1. Introduction
Bulimia nervosa (BN) is a severe psychiatric disorder that is characterized by (1) eating, in a discrete period of time, an amount of food that is definitely larger than most people would eat during similar circumstances and (2) a sense of lack of control over eating during the episode. Those binge-eating episodes are typically followed by guilt and shame, which trigger compensatory behaviors to avoid weight gain, such as self-induced vomiting, use of laxatives or diuretics, fasting, or exercise [Citation1]. BN is associated with increased mortality for all causes of death including suicide and often has a chronic course. Lifetime prevalence estimate for BN is 1.0%, and comorbidity is high (80% with an anxiety disorder, 70% with a mood disorder, 60% with an impulse control disorder, 40% with substance use disorder) [Citation2]. Cognitive behavioral therapy, interpersonal therapy, and the selective serotonin reuptake inhibitor (SSRI) fluoxetine are the treatments of choice, but about 50% have a chronic course or are only partially recovered. Thus, treatment effects are modest, and we lack pharmacological interventions that target BN as standalone treatments or in conjunction with behavioral interventions. The goal of this editorial is to briefly review behaviors and pathophysiology that have been associated with BN and identify and discuss pharmacological treatment targets.
2. Behavioral traits as vulnerability factors
The underlying etiology of BN is not well understood. BN has been associated with inadequate mechanisms to control food intake beyond one's physiological needs, and behavioral traits could contribute [Citation3]. One of those traits is altered emotion regulation, the process responsible for monitoring, evaluating, and modifying emotional reactions. Individuals with BN have difficulties modulating strong emotions and controlling rash, impulsive response. Most, but not all studies suggest that negative affect precedes binge-eating episodes, followed by initial relief. Impulsivity is a trait to react rapidly to stimuli without regard for potential negative consequences. Binge-eating behaviors frequently occur impulsively in response to external or internal triggers, and increased impulsivity has been found in BN. Negative urgency, the tendency to experience strong impulses under the influence of negative emotions or to act rashly when distressed, has also been associated with BN. Another trait, sensitivity to reward, described in the reinforcement sensitivity theory, was elevated in BN and it has been hypothesized that an imbalance between reward sensitivity, impulsivity, and inhibition are mechanistically involved in driving binge-eating episodes.
3. Neurocircuitry of emotion regulation, impulsiveness, and cognitive control
A complex interplay exists between emotion regulation and cognitive control. Emotions affect attention, drive cognitive bias, and may interrupt proper decision-making, while attention to specific goals can control emotions and override strong feelings [Citation4]. Control of food cravings is thought to involve prefrontal cortical areas, whereas greater caloric intake has been related to higher activation in the gustatory cortex and brain regions for reward computation. Studies in BN found reduced prefrontal cortical activity when viewing food pictures, hypoactivity in brain areas involved in self-regulation and impulse control, such as prefrontal cortex or insula, and positive correlations between negative affect and striatal brain response during food anticipation [Citation3]. Altogether, research suggests altered brain function related to emotion regulation in BN, but the literature is small.
4. Neurotransmitters and hormones
Dopamine, serotonin, acetylcholine, and norepinephrine have been associated with cognitive control and impulse control in frontal cortical circuits [Citation5]. Studies in BN found that serotonin 1A and dopamine D2/3 receptor binding correlated with anxiety and behavior inhibition [Citation3]. Hormones such as ghrelin, leptin, sex hormones, and cortisol can also influence food intake behaviors [Citation6]. Basic research on the gut-brain axis showed that stress, via cortisol and gut hormone activation, leads to dopamine mediated altered food intake implicating those feedback mechanisms but how they contribute to BN is still elusive [Citation3].
5. Neurobiology of reward processing
Brain reward circuits are key to the motivational aspects of food approach. Individuals with high versus low food addiction scores showed greater prefrontal cortex and caudate activation during food anticipation, but lower orbitofrontal activation in response to food receipt; this suggested that the tendency to overeat is associated with high reward system response to expectation (stimulating food approach), but reduced behavior-inhibition associated cortex response (low food intake inhibition) [Citation7]. Brain response in BN after recovery showed deficits when distinguishing gain versus loss in a monetary reward paradigm, suggesting distinct reward circuit alterations depending on stimulus saliency [Citation3]. A study in BN that used the dopamine-related prediction error reward learning paradigm found a weaker response to unexpected stimulus receipt or omission [Citation8]. In another study, negative affect correlated positively with putamen and caudate activation during food anticipation and it was hypothesized that negative affect may increase the reward value of food in BN [Citation9].
6. Targets for pharmacological intervention
The above-reviewed literature suggests several key areas of underlying disturbances in BN. Those are behavioral traits for emotional instability, alterations in brain regions that process self-regulation and impulse control, and the neurotransmitter dopamine and serotonin as well as gut hormones as potential vulnerabilities to develop and maintain BN behaviors (). The importance of SSRIs is supported by the effectiveness of fluoxetine at a high dose for the treatment of BN [Citation10]. The mechanism of fluoxetine’s action is uncertain, but effects on mood and anxiety likely play a role, since antidepressant/antianxiety medication, in general, is superior to placebo in improving BN [Citation11]. Only one true long-term study exists, conducted over 52 weeks, and while fluoxetine was superior, attrition rate was very high with over 80% [Citation12]. In that study, there was worsening over time on all measures of efficacy, and the authors suggested that fluoxetine alone may not be an adequate treatment after acute response in most patients. This makes the search for other medication approaches ever more important.
Figure 1. Vulnerability factors that contribute to the behavior chain of binge eating and purging behaviors in bulimia nervosa
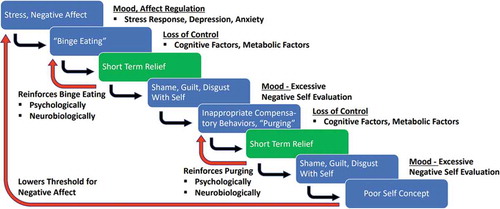
Mood instability in BN suggests that mood stabilizers could be of help. Small, uncontrolled studies suggested that the mood stabilizer lamotrigine could be beneficial in controlling BN behaviors [Citation13]. A case report suggested that the stimulant methylphenidate was beneficial in a patient with comorbid ADHD, targeting executive function and impulse control circuits [Citation14]. Stimulants have been used with success in binge-eating disorder and further investigation is warranted [Citation3]. Several neurotransmitter specific drugs that target appetite, reward system, or hormone regulation, are being considered as potential therapeutic agents, but this search has overall been disappointing [Citation15]. Different reasons may be the case. First, BN is a multifactorial problem and one drug may not be sufficient to target the biological, psychological, and social factors. Second, we need to understand BN’s underlying pathophysiology better first to apply pharmacological treatments more effectively. Third, we will need to identify a range of treatment modules that in concert address the many illness-contributing factors to overcome the illness. Those treatments will include various psychotherapies (for instance interventions for eating disorder-specific behavior management, general emotion regulation, etc.) and psychopharmacological interventions that target mood instability, anxiety, and impulse control, depending on the severity and need. Importantly, binge eating, and purging modulate brain biology and different treatments may become indicated at different time points during the course of illness. Thus, psychopharmacology in BN clearly has a role, but usually not as the only treatment for BN at this point, and often to address comorbidity and BN-related behaviors such as labile emotions and impulse control.
7. Expert opinion
The only approved medication for BN is fluoxetine, either started at or up-titrated over several days to 60 mg daily [Citation10]. This should be considered for someone with a severe form of the illness. There are several caveats though. Many patients are hesitant to start medication or agreeing to fast up-titration and gradual medication increase is more tolerable. BN is also associated with high rates of depression and SSRI aggressive dose increase may trigger suicidal behavior. Furthermore, the treatment effect may not be sustained and other treatments may be needed [Citation12]. Thus, BN patients should be referred to psychotherapy that uses evidence-based strategies such as BN focused cognitive behavior therapy. Comorbid depressive, anxiety, obsessive-compulsive and impulse control disorders are common, and a substantial number of patients have a substance use disorder [Citation2]. Another comorbid condition that is often not readily disclosed is post-traumatic stress disorder, which can trigger BN behaviors. Although systematic research on this topic is lacking, my clinical experience is that diligently identifying and treating those conditions with approved treatment regimens supports overall clinical outcome including that of BN. In the case of comorbid ADHD, there is the concern that prescription of a stimulant or other possibly appetite-reducing medication may be used to avoid normal food intake and promote the eating disorder drive to lose weight. Thus, careful weight monitoring is important. While I do not hesitate to treat ADHD in BN, it is usually the last comorbidity to address after depression, anxiety, and OCD have stabilized, as those may also contribute to inattention and distractibility. Individuals with substance use history may not be good candidates for stimulant treatment but other non-habit forming treatments might be a consideration. If the patient continues to have a poor response, then more experiential treatments using medications that have been found overall safe in other conditions could be used. One such treatment that could be tried is lamotrigine with the rationale of mood stabilization and helping with impulse control, to strengthen cognitive control over urges to binge eat, and a low risk of weight gain, which is a frequent reason why patients with eating disorders decline medication interventions.
Declaration of interest
G Frank has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
- Hudson JI, Hiripi E, Pope HG Jr., et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007 Feb 01;61(3):348–358.
- Frank GKW, Berner LA, editors. Binge eating: A transdiagnostic psychopathology. Springer Nature Switzerland; 2020, 321 p.
- Okon-Singer H, Hendler T, Pessoa L, et al. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Front Hum Neurosci. 2015;9:58.
- Logue SF, Gould TJ. The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav. 2014 Aug;123:45–54.
- Culbert KM, Racine SE, Klump KL. Hormonal factors and disturbances in eating disorders. Curr Psychiatry Rep. 2016 Jul;18(7):65.
- Gearhardt AN, Yokum S, Orr PT, et al. Neural correlates of food addiction. Arch Gen Psychiatry. 2011 Aug;68(8):808–816.
- Frank GK, Reynolds JR, Shott ME, et al. Altered temporal difference learning in bulimia nervosa. Biol Psychiatry. 2011 Oct 15;70(8):728–735.
- Bohon C, Stice E. Negative affect and neural response to palatable food intake in bulimia nervosa. Appetite. 2012 Jun;58(3):964–970.
- (FDA) FaDA. Bulimia nervosa prescribing information. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Bacaltchuk J, Hay P. Antidepressants versus placebo for people with bulimia nervosa. Cochrane Database Syst Rev. 2003;4:CD003391.
- Romano SJ, Halmi KA, Sarkar NP, et al. A placebo-controlled study of fluoxetine in continued treatment of bulimia nervosa after successful acute fluoxetine treatment. Am J Psychiatry. 2002 Jan;159(1):96–102.
- Trunko ME, Schwartz TA, Marzola E, et al. Lamotrigine use in patients with binge eating and purging, significant affect dysregulation, and poor impulse control. Int J Eat Disord. 2014 Apr;47(3):329–334.
- Guerdjikova AI, McElroy SL. Adjunctive methylphenidate in the treatment of bulimia nervosa co-occurring with bipolar disorder and substance dependence. Innovations in Clinical Neuroscience. 2013 Feb;10(2):30–33.
- McElroy SL, Guerdjikova AI, Mori N, et al. Progress in developing pharmacologic agents to treat bulimia nervosa. CNS Drugs. 2019 Jan;33(1):31–46.
