ABSTRACT
Background
Chronic pain is often difficult to treat, and many patients are not satisfied with analgesic treatment. The authors assessed patient satisfaction with oral analgesics in patients with chronic pain in Japan.
Research design and methods
This was an observational cross-sectional study conducted in dispensing pharmacies. A patient satisfaction questionnaire survey was conducted in 781 patients prescribed one nonsteroidal anti-inflammatory drug (NSAID) or neuropathic pain (NeP) drug for at least 90 consecutive days. The primary endpoint was patient satisfaction with analgesics. The secondary endpoints were pain relief, activity of daily living (ADL) improvement and doctor–patient communication.
Results
The proportions of patients who answered ‘satisfied if anything’ or better for patient satisfaction in the NSAID and NeP drug groups were 70.0% and 65.2%, respectively, whereas those of patients who answered ‘satisfied’ were 43.3% and 29.4%, respectively. The proportions of patients with improved pain relief, ADL improvement, and good doctor–patient communication were numerically higher than those of patients who answered ‘satisfied if anything’ or better.
Conclusions
Approximately two-thirds of the patients were satisfied with current analgesics. Patient satisfaction with oral analgesics could be influenced by multiple factors.
Clinical trial registration number
UMIN000036456.
1. Introduction
In clinical practice, chronic pain is often difficult to treat, and it causes problems such as reduced activity of daily living (ADL) [Citation1]. Pain is mainly classified as nociceptive pain, neuropathic pain (NeP) and mixed pain [Citation2]. In a large-scale prescription database study on oral analgesics conducted in Japan in 2017, non-steroidal anti-inflammatory drugs (NSAIDs: loxoprofen, celecoxib, and diclofenac) and NeP drugs (pregabalin, duloxetine, and neurotropin) were widely prescribed for patients with chronic pain in Japan [Citation3]. Although the therapeutic goal in patients with chronic pain is to increase patient satisfaction with analgesics, many patients are not satisfied with analgesic treatment. In fact, general physicians reported that the most common reason for dissatisfaction in patients with osteoarthritis treated with NSAIDs was either incomplete pain relief or breakthrough pain [Citation4]. Furthermore, up to 79% of the patients with chronic noncancer pain have been reported to be treated inappropriately [Citation5]. Few recent studies on pain relief and satisfaction in patients with chronic pain treated with conventional NSAIDs and NeP drugs have been reported. As patient satisfaction with oral analgesics could be influenced by pain relief and ADL improvement along with other factors such as doctor–patient communication, investigating the relationship between these factors and patient satisfaction with analgesics may be useful for the treatment of chronic pain with analgesics.
The objective of this study was to assess patient satisfaction with NSAIDs or NeP drugs in patients with chronic pain in Japan.
2. Patients and methods
2.1. Patients
The main inclusion criteria were as follows: (1) patients aged ≥20 years at the time when informed consent was obtained, (2) patients who were prescribed one NSAID or NeP drug for at least 90 consecutive days, regardless of concomitant use of drugs other than analgesics (we regarded patients who met this criterion as those with chronic pain because chronic pain is defined as pain that persists or recurs for more than 3 months [Citation6]), (3) patients who visited the pharmacy for pain relief, (4) patients with sufficient communication skills to participate in this study, and (5) patients who agreed to participate in the study. The main exclusion criteria were as follows: (1) patients who received oral analgesics (including OTC drugs) other than the study-targeted drugs or injections for pain relief within the past 90 days at the time when informed consent was obtained and (2) patients who were pregnant or possibly pregnant.
2.2. Ethics
This study (clinical trial registration number: UMIN000036456) was conducted in compliance with the Declaration of Helsinki [Citation7], the revised Personal Information Protection Law ‘Act on the Protection of Personal Information’ [Citation8], the amended Act on the Protection of Personal Information [Citation9], the Ethical Guidelines for Medical and Health Research Involving Human Subjects [Citation10], relevant notifications, and the study protocol. The patients’ informed consent was obtained before the initiation of this study. Furthermore, approval was obtained from the ethics review board (Kitamachi Clinic, Tokyo, Japan) and the ethics review committee by a third-party organization (Clinical research promotion network Japan, Osaka, Japan). The survey data provided by a contract research organization (Japan Medical Research Institute Co., Ltd., Tokyo, Japan) were data corresponding to anonymous processing information or statistical information according to the ‘Act on the Protection of Personal Information.’
2.3. Collection of patient background information
The patient background information and patient-reported outcome (PRO) survey data were collected from 589 dispensing pharmacies nationwide (affiliated to NIHON CHOUZAI Co., Ltd., Tokyo, Japan), to avoid bias for specific regions. As these data were encrypted irreversibly using a hash function in NIHON CHOUZAI Co., Ltd, information that could identify patients was not included in the data.
Pharmacists at the dispensing pharmacies recorded the following information obtained from patients.
Gender, age (20–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years, 70–79 years and ≥80 years, and <65 years, ≥65 years to <75 years, and ≥75 years, and name of clinical department at which the patient was being treated.
Names of previous analgesics prescribed in the study site and daily prescription doses.
NeP screening score (calculated from an NeP screening questionnaire). An NeP screening questionnaire developed in Japan was used to screen patients with non-NeP and NeP (). The screening questionnaire comprised 1–7 items, and the score was evaluated by a 5-point scale ranging from 0 (never), 1 (slightly), 2 (moderately), 3 (strongly), to 4 points (very strongly); subsequently, the total score was calculated [Citation11]. The higher total score indicated a greater likelihood of patients with NeP. Patients with a total score of 9 points (sensitivity 70% and specificity 76%) or more were screened as patients with NeP [Citation11].
Table 1. NeP screening questionnaire [Citation11,Citation12].
(4)Pain score (numerical rating scale, NRS).
To indicate the current level of pain, the score was evaluated using an 11-point scale ranging from 0 (no pain) to 10 (the worst possible pain).
2.4. Study design
This observational cross-sectional study was conducted in dispensing pharmacies. The study period was 4 months between March 2019 and June 2019. This study was conducted to assess patient satisfaction, pain relief, ADL improvement, and doctor–patient communication with analgesics in patients who were prescribed one NSAID or NeP drug for at least 90 consecutive days. The primary endpoint was patient satisfaction with oral analgesics. The secondary endpoints were pain relief, ADL improvement, and doctor–patient communication. The main procedures from screening and registration of target patients to obtaining the survey results were as follows, and all procedures were performed on the same day.
Pharmacist in the study site selected patients prescribed one NSAID or NeP drug using prescriptions and drug history records.
Pharmacist explained the study to the candidate patients and obtained informed consent to participate.
After registration, the eligible patients completed the NeP screening questionnaire and PRO questionnaire (patient satisfaction, pain relief, ADL improvement, and doctor–patient communication) and submitted their results to the pharmacists.
2.5. Targeted drugs
The following NSAIDs and NeP drugs were selected as the target drugs. Patients who were prescribed just one of these oral analgesics.
(1) NSAID group
Acemetacin, amfenac sodium, ampiroxicam, aspirin, aspirin dialuminate combination, celecoxib, diclofenac, etodolac, flufenamate aluminum, flurbiprofen, ibuprofen, indomethacin farnesyl, lornoxicam, loxoprofen, mefenamic acid, mofezolac, meloxicam, nabumetone, naproxen, oxaprozin, proglumetacin, sulindac, tiaprofenic acid, tiaramide, and zaltoprofen
(2) NeP drug group
Amitriptyline, carbamazepine, duloxetine, epalrestat, neurotropin, and pregabalin
2.6. Evaluation methods
Patient satisfaction, pain relief, ADL improvement, and doctor–patient communication in patients prescribed an NSAID or NeP drug were assessed by following questions ().
Table 2. Evaluation of patient satisfaction, pain relief, ADL improvement, and doctor–patient communication.
2.7. Statistical analysis
The sample size to assess patient satisfaction with analgesics was set at 400 patients each in the NSAID and NeP drug groups to ensure accuracy (maximum width of 95% confidence interval [CI]: ± 5%). The numbers and proportions of patients who were satisfied were tabulated overall and by the drug groups. The proportions of patients who were satisfied (‘satisfied if anything’ or better) and the two-sided 95% CI by the Clopper–Pearson method were calculated for each drug group in the NeP or non-NeP patient groups. For pain relief, ADL improvement and doctor–patient communication, the numbers and proportions of patients were calculated for each drug group.
No comparative tests between the NSAID and NeP drug groups were performed.
In the subgroup analysis, patient satisfaction and doctor–patient communication were assessed in patients classified into the NeP and non-NeP patient groups using NeP screening scores.
All statistical analyses were performed using the SAS statistical package version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient disposition
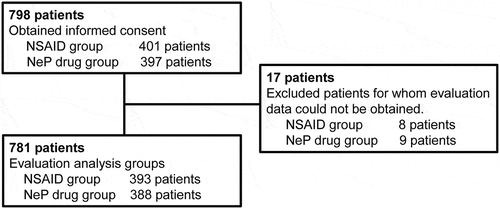
shows the disposition of patients who were prescribed analgesics. Pharmacists in each study site obtained informed consent from 798 patients to participate in the study. Seventeen patients for whom evaluation data could not be obtained were excluded from the study. Total number of patients included in the evaluation analysis group was 781 (393 and 388 patients in the NSAID and NeP drug groups, respectively).
3.2. Patient characteristics
Patient characteristics are shown in . Male and female patients accounted for 37.1% and 62.9%, respectively. The mean age was 70.1 ± 12.9 years, and the proportion of patients aged 65 years or older was 72.6%. By clinical departments, the proportions of patients treated in orthopedic surgery and internal medicine were 45.8% and 31.8%, respectively. The NeP screening scores (mean) were 5.3 and 6.8 for the NSAID and NeP drug groups, respectively. The NRS (mean) was 4.0 for the two drug groups.
Table 3. Patient characteristics.
3.3. Number and proportion of patients by drugs, and daily prescribed doses
The number and proportion of patients by drugs and daily prescription dose are shown in . Loxoprofen (44.8%) and celecoxib (39.9%) were mainly prescribed for patients in the NSAID group. The main drugs prescribed for patients in the NeP drug group were pregabalin (66.8%), duloxetine (15.2%) and neurotropin (10.3%). The daily prescription doses of loxoprofen, diclofenac, pregabalin, duloxetine, and neurotropin were lower than the approved doses.
Table 4. Number and proportion of patients by drugs and daily prescription doses.
3.4. Endpoints
3.4.1. Patient satisfaction (primary endpoint)
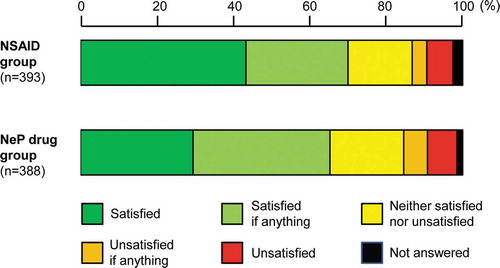
In the NSAID and NeP drug groups of all the patients, the proportions of patients who answered ‘satisfied if anything’ or better were 70.0% and 65.2%, respectively, whereas those of patients in the two drug groups who answered ‘satisfied’ were 43.3% and 29.4%, respectively, showing a numerical difference ().
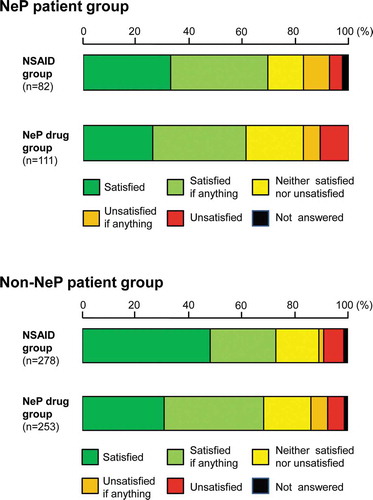
In the subgroup analysis, patient satisfaction with NSAIDs or NeP drugs in the NeP patient group (classified by NeP screening score of ≥9 points) and non-NeP patient group (≤8 points) was assessed ( and ). In the NeP patient group, the proportions of patient satisfaction (‘satisfied if anything’ or better) in the NSAID and NeP drug groups were 69.5% and 61.3%, respectively, whereas those in the non-NeP patient group were 73.0% and 68.4%, respectively. No large numerical difference between the two groups was observed. A numerical difference in the proportions of patients who answered ‘satisfied’ in the two drug groups of the non-NeP patient group (48.2% and 30.8%, respectively) was observed. The proportions of patients who answered ‘satisfied’ in the two drug groups in the NeP patient group were 32.9% and 26.1%, respectively.
Table 5. Patient satisfaction with NSAIDs and NeP drugs in the NeP or non-NeP patient groups.
Additionally, 42.5% of the NeP patients (n = 82) and 47.6% of the non-NeP patients (n = 253) were prescribed NSAIDs and NeP drugs, respectively (). On the contrary, 57.5% of the NeP patients (n = 111) and 52.4% of the non-NeP patients (n = 278) were prescribed NeP drugs and NSAIDs, respectively.
3.4.2. Pain relief
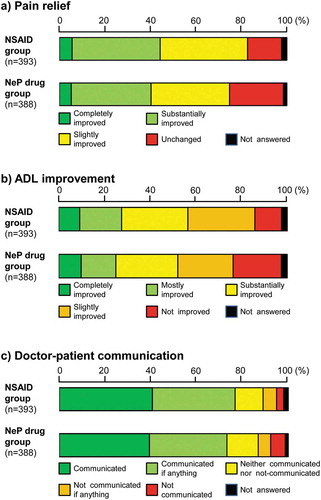
In the NSAID and NeP drug groups, the proportions of patients who answered ‘slightly improved or better’ were 82.7% and 74.8%, respectively ()).
3.4.3. ADL improvement
In the NSAID and NeP drug groups, 85.8% and 76.3% of the patients answered ‘slightly improved’ or better, respectively ()).
3.4.4. Doctor–patient communication
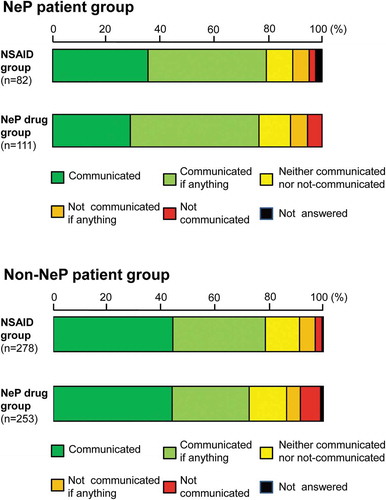
The proportions of patients who answered ‘communicated if anything’ or better in the NSAID and NeP drug groups were almost the same at 76.8% and 73.2%, respectively. Approximately 40% of the patients in the two drug groups answered ‘communicated.’ ()).
In the subgroup analysis, doctor–patient communication in the NSAID and NeP drug groups of the NeP and non-NeP patient groups was assessed (). The results revealed that 79.3% and 76.6% of the NeP patients who were prescribed NSAIDs and NeP drugs, respectively, answered ‘communicated if anything’ or better, whereas 78.4% and 72.3% of the non-NeP patients prescribed NSAIDs and NeP drugs answered ‘communicated if anything’ or better, respectively. No large numerical difference between the two drug groups of the NeP and non-NeP patient groups was observed.
4. Discussion
This observational cross-sectional study was conducted to assess patient satisfaction with oral analgesics in patients with non-NeP and NeP in Japan. The results clarified patient satisfaction, pain relief, ADL improvement, and doctor–patient communication in patients with chronic pain who were prescribed NSAID or NeP drug.
4.1. Endpoints
4.1.1. Patient satisfaction (primary endpoint)
Patient satisfaction with oral analgesics as the primary endpoint was assessed, because the therapeutic goal in patients with chronic pain is to increase patient satisfaction. The proportions of patients who answered ‘satisfied if anything’ or better in the NSAID and NeP drug groups were 70.0% and 65.2%, respectively, in patients with chronic pain prescribed analgesics for at least 90 consecutive days. Patient satisfaction (‘satisfied’ and ‘satisfied if anything’) with oral analgesics prescribed within 5 years in patients with chronic pain in 2012 and 2017 has been reported to be approximately 59% in Japan [Citation13]. The patient satisfaction in our study was numerically slightly higher than that of this previous study. One of the reasons for the difference may be that our study included more patients with good conditions of pain and ADL who had continued to receive the same drug without switching to other analgesics or co-prescription for 90 days or more. In this study, the proportions of patients who answered ‘satisfied’ in the NSAID and NeP drug groups were 43.3% and 29.4%, respectively. In the subgroup analysis, the proportions of patients who answered ‘satisfied’ in the NSAID and NeP drug groups of the non-NeP patient group were 48.2% and 30.8%, respectively, whereas those in the two drug groups of the NeP patient group were 32.9% and 26.1%, respectively, no large numerical difference was observed. The reason for this numerical difference in patient satisfaction between the two drug groups in the non-NeP patient group was unclear. It could be because NSAIDs are commonly effective on nociceptive pain, but ineffective on NeP with nerve damage [Citation14]. Therefore, the good pain relief with NSAIDs in the non-NeP patient group compared with that in the NeP drug group may increase patient satisfaction. Additionally, the proportions of patients who answered ‘satisfied’ and ‘satisfied if anything’ or better in the NeP drug groups of the non-NeP patient group were 30.8% and 68.3%, respectively. Although duloxetine and neurotropin are indicated for neuropathic and nociceptive painful conditions [Citation15,Citation16], it is considered that pain relief effect in patients with nociceptive pain treated with these NeP drugs in the non-NeP patient group may increase patient satisfaction.
In the patient satisfaction assessment using the subgroup analysis, 42.5% of the NeP patients and 47.6% of the non-NeP patients were prescribed NSAIDs and NeP drugs, respectively. It is unclear whether these patients were not prescribed appropriate analgesics, because the accurate diagnosis of NeP and non-NeP based on only NeP screening score (cutoff value: 9 points) could not be made. Therefore, it is required to accurately diagnose NeP and non-NeP using not only the screening scores but also disease name and objective findings to increase patient satisfaction.
4.1.2. Pain relief, ADL improvement, and good doctor–patient communication (secondary endpoints)
As pain relief, ADL improvement and good doctor–patient communication may have a considerable influence on patient satisfaction, we assessed the improvement in these factors in patients prescribed NSAIDs and NeP drugs. The proportions of patients with improved pain relief, ADL, and doctor–patient communication in the NSAID group were 76.8–85.8%, whereas those in the NeP drug group were 73.2–76.3%. They were numerically higher than those of patient satisfaction (70.0% and 65.2%). These results suggest that patient satisfaction with oral analgesics could be influenced by these multiple factors.
This study had some limitations. The patients were prescribed one NSAID or NeP drug for at least 90 consecutive days, which may have resulted in the inclusion of more patients with good condition of pain and ADL. As this survey was conducted at dispensing pharmacies, the disease data of individual patients such as hypertension and diabetes based on doctor’s diagnosis, safety data, and efficacy data other than pain relief and ADL improvement were not available. The accurate diagnosis of NeP and non-NeP could not be made because the patients’ disease name and objective findings were not included in patients’ data. The NeP screening scores (cutoff value: 9 points) were used to classify the patients suspected to have NeP and non-NeP but could not exclude the possibility that patients with non-NeP and NeP were mixed in the NeP and non-NeP patient groups, respectively. Although duloxetine and neurotropin were classified as NeP drugs, as they have indications for neuropathic and nociceptive painful conditions, data of patients with nociceptive pain may be mixed with those of patients from the NeP drug group.
5. Conclusions
This observational cross-sectional study was conducted to assess patient satisfaction with NSAIDs and NeP drugs in patients with chronic pain in Japan. Approximately two-third of the patients were satisfied with current analgesics. Patient satisfaction may be influenced by several factors. As there is the possibility of improper administration of analgesics to patients who seemed to have NeP using the NeP screening score, not only a screening questionnaire but also accurate clinical diagnosis is required.
Declaration of interest
T Ushida has received consulting fees for the performance of the study from Daiichi Sankyo Co., Ltd. K Okuizumi, T Inoue, D Matsui, K Shiosakai, and M Yokoyama are all full-time employees of Daiichi Sankyo Co., Ltd. K Takeda is an employee of the Japan Medical Research Institute Co., Ltd., while K Fukuoka is a full-time employee of Nihon Chouzai Co., Ltd. Finally, O Nakagawa is a president of The Agent LLC. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee declares that they are Consultant, Speaker, and Researcher for US World Meds, Salix, Enalare, Scilex, and Neumentum. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgments
Daiichi Sankyo Co., Ltd. participated in the study design, decision to publish, and preparation of the manuscript. The Japan Medical Research Institute Co., Ltd., Nihon Chouzai Co., Ltd., and The Agent LLC. have also participated in the data collection and analysis as well as the preparation of the manuscript, study design, and decision to publish. Medical writing assistance was utilized in the manuscript and was provided by Honyaku Center Inc. during the preparation of this manuscript.
Additional information
Funding
References
- Buchman AS, Shah RC, Leurgans SE, et al. Musculoskeletal pain and incident disability in community-dwelling older adults. Arthritis Care Res (Hoboken). 2010;62:1287–1293.
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819.
- Ushida T, Matsui D, Inoue T, et al. Recent prescription status of oral analgesics in Japan in real-world clinical settings: retrospective study using a large-scale prescription database. Expert Opin Pharmacother. 2019;20:2041–2052.
- Crichton B, Green M. GP and patient perspectives on treatment with non-steroidal anti-inflammatory drugs for the treatment of pain in osteoarthritis. Curr Med Res Opin. 2002;18:92–96.
- Bekkering GE, Bala MM, Reid K, et al. Epidemiology of chronic pain and its treatment in the Netherlands. Neth J Med. 2011;69:141–153.
- Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:19–27.
- World Medical Association (WMA). WMA declaration of Helsinki -ethical principles for medical research involving human subjects. [cited 2019 Feb 12]. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- Act on the protection of personal information (Act No. 57 of 2003). [cited 2019 Oct 10]. Japanese. Available from: https://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=415AC0000000057
- Amended act on the protection of personal information (May 30, 2017). [cited 2019 Oct 10]. Japanese. Available from: https://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=415AC0000000057
- Ethical guidelines for medical and health research involving human subjects. [cited 2019 Oct 10]. Available from: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf
- Ogawa S. Development of new screening questionnaire to identify neuropathic components in Japanese patients with chronic pain. Pain Clinic. 2010;31:1187–1194. Japanese.
- Japan Society of Pain Clinicians, eds. Guidelines for the pharmacologic management of neuropathic pain. 2nd ed. Tokyo, Japan: Shinko Trading Co., Ltd.; 2016. [cited 2019 Oct 10]. Japanese/English (Japanese Guideline, p34, Figure 2/English Guideline, p156, Figure 2). Available from: http://minds4.jcqhc.or.jp/minds/Pharmacologic-management-of-neuropathic-pain/Pharmacologic-management-of-neuropathic-pain.pdf
- Pfizer Japan Inc. Status of chronic pain in 47 prefectures: a comparative study between 2012 and 2017. Reference Material. [ cited 2019 October 10]. Japanese. Available from: https://www.pfizer.co.jp/pfizer/company/press/2017/documents/2017082302.pdf
- Ogawa S, eds. Neuropathic pain. Tokyo, Japan: Nanzando Co., Ltd.; 2010. Japanese.
- Eli Lilly Japan K.K. Cymbalta (duloxetine) Capsules, package insert. Japanese. [cited 2019 May 24]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/340018_1179052M1022_2_21
- NIPPON ZOKI Pharmaceutical Co., Ltd. Neurotropin tab. 4 N.U., package insert. Japanese. [cited 2019 Feb 12]. Available from: https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/530288_1149023F1036_1_05