1. Introduction
Killing or inhibiting the growth of pathogenic microorganisms in vivo has been made possible by the revolutionary discovery of antimicrobial substances that are tolerated by the human body [Citation1,Citation2]. In the first half of the past century, the mortality of gangrene, streptococcal pneumonia, and other deadly bacterial infections started to decrease, thereby certifying the unprecedented impact of antimicrobials on the history of mankind [Citation2,Citation3]. In addition, antimicrobials also helped other important innovations of human medicine to achieve an enduring success, by allowing the cure of severe infections that follow and exploit procedure-related breaches in our defenses (e.g. after major surgery, or chemotherapy for cancer) [Citation4,Citation5].
However, the spread of antimicrobial resistance (AMR) threatens all these positive effects of antimicrobials on humans’ health [Citation6–8]. This is simple to say, but hard both to explain and to counteract, because of the many participating actors. Indeed, acquisition of colonization by multidrug-resistant (MDR) organisms and the development of MDR infections are influenced by a complex interplay of antimicrobial use, stochastic mutations, patient-level risk factors for colonization/infection, and level of adequateness of infection-control measures [Citation9–11]. In the following paragraphs, we provide some brief examples illustrating peculiar but also intriguing challenges of facing AMR in 2020. While the importance of a One Health approach to AMR should be reminded and stressed [Citation12,Citation13], here we focus in particular on the impact of AMR in humans.
1.1. Facing the clinical impact of AMR in 2020
Clinicians facing the threat of AMR are interested in several things: (i) prevent patients from becoming colonized/infected by MDR organisms; (ii) improving accuracy and anticipating etiological diagnosis and detection of resistance in patients with MDR infections; (iii) improving survival of patients with MDR infections. These tasks are all complex and multifaceted, and they involve dynamic risk calculations that may sometimes present with a large degree of uncertainty. However, intriguing strategies are starting to appear that, if further developed, will help us in improving and optimizing our clinical approach to AMR.
As a first concept, it should be reminded that the most secure way not to die of MDR infection is not to develop MDR infection. In this regard, besides classical infection-control approaches, the development of antibacterial vaccines may be a potential way to curtail mortality rates of MDR infections. The idea of bacterial vaccines is certainly not new. However, several bacterial vaccine trials have failed in the past because of fluctuating reproducibility of pre-clinical models, difficulty of a priori sample size calculations, rarity of measured outcomes (among patients at risk, only a few will eventually develop MDR infections, and thus, large trials are needed), confounding effect of other intended (e.g. infection-control measures) or unintended (e.g. antibacterial therapies, center-level variables) fixed or random preventive factors, and possible unfeasibility in developing vaccines against specific targets of interest in potential MDR pathogens [Citation14,Citation15]. Against this backdrop, there are a few preclinical projects aimed at developing, for example, vaccines against Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii [Citation15]. All things considered, a peculiar challenge in the following decade will be that of understanding if and when bacterial vaccines could really help clinicians to prevent AMR.
Another approach to improve the prevention of MDR infections is to achieve a better knowledge of (and to better track) how MDR organisms spread in the community and in the health-care setting. In this regard, further advancements in the clinical applicability of ‘omics’ approaches are much awaited to unravel the complex interplay of resistance gene transmission, resistance gene expression, metagenomic aspects, and host factors in influencing the spread of AMR, and further improving and optimizing our preventive measures [Citation16–18].
Both rapid PCR-based/phenotypical diagnostic tests and genomic approaches through whole-genome sequencing are expected to improve and accelerate the diagnosis of MDR infections, thereby allowing both early isolation of patients and early adequate treatment. In turn, this could lead to beneficial effects both on patients‘ survival (patient-level effect) and on the epidemiology of resistance (population-level effect) [Citation19,Citation20]. In our opinion, a critical factor to be considered regarding the use of rapid diagnostic tests in 2020 and beyond is that of always tailoring their implementation to the local characteristics and needs of every single hospitals (e.g. laboratory staff availability and expertise, possible prioritization to specific categories of patients, choice of specific rapid tests by coupling their spectrum of pathogen/resistance identification with the local microbiological epidemiology) in order to maximize their cost-effectiveness [Citation20].
Finally, once an MDR infection is rapidly diagnosed, clinicians also want to maximize the efficacy (and effectiveness, in real life) of antibiotic therapy, at the same time reducing toxicity as much as possible. This was frequently unfeasible for the most part of the last two decades in the case of infections due to MDR Gram-negative bacteria (MDR-GNB) since the paucity of available active agents against these organisms forced clinicians to renew the use of old antibiotics that, albeit very useful in the absence of dependable alternatives, has shown partial efficacy and nonnegligible toxicity (e.g. polymyxins) [Citation21,Citation22]. In an ideal scenario, these drugs should be reserved as last-resort options and not used as first-line treatments. Fortunately, in the last few years, some novel agents belonging to old classes (e.g., β-lactam/β-lactamase inhibitors) have become available that have improved our ability to effectively treat some MDR-GNB infections, with also a good tolerability profile [Citation21,Citation23,Citation24]. Nevertheless, although uncommon, resistance to novel agents has already been described [Citation25–27], as in part expected because of their belonging to already existing antibiotic classes and the inherent and long-known resilience of bacteria under antibiotic selective pressure [Citation2,Citation28]. From this perspective, important challenges in the next decade will be not only to preserve the activity of these precious novel agents in the long term but also to complete clinical development and achieve approval of other alternative or complementary therapeutic strategies based on novel mechanisms of actions, such as direct-acting small molecules active on novel targets (e.g., LpxC inhibitors, antimicrobial peptides), potentiators of antimicrobial activity (e.g., efflux pump inhibitors), phage and phage-derived molecules (e.g., endolysins), inhibitors of virulence factors, monoclonal antibodies, and microbiota-modulating therapies [Citation15,Citation29]. Very importantly, in the next decade, any possible successful development and use of novel drugs will increasingly depend not only upon efficacy and safety but also upon the availability of economic incentives for their development and for guaranteeing sufficient post-marketing profitability and avoid bankruptcy (see below).
1.2. Facing the societal and economic impact of AMR in 2020
AMR should better be viewed as a tragedy of the commons, thereby intuitively making its societal impact of major interest. In this regard, the dichotomy of concomitantly guaranteeing the best available treatment for the single patient and preventing the emergence and spread of resistance to other patients is a rather unique challenge in medicine. From this perspective, it appears imperative to concert supranational, public-private initiatives to promote solid measures at all levels of our fight against AMR. For example, the recent bankruptcy of Achaogen (the industry that produced plazomicin) has brought attention to the fact that there is a necessity not only of push incentives to allow small- and medium-sized enterprises to enter antimicrobial research and development but also of pull incentives to ensure novel approved antimicrobials are profitable (also not to discourage investors). The problem is not of simple solution (again, because of the dichotomy of needing both to use and to preserve novel agents) and novel revenue models are under evaluation (e.g., the DRIVE-AB project) [Citation30,Citation31]. The first subscription-style payment model (in which pharmaceutical companies are paid upfront based on the value their product provides to the National Health System and not on how much the product is used) has been recently launched in the UK [Citation32].
Besides the important role of economic aspects in optimizing antimicrobial development, also estimating the favorable economic impact of initiatives to counteract AMR on either health care or society remains a partly unmet challenge since existing economic estimates vary markedly across studies [Citation33–35]. In this regard, we would like to share a personal impression and bring the attention of readers to what we feel is a critical, common denominator: the reliability of economic models greatly depends on the reliability of the measured impact of actions aimed at counteracting AMR and/or improving clinical outcomes. That is, first we need to know if (and how) our infection-control/antimicrobial stewardship measures truly reduce the risk of developing colonization/infection by resistant organisms and/or improve antimicrobial use and relevant patients’ outcomes (e.g., survival). For example, we recently reviewed the therapeutic implications of using rapid microbiological tests for the diagnosis of bloodstream infections due to MDR-GNB [Citation20]. The potential impact on patients’ health of such tests is indeed relevant, since they may anticipate diagnosis, treatment, and infection-control measures in patients with MDR-GNB bloodstream infections (BSI). However, we realized that several studies estimated only the potential clinical and economic advantages of reducing the diagnostic turnaround time (i.e., the time elapsing from the blood draw to the identification of the causative agent and/or the phenotypical/molecular antibiogram) in ideal conditions, without considering the impact of locally relevant real-life variables. Indeed, several factors may considerably affect the true advantage in terms clinical outcomes, antimicrobial use, and cost-effectiveness of rapid tests in real-life and in different settings: (i) the local microbiological epidemiology; (ii) the local prevalence of the various resistance mechanisms; (iii) the local antimicrobial empirical therapy protocols; (iv) and the laboratory staff availability on night and weekend shifts [Citation20]. In turn, estimating any possible microeconomic or macroeconomic advantage of implementing rapid microbiological tests in clinical practice without taking into account the impact of these real-life factors and their variance across regions and centers would likely carry a significant risk of model unreliability.
2. Expert opinion
In the previous paragraphs, we briefly presented a perspective on the expected clinical, societal, and economic challenges of AMR at the dawn of 2020. Now, imagine a highly contagious viral disease rapidly spreading across continents and causing a severe acute respiratory syndrome in a small proportion of patients, yet highly relevant in terms of absolute numbers and surpassing intensive care unit (ICU) bed capacity [Citation36]. A novel ICU is rapidly built and/or made available in your hospital to guarantee mechanical ventilation and intensive care assistance for all these patients. They almost entirely come from the community, have none or little previous contacts with the healthcare system, and many of them are not colonized by MDR organisms (although the prevalence of MDR organisms in the community may be non-negligible in some areas and prevention of further community spread of AMR is also of utmost importance [Citation37,Citation38]). Although affected by a viral disease, most of them are treated with antibiotics since admission, because of the atypical clinical presentation and the laboratory values that resemble bacterial sepsis, thereby frequently precluding to rapidly exclude concomitant bacterial infections [Citation39]. Facilitated by an ICU stay frequently longer than 10 days, several of them will develop ICU-acquired BSI and/or ventilator-associated pneumonia (VAP). Initially, these superinfections will be caused by multi-susceptible organisms from the patients’ own microbiota. Then, after a variable period of weeks to months, BSI and VAP caused by bacteria resistant to commonly used empirical antibiotics will start to emerge, both in patients with prolonged length of stay and in novel patients. Indeed, owing to the difficulty in implementing further infection-control measures, cross-transmission of MDR organisms within the ICU may occur. This because personal protective equipment (PPE) is employed to protect the ICU staff from acquiring the viral disease (while the same PPE is used for assisting different patients), and adopting further measures and PPE for avoiding cross-transmission of MDR organisms may prove impossible because of possible shortages in either equipment or personnel, as well as redeployment of infectious disease specialists and infection-control experts to the primary care of patients with the viral disease [Citation40]. Of course, this is only a hypothetical scenario still (fortunately) not supported by published data, but in our opinion, it is possible that this could become a reality in some wards in areas highly affected by the COVID-19 pandemic. In turn, this testifies to the potentially devastating impact of the COVID-19 pandemic on MDR at the different clinical, societal, and economic levels. Because of the complexity of the AMR dynamics across all these levels, we feel the impact of COVID-19 on AMR could also have unpredictable unfavorable effects in the long term, which we will have to deal with in the near future. For example, it cannot be excluded that an uncontrolled spread of AMR during the peak of the COVID-19 pandemic could contribute to create or deepen impoverishment and inequalities.
Therefore, we think there is a critical need to expand our knowledge on the true risk of bacterial superinfection in COVID-19 patients, in order to reduce the high rates of empirical antibiotics administered in the first months of the pandemic (they were frequently administered to more than 70% of critically ill COVID-19 patients in the first reported experiences [Citation41–44]). Furthermore, we think diagnostic efforts should be both maximized and improved, by guaranteeing secure procedures for collecting and processing respiratory samples, as well as by implementing rapid PCR-based/phenotypical diagnostic tests for etiological diagnosis and rapid de-escalation/withdrawal of antibiotic therapy. Indirect measures such as developing dedicated guidelines for antimicrobials use in COVID-19 patients, increasing public awareness, being logistically able to preserve sufficient bed capacity, and avoiding excessive re-deployment of infection-control and antimicrobial stewardship experts during possible future peaks of this or other pandemics could also be crucial from the perspective of health-care systems to prevent an unintended spread of AMR [Citation45]. Finally, automated collection of large data from electronic records to exploit possible advantages of machine learning techniques may also improve our ability to counteract both the clinical and societal impact of AMR, although both machine learning algorithms and automated data collection are not exempt from biases, with legal and ethical aspects also needing standardization [Citation46,Citation47].
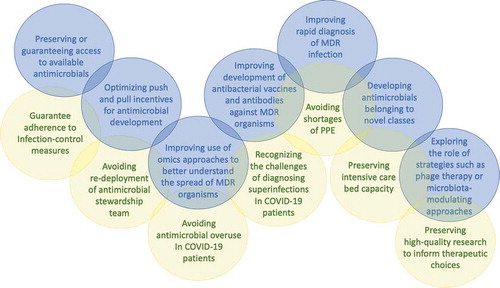
In conclusion, this brief editorial reflects our personal perspective on the novel challenges posed by AMR in 2020, a time when an unanticipated emergency is forcing us to re-discuss and partly renew our approach to estimating and counteracting AMR in the long run (see ). Without doing this, we will probably jeopardize all the important progress in antimicrobial stewardship and reliability of AMR modeling achieved in the last decade.
Figure 1. Examples of novel challenges posed by antimicrobial resistance in 2020. Blue circles represent general novel challenges posed by antimicrobial resistance in 2020, according to current scientific progress in terms of prevention, diagnosis, and treatment of infections due to multidrug-resistant organisms. Yellow circles represent pressing antimicrobial resistance challenges indirectly posed by the COVID-19 pandemic, that dynamically overlap with existing baseline challenges. MDR, multidrug-resistant; PPE, personal protective equipment
Declaration of interest
DR Giacobbe reports honoraria from Stepstone Pharma GmbH as well as an unconditional grant from Merck Sharp and Dohme Italia and Correvio Italia. Furthermore, M Bassetti reports, outside of this submitted work, having received funding for scientific advisory board participation, travel and for speaking from Angelini, AstraZeneca, Basilea, Bayer, BioMerieux, Cidara, Correvio, Cubist, Menarini, Molteni, Merck Sharp and Dohme, Nabriva, Paratek, Pfizer, Roche, Shionogi, Tetraphase, ThermoFisher, and The Medicine Company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Referee disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529:336–343.
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433.
- Abraham EP, Chain E. An enzyme from bacteria able to destroy penicillin. 1940. Rev Infect Dis. 1988;10:677–678.
- Fauci AS, Marston D. The perpetual challenge of antimicrobial resistance. JAMA. 2014;311:1853–1854.
- Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl 4):S240–245.
- Bassetti M, Poulakou G, Ruppe E, et al. Antimicrobial resistance in the next 30 years, humankind, bugs and drugs: a visionary approach. Intensive Care Med. 2017;43:1464–1475.
- Courvalin P. Why is antibiotic resistance a deadly emerging disease? Clin Microbiol Infect. 2016;22:405–407.
- Barriere SL. Clinical, economic and societal impact of antibiotic resistance. Expert Opin Pharmacother. 2015;16:151–153.
- Allegranzi B, Kilpatrick C, Storr J, et al. Global infection prevention and control priorities 2018-22: a call for action. Lancet Glob Health. 2017;5:e1178–e1180.
- Bassetti M, Giacobbe DR, Vena A, et al. Challenges and research priorities to progress the impact of antimicrobial stewardship. Drugs Context. 2019;8:212600.
- Giacobbe DR, Del Bono V, Mikulska M, et al. Impact of a mixed educational and semi-restrictive antimicrobial stewardship project in a large teaching hospital in Northern Italy. Infection. 2017;45:849–856.
- Hernando-Amado S, Coque TM, Baquero F, et al. Defining and combating antibiotic resistance from one health and global health perspectives. Nat Microbiol. 2019;4:1432–1442.
- McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol Spectr. 2018;6:ARBA-0009-2017.
- Redi D, Raffaelli CS, Rossetti B, et al. Staphylococcus aureus vaccine preclinical and clinical development: current state of the art. New Microbiol. 2018;41:208–213.
- Theuretzbacher U, Outterson K, Engel A, et al. The global preclinical antibacterial pipeline. Nat Rev Microbiol. 2020;18:275–285.
- Hendriksen RS, Munk P, Njage P, et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat Commun. 2019;10:1124.
- Wyres KL, Lam MMC, Holt KE. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol. 2020;18:344–359.
- Shendure J, Balasubramanian S, Church GM, et al. DNA sequencing at 40: past, present and future. Nature. 2017;550:345–353.
- Dubourg G, Raoult D, Fenollar F. Emerging methodologies for pathogen identification in bloodstream infections: an update. Expert Rev Mol Diagn. 2019;19:161–173.
- Giacobbe DR, Giani T, Bassetti M, et al. Rapid microbiological tests for bloodstream infections due to multidrug resistant Gram-negative bacteria: therapeutic implications. Clin Microbiol Infect. 2020;26:713–722.
- Bassetti M, Giacobbe DR. Judging the appropriate therapy for carbapenem-resistant Acinetobacter infections. Expert Opin Pharmacother. 2020;21:135–138.
- Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39:10–39.
- Giacobbe DR, Mikulska M, Viscoli C. Recent advances in the pharmacological management of infections due to multidrug-resistant gram-negative bacteria. Expert Rev Clin Pharmacol. 2018;11:1219–1236.
- Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam,meropenem/ vaborbactam,or both? Clinical and formulary considerations. Clin Infect Dis. 2019;68:519–524.
- Berrazeg M, Jeannot K, Ntsogo Enguene VY, et al. Mutations in beta-Lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob Agents Chemother. 2015;59:6248–6255.
- Shields RK, Chen L, Cheng S, et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae Infections. Antimicrob Agents Chemother. 2017;61:e02097–16.
- Sun D, Rubio-Aparicio D, Nelson K, et al. Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61:e01694–17.
- Marston HD, Dixon DM, Knisely JM, et al. Antimicrobial resistance. JAMA. 2016;316:1193–1204.
- Pollock J, Low AS, McHugh RE, et al. Alternatives to antibiotics in a one health context and the role genomics can play in reducing antimicrobial use. Clin Microbiol Infect. 2020; 10.1016/j.cmi.2020.02.028.
- Innovative Medicine Inititative. DRIVE AB report. Revitalizing the antibiotic pipeline: stimulating innovation while driving sustainable use and global access. 2018 [ accessed 2020 May 29]; Available at: http://drive-ab.eu/wp-content/uploads/2018/01/CHHJ5467-Drive-AB-Main-Report-180319-WEB.pdf
- Ardal C, Balasegaram M, Laxminarayan R, et al. Antibiotic development – economic, regulatory and societal challenges. Nat Rev Microbiol. 2020;18:267–274.
- Mahase E. UK launches subscription style model for antibiotics to encourage new development. BMJ. 2020;369:m2468.
- OECD and ECDC. Antimicrobial resistance: tackling the burden in the European Union 2019 [ accessed 2020 May 27]; Available at: http://www.oecd.org/health/health-systems/AMR-Tackling-the-Burden-in-the-EU-OECD-ECDC-Briefing-Note-2019.pdf
- Review on antimicrobial resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2016 [ accessed 2020 May 27]; Available at: https://amr-review.org/
- Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect. 2014;20(10):973–980.
- Bassetti M, Giacobbe DR, Aliberti S, et al. Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: current position of the Italian Society of Anti-infective Therapy (SITA) and the Italian society of Pulmonology (SIP). Clin Microbiol Infect. 2020;26(7):880–894. .
- Cilloniz C, Dominedo C, Torres A. Multidrug resistant gram-negative bacteria in community-acquired pneumonia. Crit Care. 2019;23:79.
- van Duin D, Paterson DL. Multidrug-resistant bacteria in the community: trends and lessons learned. Infect Dis Clin North Am. 2016;30:377–390.
- Hsu J. How COVID-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983.
- Rawson TM, Moore LSP, Castro-Sanchez E, et al., COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75(7):1681–1684.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
- Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020; DOI:10.1093/cid/ciaa530.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062.
- Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;e13319. DOI:10.1111/eci.13319
- Rawson TM, Ming D, Ahmad R, et al. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020;18:409–410.
- Giacobbe DR, Mora S, Giacomini M, et al. Machine learning and multidrug-resistant gram-negative bacteria: an interesting combination for current and future research. Antibiotics (Basel). 2020;9:54.
- Prosperi M, Guo Y, Sperrin M, et al. Causal inference and counterfactual prediction in machine learning for actionable healthcare. Nature Mach Intell. 2020;2:369–375.
