top200Drugs.aspx
- Daiichi Sankyo Co., Ltd. Loxonin (loxoprofen) Tablets, Fine Granules, pharmaceutical product interview form. Japanese. [cited 2019 Feb 12]. Available from: http://www.info.pmda.go.jp/go/interview/1/430574_1149019C1149_1_L10_1F.pdf
- Daiichi Sankyo Co., Ltd. Loxonin (loxoprofen) Tablets, Fine Granules, package insert. Japanese. [cited 2019 Feb 12]. Available from: http://database.japic.or.jp/pdf/newPINS/00057032.pdf
- Astellas Pharma Inc. Celecox (celecoxib) Tablets 100mg, 200mg, package insert. Japanese. [cited 2019 Feb 12]. Available from: http://www.info.pmda.go.jp/downfiles/ph/PDF/800126_1149037F1020_1_15.pdf
- Kawaguchi I, Kamae I, Soen S, et al. Cost-effectiveness analysis of celecoxib in the treatment of patients with chronic pain in Japan. J Health Care Med Commun. 2014;24:289–302.
- Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102.
- Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519–2529.
- Peura DA, Goldkind L. Balancing the gastrointestinal benefits and risks of nonselective NSAIDs. Arthritis Res Ther. 2005;7:S7–S13.
- Pfizer Japan Inc. LYRICA- pregabalin capsule PRESCRIBING INFORMATION. [cited 2019 Feb 12]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=561
- Hirakata M, Yoshida S, Tanaka-Mizuno S, et al. Pregabalin prescription for neuropathic pain and fibromyalgia: a descriptive study using administrative database in Japan. Pain Res Manag. 2018;2786151:[10 p.].
- TSUMURA & Co. TSUMURA Shakuyaku-kanzo-to Extract Granules for ethical use, package insert. Japanese. [cited 2019 Feb 12]. Available from: http://www.info.pmda.go.jp/downfiles/ph/PDF/460026_5200067D1049_1_15.pdf
- Daiichi Sankyo Espha Co., Ltd. Toaraset combination Tablets (DSEP), package insert. Japanese. [cited 2019 Feb 12]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/430773_1149117F1039_1_01
- Shionogi & Co., Ltd. PL combination (acetaminophen combinations) Granules, package insert. Japanese. [cited 2019 Jun 07]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/343018_1180107D1131_2_01