ABSTRACT
Introduction
Patisiran and inotersen are two therapies approved for the treatment of hereditary transthyretin-mediated (hATTR) amyloidosis with polyneuropathy, a rapidly progressive disease with a substantial clinical burden. This analysis indirectly compares the efficacy of patisiran and inotersen on neuropathy and quality of life (QOL).
Methods
Published results from the NEURO-TTR study of inotersen and individual patient data from the APOLLO study of patisiran were used. Indirect comparisons were conducted for 15-month changes in neuropathy and QOL endpoints: modified Neuropathy Impairment Score +7 (mNIS+7Ionis,), Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QOL-DN) questionnaire, body mass index (BMI), and Polyneuropathy Disability (PND) score. Analyses were conducted under different assumptions about the impact of missing data and to adjust for baseline differences between studies.
Results
Patisiran showed significantly greater treatment effects than inotersen for mNIS+7Ionis (mean difference: −12.3 [95% confidence interval: −21.4, −3.3]), Norfolk QOL-DN (−11.3 [−19.8, −2.9]), and BMI (1.0 [0.4, 1.7]). The proportion of patients with improvement or no change from baseline on PND score was higher for patisiran-treated patients (odds ratio: 8.9 [4.6, 17.5]). Results were consistent and robust across analyses and methods.
Conclusions
Patisiran demonstrated greater treatment effects on neuropathy and QOL than inotersen in patients with hATTR amyloidosis with polyneuropathy.
1. Introduction
Hereditary transthyretin-mediated (hATTR) amyloidosis is a rapidly progressive, inherited, multisystem disease associated with substantial disability, morbidity, and mortality [Citation1]. hATTR amyloidosis is caused by mutations in the transthyretin (TTR) gene, which result in the production of misfolded TTR proteins that accumulate in tissues and organs including the nerves, heart, and gastrointestinal tract [Citation2,Citation3]. It is estimated that approximately 50,000 patients worldwide have hATTR amyloidosis [Citation1].
The clinical presentation of hATTR amyloidosis is heterogeneous and the disease can include a wide range of manifestations that progressively impair sensorimotor, autonomic, and cardiovascular function [Citation1,3–7]. Impacts on peripheral sensory-motor function include progressive impairment of ability to sense pain, temperature, and touch, neuropathic pain, and muscle weakness [Citation3]. Deterioration in ambulatory ability is an important indicator of disease progression for hATTR amyloidosis, underscored by its use in two commonly used staging measures for this condition: Polyneuropathy Disability (PND) score and Familial Amyloidotic Polyneuropathy (FAP) Stage [Citation8,Citation9]. Patients with hATTR amyloidosis may also experience potentially severe autonomic dysfunction and gastrointestinal manifestations [5–7]. Overall, the multisystem and progressive nature of the disease substantially limits patients’ ability to work, socialize, and perform daily activities, resulting in profound impairment of independence and quality of life [Citation10,Citation11].
Currently approved treatment options for patients with hATTR amyloidosis with polyneuropathy include patisiran, inotersen, and tafamidis [12–14]. Patisiran, a ribonucleic acid interference therapeutic, and inotersen, an antisense oligonucleotide, have both been approved for the treatment of hATTR amyloidosis with polyneuropathy by multiple regulatory agencies worldwide [13–20]. Patisiran is administered via intravenous infusion (weight <100 kg, 0.3 mg/kg; weight ≥100 kg, 30 mg) every 3 weeks, and inotersen is administered via subcutaneous injection (284 mg) weekly [Citation13,Citation14]. An indirect treatment comparison analysis suggested that patisiran had greater treatment benefit than tafamidis on neuropathy and quality of life outcomes in patients with Stage 1 symptomatic polyneuropathy [Citation21]. Given this previous research, the present study focuses only on comparing efficacy between patisiran and inotersen.
Patisiran and inotersen both inhibit the production of mutant and wild-type TTR thereby reducing amyloid deposition. In their pivotal, randomized, placebo-controlled Phase 3 trials, both therapies demonstrated efficacy against placebo on the modified Neuropathy Impairment Score +7 (mNIS+7) and Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QOL-DN) questionnaire [Citation22,Citation23], which were primary or key secondary endpoints measuring disease progression and the impact of polyneuropathy on quality of life.
Both patisiran and inotersen represent important advances in the treatment of hATTR amyloidosis with polyneuropathy relative to current standard of care and present effective therapeutic options for a population with significant unmet need. However, differences between these two therapies in the magnitude of neuropathy and quality of life improvements observed relative to placebo in their respective trials suggest that there may be important differences in their comparative efficacy. Due to the progressive nature of the disease and the need for early treatment, understanding the comparative efficacy of these different therapies is crucial to inform decision-making by patients, clinicians, payers, and other stakeholders. In the absence of head-to-head trials, indirect treatment comparisons approaches are commonly used to assess relative effectiveness of different therapies using a common comparator [Citation24,Citation25]. In this study, we add to the literature by indirectly comparing the relative efficacy of patisiran and inotersen for hATTR amyloidosis with polyneuropathy.
2. Methods
The feasibility of conducting indirect comparisons was assessed and confirmed prior to designing the analyses described below. Statistical methods used and outcomes analyzed were prespecified prior to the conduct of the indirect comparisons. A summary of the feasibility assessment and a detailed analysis plan are included in Appendix A.
2.1. Data sources
The APOLLO study was a Phase 3, multicenter, randomized, double-blind, placebo-controlled trial in which 225 patients with hATTR amyloidosis with polyneuropathy were randomized to receive placebo (N = 77) or patisiran (N = 148) [Citation22]. Individual patient data from the APOLLO study were used for the indirect comparisons.
The NEURO-TTR study was a Phase 3, multicenter, randomized, double-blind, placebo-controlled trial in which 172 patients with hATTR amyloidosis with polyneuropathy were randomized to receive placebo (N = 60) or inotersen (N = 112) [Citation23]. To ensure all relevant data on inotersen were captured, a literature scan of bibliographic databases and conference repositories was performed to identify published evidence on the efficacy of inotersen for the treatment of hATTR amyloidosis with polyneuropathy (Appendix B: Supplemental Methods and Tables B.1-B.3). Identified reports and conference abstracts were examined in detail and those that reported data on efficacy of inotersen were selected for data extraction [Citation13,Citation15,Citation19,Citation23,Citation26].
This study was conducted according to the principles outlined in the Declaration of Helsinki. Because only previously published data from clinical trials were analyzed, no institutional board review was required.
2.2. Outcomes
The outcomes analyzed were those related to neuropathic disease manifestations and quality of life that were common to both trials and reported in publicly available NEURO-TTR trial documents. The outcomes used were mNIS+7Ionis [Citation27], Norfolk QOL-DN [Citation28], body mass index (BMI), and PND score [Citation8]. BMI was analyzed instead of modified BMI (mBMI), a secondary endpoint in both studies, because mBMI results from NEURO-TTR were not reported for all analyses relevant to the indirect comparisons conducted here [Citation26]. Collectively, these outcomes capture the polyneuropathy manifestations of hATTR amyloidosis and their impact on quality of life.
2.3. Differences between the APOLLO and NEURO-TTR studies
The APOLLO and NEURO-TTR studies had comparable inclusion and exclusion criteria, neuropathy endpoints, and were largely similar in terms of trial design and methodology (Appendix C: Table C.1). However, a few differences existed, namely: the versions of the mNIS+7 used, the follow-up durations, and the distribution of certain patient characteristics at baseline.
2.3.1. Differences in the versions of mNIS+7
In all indirect comparisons presented in this analysis, the mNIS+7 endpoint defined in the NEURO-TTR study (i.e., mNIS+7Ionis) was used to facilitate comparisons of patisiran and inotersen on exactly the same measure. This approach was taken due to the minor differences between mNIS+7Ionis (used in the NEURO-TTR study) and mNIS+7Alnylam (used in the APOLLO study), related to their scoring of sensation and autonomic function (Appendix C: Figure C.1) [Citation27,Citation29]. The mNIS+7Ionis was chosen instead of the mNIS+7Alnylam in these analyses due to data availability. The mNIS+7Alnylam results were unavailable from our review of publications of the NEURO-TTR study. In contrast, the mNIS+7Ionis results from the NEURO-TTR study were publicly available, and mNIS+7Ionis could be precisely calculated for each patient in the APOLLO study using components of the mNIS+7Alnylam and NIS+7 measures collected in the study.
2.3.2. Differences in follow-up duration
The NEURO-TTR study was a 15-month trial while the APOLLO study was an 18-month trial. To facilitate comparisons over the same time frame as in the NEURO-TTR study, 15-month changes on mNIS+7Ionis and Norfolk QOL-DN were estimated for patients in the APOLLO study using a linear mixed effects regression model as described in Appendix A. Interpolation was not needed for BMI as it was measured at approximately 15 months in the APOLLO study.
2.3.3. Differences in baseline characteristics
Baseline differences in certain demographic and disease characteristics were noted between the APOLLO and NEURO-TTR study populations (). As imbalances in these baseline characteristics may contribute to differences in outcomes between the two studies, adjustment is needed prior to conducting indirect comparisons. Therefore, matching-adjusted indirect comparisons (MAIC) [Citation30,Citation31], a method that adjusts for observed imbalances in baseline characteristics, was also used in this study. The MAIC method reweighted patients in the APOLLO study so that, on average, after reweighting, baseline characteristics were the same as in the NEURO-TTR study. Indirect comparisons were then conducted between this reweighted APOLLO study population and the NEURO-TTR study population.
Table 1. Baseline characteristics of APOLLO and NEURO-TTR studies before and after matching.a,b.
2.4. Indirect comparison methods
Indirect comparisons of patisiran versus inotersen were conducted for each outcome by comparing the mean differences for each therapy relative to placebo obtained from the direct comparisons in their respective trials. This was conducted using Bucher and MAIC methods (Appendix A). Differences in mean 15-month changes from baseline on mNIS+7Ionis, Norfolk QOL-DN total score, and BMI were compared between patisiran- and inotersen-treated patients.
The proportions of patients with improvement or no change in neuropathy (defined as a change from baseline in mNIS+7Ionis ≤ 0), and quality of life (defined as a change from baseline in Norfolk QOL-total DN score ≤ 0), were compared between therapies. The proportion with improvement or no change from baseline on PND score (defined as having either a lower or equal PND score as at baseline) was also compared. As the linear modeling approach cannot be applied to categorical outcome measures, comparisons of PND score were only performed for 18-month changes in the APOLLO study vs. 15-month changes in the NEURO-TTR study.
2.5. Primary and secondary analyses
The indirect comparisons were based on two methods of analyzing the APOLLO and NEURO-TTR study data. Primary analyses in this indirect comparison were based on explicit imputation of missing data in the underlying trials using jump to reference imputation for continuous outcomes or nonresponder imputation for binary outcomes. The jump to reference imputation approach applied a placebo-like response for patients with missing data due to treatment discontinuation, based on the assumption that observed treatment effects in these patients would not be maintained after discontinuation. Nonresponder imputation, in this context, assumes that patients with missing data had worsened relative to baseline. These types of analyses are often considered by regulators when assessing efficacy, especially when discontinuation is safety related and more common in the active treatment arm [Citation32]. In the NEURO-TTR study, 22% of inotersen arm patients and 13% of placebo arm patients discontinued treatment, compared to 7% and 28% in the patisiran and placebo arms of the APOLLO study. Given this observed pattern of treatment discontinuation in the NEURO-TTR study, regulators have prioritized results obtained from jump to reference analyses in labeling information for inotersen [Citation15,Citation20,Citation26].
Indirect comparisons based on the results from mixed model repeated measures analyses of observed data that did not explicitly impute missing data were considered secondary analyses. This method assumes that patients who discontinued behaved similarly to those who did not, and that observed treatment effects were maintained after treatment discontinuation. This approach was used in the prespecified efficacy analyses versus placebo conducted in the APOLLO and NEURO-TTR studies. However, indirect comparisons based on the results from this method were considered secondary because this assumption may be less appropriate when discontinuation is safety-related [Citation26].
A sensitivity analysis additionally adjusting for baseline differences in the presence of cardiomyopathy between the APOLLO and NEURO-TTR study populations using the MAIC method was also performed (Appendix A).
The list of analyses conducted is summarized in .
Table 2. Summary of conducted Bucher and MAIC analyses
3. Results
After reweighting, baseline characteristics in the APOLLO study population were balanced with those of the NEURO-TTR study population across both the treatment and placebo arms. The effective sample sizes of the patisiran and placebo arms after reweighting were 90 and 47, respectively. The Bucher analyses described below were conducted between the APOLLO and NEURO-TTR studies without any reweighting. The MAIC analyses described below were conducted between the reweighted APOLLO study population and the NEURO-TTR study population.
3.1. Indirect comparisons
In the primary indirect comparison analyses, mean 15-month changes from baseline in mNIS+7Ionis favored patisiran compared to inotersen in the Bucher analyses (−16.2 points, p = 0.001). The MAIC results similarly favored patisiran (−12.3 points, p = 0.007) (). The proportion of patients experiencing improvement or no change on this measure was significantly higher for patisiran than for inotersen as shown by the risk difference, risk ratios, and odds ratios. The odds of improvement in mNIS+7Ionis were significantly higher for patients treated with patisiran relative to inotersen in both the Bucher (odds ratio = 43.6, p < 0.001) and the MAIC (odds ratio = 193.1, p < 0.001) analyses (Appendix C: Table C.2).
Figure 1. Mean differences between patisiran and inotersen for 15-month change from baseline on mNIS+7Ionis under the Bucher and MAIC analyses
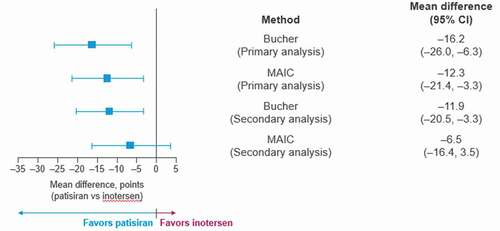
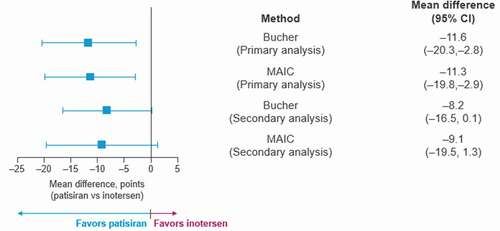
Similarly, mean 15-month changes from baseline on Norfolk QOL-DN also favored patisiran compared to inotersen in both the Bucher (−11.6 points, p = 0.009) and MAIC (−11.3, p = 0.009) analyses (). The proportion of patients experiencing improvement in quality of life relative to baseline was higher for patisiran than for inotersen as shown by the risk difference, risk ratios, and odds ratios. The odds of improvement in Norfolk QOL-DN were significantly higher for patients treated with patisiran relative to inotersen in both the Bucher (odds ratio = 14.7, p < 0.001) and the MAIC (odds ratio = 18.1, p < 0.001) analyses (Appendix C: Table C.2).
Figure 2. Mean differences between patisiran and inotersen for 15-month change from baseline on Norfolk QOL-DN total score under the Bucher and MAIC analyses
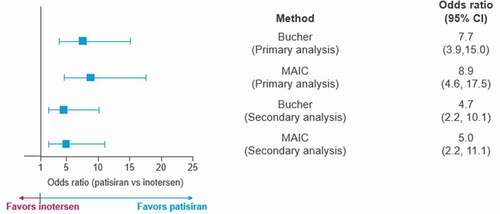
Mean differences in BMI at 15 months relative to baseline were also more favorable for patisiran compared to inotersen in both the Bucher (0.7 kg/m2, p = 0.033) and the MAIC (1.0 kg/m2, p = 0.002) analyses (). The proportion of patients experiencing an improvement or no change in PND score was also significantly higher among those receiving patisiran at 18 months compared to inotersen at 15 months in both the Bucher (odds ratio = 7.7, p < 0.001) and MAIC (odds ratio = 8.9, p < 0.001) analyses ().
Figure 3. Mean differences between patisiran and inotersen on 15-month change in BMI under the Bucher and MAIC analyses
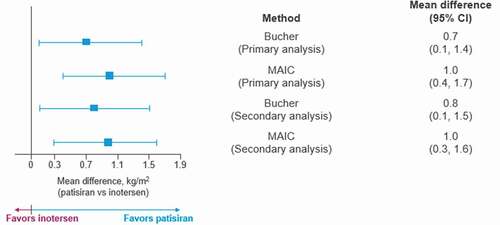
Figure 4. Odds ratios between patisiran and inotersen on PND score under the Bucher and MAIC analyses
Similar results were observed in the secondary and sensitivity analyses (, Appendix C: Tables C.2 and C.3).
4. Discussion
Patisiran and inotersen have both demonstrated efficacy in placebo-controlled trials and represent important advances in the treatment of hATTR amyloidosis with polyneuropathy. Timely and appropriate disease management and treatment selection are imperative for patients with hATTR amyloidosis with polyneuropathy given the multisystem manifestations of the disease and its progressively disabling and life-threatening impact on patients. For these reasons, the choice of therapy is paramount to preventing disability associated with this disease. In the absence of direct, head-to-head trials, indirect comparisons of these therapies, as described here, can be used to reliably assess their comparative treatment effect and provide evidence to inform treatment decision making [Citation33,Citation34].
The results of this study consistently indicated greater treatment effects for patisiran compared to inotersen across all measures analyzed, which encompassed a broad array of polyneuropathy measures. The mNIS+7 is a composite clinical measure of motor strength, sensation, reflexes, nerve conduction, and autonomic function, developed specifically for patients with hATTR amyloidosis [Citation27,Citation29], and has been shown to be correlated with other markers of disease progression such as PND score [Citation27,Citation35]. The Norfolk QOL-DN is a patient-reported measure that captures small and large fiber impairments, autonomic function, symptoms, and impacts on activities of daily living [Citation28]. It is a validated indicator of disease severity in hATTR amyloidosis with polyneuropathy, correlating well with the mNIS+7 and PND score [Citation28,Citation35]. BMI is a measure of nutritional status that reflects unintentional weight loss due to autonomic and gastrointestinal manifestations [Citation36]. Finally, the PND score is a measure of ambulatory ability that is widely used in clinical staging of the disease that captures the degree of functional impairment due to polyneuropathy. Shifts between PND scores reflect clinically meaningful progression of neuropathy [Citation35]. Collectively, these measures capture the multisystem disease manifestations and impact on disease burden that are relevant for patients with hATTR amyloidosis with polyneuropathy.
These findings were robust across multiple analyses and methods of comparison. The benefits for patisiran were more pronounced in the primary analyses, which were based on the assumption of a placebo-like response among patients who discontinue the study regimen in each trial. As discontinuation of the trial regimen was more common for patients receiving inotersen versus placebo in the NEURO-TTR study, results obtained under these assumptions were considered by regulatory agencies to reflect the most appropriate estimate of the efficacy of inotersen [Citation15,Citation26]. Secondary indirect comparison analyses, based on the assumption that the treatment effect for patients who discontinue is the same as those who remained in the study, were also all directionally in favor of patisiran.
The results were also consistent across both Bucher and MAIC analyses, two well-established and valid methods of indirect comparison. Prior meta-analyses comparing the results obtained by direct treatment comparisons or by Bucher indirect comparisons [Citation33] found no statistically significant differences in 93% of trials evaluated [Citation34]. Similarly, MAIC is a widely used approach to adjust for population differences when conducting indirect comparisons [Citation37]. Notably, patients in the NEURO-TTR study had milder severity of neuropathy and quality of life impairment at baseline compared to patients in the APOLLO study. We observed that both the analyses without (Bucher) and with (MAIC) adjustment for baseline differences gave very similar results across the primary and secondary analyses. This suggests that the findings regarding the estimated indirect treatment effects between patisiran and inotersen are robust, regardless of the differences in disease severity between the trial populations. Sensitivity MAIC analyses additionally adjusted for differences in the proportion of patients with cardiomyopathy (as defined by the prespecified cardiac subpopulations in the each respective study) at baseline between the APOLLO and NEURO-TTR studies also indicated a consistently greater treatment effect of patisiran relative to inotersen (Appendix C: Table C.3).
The mean differences between patisiran and inotersen on mNIS+7Ionis obtained from the different analyses in this study range between 6 and 15 points, and are greater than the posited minimal clinically meaningful change of 2 points on the mNIS+7Ionis [Citation23]. This suggests that the benefit of patisiran relative to inotersen on neuropathy is clinically meaningful. The favorable treatment effects of patisiran relative to inotersen observed in these indirect comparisons are also consistent with the average changes observed in their respective trials. In both studies, neuropathy and quality of life rapidly worsened in placebo-treated patients. On average, inotersen-treated patients in the NEURO-TTR study experienced worsening in neuropathy (i.e. change from baseline in mNIS+7Ionis total scores was 5.8 points, 95% confidence intervals [CI]: 1.6, 10.0) and slight declines in quality of life (i.e. change from baseline in Norfolk QOL-DN total scores was 1.0 points, 95% CI: −3.2, 5.2) relative to baseline over 15 months. Conversely, patisiran-treated patients in the APOLLO study on average showed significant improvement on these measures (i.e. change from baseline in mNIS+7Alnylam and Norfolk QOL-DN total scores) relative to baseline over 18 months.
Safety outcomes were not compared in this study due to likely differences between sponsors and studies in the ascertainment, definition, and assessment of adverse events. In the product labeling for inotersen, the two most important warnings and precautions are thrombocytopenia and glomerulonephritis [Citation13,Citation15,Citation19], while infusion-related reactions and reduced serum vitamin A levels and required supplementation are the two most important warnings and precautions in product labeling for patisiran [Citation14,Citation16,Citation20]. In the APOLLO study, 3 (2%) and 7 (9%) patients discontinued patisiran and placebo due to adverse events, respectively. In the NEURO-TTR study, 16 (14%) and 1 (2%) patients discontinued inotersen and placebo due to adverse events. As the higher occurrence of AE-related discontinuation in the inotersen arm could have influenced efficacy in NEURO-TTR, efficacy estimates from analyses explicitly accounting for discontinuation were considered to be the most appropriate measure of treatment effect by some regulatory agencies [Citation26].
This study has several important strengths, including the conduct of the indirect comparisons using both Bucher and MAIC methods and under different analytic assumptions about the impact of missing data in the underlying trials. This study was based on the pivotal trials for patisiran and inotersen and reflects the largest and highest quality sources of data available for the clinical evaluation of these drugs. While the pivotal trials were generally similar in design, endpoints, and analytic approaches, use of the individual patient data from the APOLLO study enabled several additional analytic steps to be taken to account for differences between the two studies that could bias the assessment of the comparative efficacy of patisiran and inotersen. Although the indirect comparison analyses here are based on the results from only one trial of each treatment, the consistency across the different outcomes, analyses and methods used suggests the estimated treatment benefit of patisiran relative to inotersen is unlikely to be explained by chance.
Nonetheless, there are limitations to indirect treatment comparisons. In this study, adjustment variables used in the MAIC analyses were limited to those for which published summary statistics from the inotersen trial are available. While a relatively comprehensive list of variables was included in this analysis, differences in unmeasured factors may exist and can only be accounted for in head-to-head randomized trials directly comparing patisiran and inotersen. While we used statistical modeling to estimate 15-month changes in the APOLLO study for mNIS+7Ionis and Norfolk QOL-DN, this was not possible for PND score. Results on PND score should be interpreted keeping this 3-month difference in trial durations in mind. Additionally, indirect treatment comparisons could not be conducted for the version of the mNIS+7Alnylam included in the APOLLO study because the data were not available in the publications of the NEURO-TTR study.
5. Expert opinion
Patisiran and inotersen are two therapies approved for treatment of hereditary transthyretin-mediated (hATTR) amyloidosis with polyneuropathy, a rapidly progressive disease with a substantial clinical burden. Data from the NEURO-TTR study of inotersen and the APOLLO study of patisiran provide the opportunity to indirectly compare the two treatments in terms of efficacy on validated measures of neuropathy and quality of life (QOL). This indirect comparison study found that patisiran demonstrated greater treatment effects on neuropathy and QOL compared with inotersen in patients with hATTR amyloidosis with polyneuropathy. The wide range of analyses described provides a comprehensive evidence base for patients, physicians, and other stakeholders to evaluate the comparative efficacy of patisiran and inotersen to inform treatment decision-making.
6. Conclusions
The wide range of analyses described here provides a comprehensive evidence base for patients, physicians, and other stakeholders to evaluate the comparative efficacy of patisiran and inotersen to inform treatment decision-making. Overall, the totality of evidence across the variety of indirect comparisons conducted suggests that patisiran has greater treatment effect than inotersen for neuropathy and quality of life outcomes in patients with hATTR amyloidosis with polyneuropathy.
Declaration of interest
P Gorevic received grant support from Alnylam Pharmaceuticals, Ionis, and Pfizer for the conducting of phase 3 studies on patisiran, inotersen, and tafamimdis. H Lin is an employee of Alnylam Pharmaceuticals and holds stocks/options. J Franklin is a former employee of Alnylam Pharmaceuticals and was employed during the development of the manuscript by them. J Franklin also holds stocks and options with them. Furthermore, she is currently employed as a Program Manager at Voyager Therapeutics. J Chen is an employee of Alnylam Pharmaceuticals and holds stocks/options. G Sajeev is an employee of Analysis Group Inc, which has received consulting fees from Alnylam Pharmacetucals. JCH Wang is also an employee of Analysis Group Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One referee declares that they consult for both Alnylam and Akcea and received payment from both companies for participating in advisory boards, given educational talk and research support. Another referee declares receiving speaker’s honoraria from Pfizer Inc. Furthermore, another referee declares both working on both phase 3 trial publications on patisiran and inotersen. Additionally, peer reviewers on this manuscript have received an honorarium from Expert Opinion on Pharmacotherapy for their review work but have no other relevant financial relationships to disclose.
Author contributions
Hollis Lin, Jaclyn Franklin, Jihong Chen, Gautam Sajeev, and Jessie CH Wang were involved in the conception and design. All authors were involved in the analysis, interpretation of data, drafting and revision of the paper. All authors reviewed and approved the final version of the manuscript to be published and all authors agree to be accountable for all aspects of the work.
Data Availability Statement: Due to the sensitive nature of the data used in this study, the dataset will not be made available to other researchers. However, requests from qualified researchers for additional analyses may be sent to Alnylam Pharmaceuticals ([email protected]).
Lin_supplement.docx
Download MS Word (722 KB)Acknowledgments
Medical writing assistance was provided by Shelley A. Batts, PhD, an employee of Analysis Group, Inc. Funding for this service was provided by the sponsor, Alnylam Pharmaceuticals. The authors would like to thank T Brannagan of Columbia University Medical Center for their assistance with research and the analysis and L Obici from the Amyloidosis Research and Treatment Center for their insightful discussion along with the development of the original abstract and poster presentation.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Gertz MA. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;23(suppl 7):S107–S112.
- Vita G, Vita GL, Stancanelli C, et al. Genetic neuromuscular disorders: living the era of a therapeutic revolution. Part 1: peripheral neuropathies. Neurol Sci. 2019;40(4):661–669.
- Ando Y, Coelho T, Berk JL, et al. Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis. 2013;8:31. PubMed PMID: 23425518; PubMed Central PMCID: PMCPMC3584981.
- Hawkins PN, Ando Y, Dispenzeri A, et al. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015 Nov 17;47(8):625–638. .
- Gonzalez-Duarte A. Autonomic involvement in hereditary transthyretin amyloidosis (hATTR amyloidosis). Clin Auton Res. 2019;29(2):245–251.
- Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. 2011;10(12):1086–1097.
- Parman Y, Adams D, Obici L, et al. Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol. 2016;29(Suppl 1):S3.
- Yamamoto S, Wilczek HE, Nowak G, et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant. 2007;7(11):2597–2604. PubMed PMID: 17868062.
- Coutinho P, Martins da Silva A, Lopes Lima J, et al. Forty years of experience with type I amyloid neuropathy. Review of 483 cases. Amyloid and Amyloidosis; 1980, 88–98.
- Berk JL, Lin H, Agarwal S, et al. Impact of hereditary transthyretin-mediated amyloidosis on daily living and work productivity: baseline results from APOLLO. 16th International Symposium on Amyloidosis; 2018 Mar 26–29; Kumamoto, Japan.
- Yarlas A, Gertz MA, Dasgupta NR, et al. Burden of hereditary transthyretin amyloidosis on quality of life. Muscle Nerve. 2019;60(2):169–175.
- European Medicines Agency (EMA). Vyndaqel (tafamidis) 20 mg soft capsules: summary of product characteristics. 2013 [cited 2019 Jul 17]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/vyndaqel
- United States Food and Drug Administration (FDA). Highlights of prescribing information: tegsedi (inotersen). 2018 [cited 2018 Oct 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211172lbl.pdf
- United States Food and Drug Administration (FDA). Highlights of prescribing information: onpattro (patisiran). 2018 [cited 2018 Oct 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210922s000lbl.pdf
- Health Canada. Product monograph and patient information: tegsedi (Inotersen). 2018 [cited 2018 Nov 14]. Available from: https://pdf.hres.ca/dpd_pm/00047651.PDF
- Health Canada. Product monograph including patient medication information, Onpattro (patisiran). [cited 2020 Apr 9]. Available from: https://pdf.hres.ca/dpd_pm/00052462.PDF
- PTC Therapeutics. PTC Therapeutics provides corporate update and highlights pipeline progress at 2020 J.P. Morgan Healthcare conference. [cited 2020 Apr 9]. Available from: http://ir.ptcbio.com/news-releases/news-release-details/ptc-therapeutics-provides-corporate-update-and-highlights
- Alnylam Pharmaceuticals. Alnylam announces approval in Brazil of ONPATTRO® for the treatment of hereditary ATTR amyloidosis with polyneuropathy. 2020 [cited 2020 Apr 9]. Available from: https://investors.alnylam.com/press-release?id=24606
- European Medicines Agency (EMA). Tegsedi (inotersen): summary of product characteristics. 2018 [cited 2018 Sept 27]. Available from: https://www.ema.europa.eu/en/documents/product-information/tegsedi-epar-product-information_en.pdf
- European Medicines Agency (EMA). Onpattro (patisiran): summary of product characteristics. 2018 [cited 2019 May 5]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/onpattro
- Planté-Bordeneuve V, Lin H, Gollob J, et al. An indirect treatment comparison of the efficacy of patisiran and tafamidis for the treatment of hereditary transthyretin-mediated amyloidosis with polyneuropathy. Expert Opin Pharmacother. 2019;20(4):473–481.
- Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21.
- Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31.
- Jansen JP, Trikalinos T, Cappelleri JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC good practice task force report. Value Health. 2014 Mar 1;17(2):157–173. .
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- European Medicines Agency (EMA). Public assessment report for Tegsedi (Inotersen). 2018 [cited 2018 Sept 28]. Available from: https://www.ema.europa.eu/documents/assessment-report/tegsedi-epar-public-assessment-report_en.pdf
- Dyck PJ, Kincaid JC, Dyck PJB, et al. Assessing mNIS+ 7Ionis and international neurologists’ proficiency in a familial amyloidotic polyneuropathy trial. Muscle Nerve. 2017;56(5):901–911.
- Vinik EJ, Vinik AI, Paulson JF, et al. Norfolk QOL-DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2014 Jun 1;19(2):104–114. .
- Suanprasert N, Berk J, Benson M, et al. Retrospective study of a TTR FAP cohort to modify NIS+ 7 for therapeutic trials. J Neurol Sci. 2014;344(1–2):121–128.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- Signorovitch JE, Wu EQ, Andrew PY, et al. Comparative effectiveness without head-to-head trials. Pharmacoeconomics. 2010;28(10):935–945.
- European Medicines Agency (EMA). ICH harmonised guideline E9(R1). Estimands and sensitivity analysis in clinical trials. Step 2b, version dated August 30, 2017. 2017 [cited 2020 Apr 3]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-ich-e9-r1-addendum-estimands-sensitivity-analysis-clinical-trials-guideline-statistical_en.pdf
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Song F, Altman DG, Glenny A-M, et al. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. Br Med J. 2003;326(7387):472.
- Adams D, Suhr OB, Dyck PJ, et al. Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy. BMC Neurol. 2017;17(1):181.
- Carvalho A, Rocha A, Lobato L. Liver transplantation in transthyretin amyloidosis: issues and challenges. Liver Transplant. 2015;21(3):282–292.
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211.
