ABSTRACT
Objective
To evaluate the long-term safety and effectiveness of ipragliflozin in real-world clinical practice in Japanese patients with type 2 diabetes mellitus (T2DM).
Research design and methods
This post-marketing surveillance study (STELLA-LONG TERM) included Japanese patients newly initiated on ipragliflozin between 17 July 2014 and 16 October 2015 (data lock: 30 September 2019). Survey items included demographics, treatments, adverse drug reactions (ADRs), vital signs, and laboratory variables.
Results
Of 11,424 registered patients, safety and efficacy analysis sets comprised of 11,051 and 8,763 patients, respectively. ADRs occurred in 2,129 patients (19.27%) and serious ADRs occurred in 210 patients (1.90%). Renal and urinary disorders (n = 739, 6.69%), particularly polyuria/pollakiuria (n = 612, 5.54%) and volume depletion-events, including dehydration (n = 243, 2.20%), comprised the most common ADRs. Mean (SD) change in hemoglobin A1c (─0.66 [1.25] %), fasting plasma glucose (─28.8 [50.1] mg/dL) and body weight (─3.33 [4.32] kg) from baseline to 36 months were statistically significant (P < 0.001).
Conclusions
The safety profile of long-term ipragliflozin treatment in routine clinical practice is consistent with previously reported interim data at 12 or 24 months and pre-approval clinical trials. Ipragliflozin treatment was also associated with sustained improvements in efficacy parameters for over 3 years.
1. Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a class of oral antihyperglycemic medication with an insulin-independent mechanism of glucose-lowering action. SGLT2 inhibitors suppress glucose reabsorption at renal proximal tubules, thus stimulating glycosuria, which leads to reductions in both fasting and postprandial blood glucose (and a decrease in glycated hemoglobin [HbA1c] level as a result), body weight and blood pressure in patients with type 2 diabetes mellitus (T2DM) [Citation1,Citation2]. In addition, SGLT2 inhibitors provide other benefits for patients with T2DM, including decreased cardiovascular risk, improvement of hepatic function, and renoprotective effects as demonstrated in the EMPA-REG OUTCOME, CANVAS and CREDENCE studies [Citation3–5]. Based on the results of these key trials, the European Society of Cardiology and the European Association for the Study of Diabetes recommend SGLT2 inhibitors as first-line oral medication for specific T2DM patients, such as those with high-risk or established cardiovascular disease and chronic kidney disease [Citation6]. Accordingly, the use of SGLT2 inhibitors has been rapidly increasing both globally [Citation7], as well as in Japan [Citation8].
Of the SGLT2 inhibitors currently available in Japan, ipragliflozin was the first to be approved in January 2014 [Citation9], with numerous clinical trials having previously demonstrated the safety and efficacy of ipragliflozin in Japanese T2DM patients [Citation10–20]. A long-term post-marketing surveillance study (STELLA-LONG TERM [Specified drug use resulTs survEy of ipragLifLozin treAtment in type 2 diabetic patients: LONG-TERM use]), was conducted over a period of 3 years in routine clinical practice and registered more than 11,000 patients. Reports based on interim data covering periods of up to 3 months, 12 months, and 2 years after treatment initiation, including subgroup analyses based on age, body mass index (BMI), and hepatic/renal function, have been published previously [Citation21,Citation22,Citation26]. These previous analyses based on interim data have demonstrated that ipragliflozin is well tolerated, including in the elderly and other age subgroups, and maintains glycemic control and body weight reduction for up to 24 months after treatment initiation. Since T2DM is a progressive disease requiring life-long therapy and concerns remain about the potential adverse events (AEs) of SGLT2 inhibitor therapy [Citation27,Citation28], further evaluation of long-term safety and effectiveness of ipragliflozin would provide valuable information for the treatment of T2DM patients in routine clinical practice. Accordingly, we report here the final, 36-month results of the STELLA-LONG TERM post-marketing surveillance study, which comprehensively evaluates the long-term safety and effectiveness of ipragliflozin in routine clinical practice. This represents the first report to evaluate the effectiveness and safety of an SGLT2 inhibitor over a long-term period of 3 years in Japanese patients with T2DM.
2. Patients and Methods
2.1. Study Design, Patients and Data Collection
This was an observational, multicenter post-marketing surveillance study conducted in Japan over an observation period of 3 years per patient (ClinicalTrials.gov identifier: NCT02479399). Details of the study design, patient selection criteria, and methods have been described previously in a report based on analysis of data at 3 months [Citation21]. In brief, data from Japanese patients with T2DM who were newly initiated on treatment with ipragliflozin during the registration period (17 July 2014 to 16 October 2015) were included; the cutoff date for data inclusion was 30 September 2019. Ipragliflozin 50 mg once daily was administered before or after breakfast in accordance with the approved label with dose increase or decrease permitted also according to the label at the attending physician’s discretion.
This study was conducted in compliance with Japanese Good Post-marketing Study Practice regulations. All medical institutions that agreed to provide data signed a contract with Astellas Pharma Inc. As anonymous data were collected via electronic survey forms from clinical settings, it was not deemed necessary to obtain informed consent.
2.2. Assessments
Details of the survey assessment items were described in the report based on interim data at 3 months [Citation21]. In brief, assessment items covered baseline demographic characteristics (age, sex, height, body weight, BMI, inpatient/outpatient status, duration of diabetes, diabetic complications, presence of urinary tract/genital infection, renal/hepatic function) and medication data (treatment start date, daily dose/administration frequency, treatment period, reason of termination or discontinuation of ipragliflozin, and concomitant medication type and dose).
Safety assessment items were the incidence of AEs, including serious AEs, and adverse drug reactions (ADRs). Serious AEs were defined as those AEs that were life-threatening or resulted in death, permanent or significant disability, inpatient or prolonged hospitalization, or congenital abnormality, as per the physician’s judgment. ADRs were defined as AEs for which a causal relationship to ipragliflozin could not be excluded. The time to onset, details of severity, and outcome of ADRs were also recorded. AEs and ADRs were evaluated and categorized by system organ class (SOC) and preferred term (PT) using MedDRA/J version 22.0. The incidence of three particular AEs, namely malignant tumor, cardiovascular disease and cerebrovascular disease, was evaluated according to the definitions of the J-DOIT3 study [Citation29]. ADRs of special interest included hypoglycemia, genital infection, urinary tract infection, polyuria/pollakiuria, volume depletion-related events (including dehydration), renal disorder, hepatic disorder, fracture, malignant tumor, cardiovascular disease, cerebrovascular disease, skin complications, ketoacidosis and events related to ketone-body increase, and lower limb amputation. Data were also collected on vital signs and laboratory variables as part of the safety assessment, including waist circumference, BMI, heart rate, estimated glomerular filtration rate (eGFR), red blood cell count, hemoglobin, hematocrit, blood urea nitrogen (BUN), serum and urinary albumin, serum and urinary creatinine, serum ketone bodies, fasting C-peptide, white blood cell count, total bilirubin, electrolytes (sodium, chloride, potassium, calcium, phosphorus, and magnesium), and pH, at baseline and during the observation period.
To evaluate the effectiveness of ipragliflozin, assessment items consisted of changes in HbA1c, fasting plasma glucose (FPG) levels, fasting insulin levels, body weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), liver enzymes (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], and gamma-glutamyl transpeptidase), lipid parameters (total cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, non-HDL cholesterol, and triglycerides), and uric acid from the start to the end of treatment with observations at 1, 3, 6, 12, 24 and 36 months after initiation of treatment.
2.3. Statistical Analysis
The rationale for the study period and sample size calculations were described in the previous report based on interim data [Citation21]. In general, categorical variables were represented as n (%) and continuous variables were represented as mean ± standard deviation (SD). Obviously unreliable values were excluded from analyses. One-sample t-tests were performed for evaluations of changes in laboratory variables from baseline to the observation period. Statistical significance was set at two-sided P < 0.05; adjustments for type I error based on multiple hypothesis testing were not performed.
The possibility of a relationship between risk factors, such as patient background and treatment factors, and ADRs of special interest was initially analyzed by univariate analysis stratified by patient background and treatment factors using the chi-square test, Fisher’s exact test, or Cochran–Armitage test depending on the nature (nominal/ordinal) of the categorical variable analyzed. Factors in the univariate analysis which showed P < 0.05 were included into multivariate logistic regression to estimate the odds ratio for incidence of ADRs of special interest with 95% confidence intervals and P values by the Wald chi-squared test. The final models were built using a stepwise procedure with P values of < 0.15 set for entry and staying in the model. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient Disposition
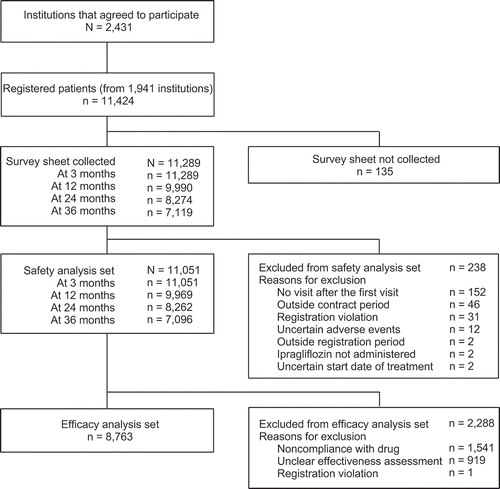
In total, 11,424 patients were registered from 2,431 medical institutions and survey forms were collected for 11,289 patients (). Of these patients, the safety analysis set was comprised of 11,051 patients. The main reasons for exclusion from the safety analysis set were lack of visit after first administration (n = 152), being outside the contract period (n = 46), and registration violation (n = 31). The efficacy analysis set was comprised of data from 8,763 patients derived from the safety analysis set. The main exclusion reasons from the efficacy analysis set were noncompliance with study drug (n = 1,541) and unclear effectiveness assessment (n = 919).
Among the safety analysis set, 4,727 patients (42.8%) discontinued ipragliflozin treatment. The main reasons for discontinuation, of which some patients had multiple reasons, were: completion and discontinuation of treatment during observation period (n = 2,801), no visit to the institution (n = 1,449), occurrence of AEs (n = 830), patient request (except for AEs, n = 771), no change or worsening of diabetes (n = 691), improvement (n = 311), and interruption of ipragliflozin treatment for more than 2 months (n = 11).
3.2. Patient Characteristics
Baseline demographic and clinical characteristics of patients included in the safety analysis set are shown in . Among these patients, the mean ± SD age was 56.9 ± 12.2 years, 60.7% of patients were male, the mean ± SD BMI was 29.08 ± 5.30 kg/m2 and the mean ± SD duration of T2DM was 7.96 ± 6.45 years. Regarding treatment status, 9,115 patients (82.5%) in the safety analysis set were treated with concomitant antidiabetic agents, which mainly included dipeptidyl peptidase-4 (DPP-4) inhibitors (n = 6,427, 58.2%), biguanides (n = 4,977, 45.0%), and sulfonylureas (SU) (n = 3,244, 29.4%) with only 882 patients (8.0%) treated with diuretics ().
Table 1. Patient demographics and characteristics at baseline
Table 2. Treatments used at baseline and during the survey period
3.3. Safety
details the incidences of ADRs classified by SOC in 11,051 patients in the safety analysis set together with reference values from 1,669 patients in pre-approval clinical trials [Citation10,Citation11,Citation12,Citation13,Citation14,Citation15,Citation16,Citation17,Citation18,Citation19]. Overall, ADRs occurred in 2,129 patients (19.27%) with major ADRs (≥1% of patients) consisting of renal and urinary disorders (n = 739, 6.69%), laboratory tests (n = 345, 3.12%), infections and infestations (n = 285, 2.58%), metabolism and nutrition disorders (n = 251, 2.27%), skin and subcutaneous tissue disorders (n = 176, 1.59%), gastrointestinal disorders (n = 139, 1.26%), reproductive system and breast disorders (n = 118, 1.07%), hepatobiliary disorders (n = 115, 1.04%), nervous system disorders (n = 112, 1.01%). Other ADRs occurred in less than 1% of patients.
Table 3. Summary of adverse drug reactions reported during the survey period
Main ADRs classified by PT that occurred in ≥0.1% patients are detailed in Table S1. Of these ADRs, those which occurred in ≥0.4% patients were pollakiuria (n = 470, 4.25%) and polyuria (n = 303, 2.74%), pruritus genital (n = 81, 0.73%), cystitis (n = 77, 0.70%), urinary tract infection (n = 74, 0.67%), dyslipidemia (n = 54, 0.49%), hypertension (n = 53, 0.48%), liver disorder (n = 52, 0.47%), renal impairment (n = 49, 0.44%), blood triglycerides increased (n = 49, 0.44%), hypoglycemia (n = 48, 0.43%), constipation (n = 45, 0.41%), and hepatic function abnormal (n = 45, 0.41%).
Serious ADRs reported during the survey period are shown in Table S2. Overall, serious ADRs occurred in 210 patients (1.90%). Major serious ADRs classified by PT that occurred in ≥0.1% patients were cerebral infarction (n = 18, 0.16%), myocardial infarction (n = 13, 0.12%) and colon cancer (n = 12, 0.11%).
The incidence of ADRs of special interest, for which the range of incidence proportion was 0.00% to 5.54%, are shown in (and classified by SOC and PT in Table S3). Among ADRs of special interest, the most common event was polyuria/pollakiuria (612 patients, 5.54%), followed by volume depletion-related events, including dehydration (243 patients, 2.20%), skin complications (198 patients, 1.79%), renal disorder (191 patients, 1.73%), urinary tract infections (170 patients, 1.54%), genital infection (161 patients, 1.46%), and hepatic disorder (133 patients, 1.20%). Minor ADRs of special interest that occurred in <1% patients were cardiovascular disease events (67 patients, 0.61%), hypoglycemia (57 patients, 0.52%), malignant tumor (51 patients, 0.46%), cerebrovascular disease events (48 patients, 0.43%), ketoacidosis, and events related to ketone-body increase (7 patients, 0.06%), and fractures (4 patients, 0.04%). No lower limb amputation events were reported. The incidence of serious ADRs of special interest, for which the range of incidence proportion was 0.01% to 0.42%, are also shown in (and classified by SOC and PT in Table S3).
Table 4. Adverse drug reactions of special interest
For three specific AEs, malignant tumor, cardiovascular disease and cerebrovascular disease, the incidence rates were calculated according to the definitions of J-DOIT3, and determined to be 4.36, 3.56 and 2.62 per 1,000 person-years, respectively.
The time to onset of ADRs of special interest is shown in Table S4. The cumulative proportion of ADRs that occurred within 30 days after the first administration of ipragliflozin was 31.47% (985 events) whereas more than half of ADRs (57.19%, 1,790 events) occurred within 180 days after the first administration of ipragliflozin. ADRs that totaled more than 5 events after 180 days were colon cancer (13 events, 0.12%), allergic rhinitis (7 events, 0.06%), gastric ulcer (6 events, 0.05%), as well as gastric cancer and tension bladder (5 events, 0.05% each).
ADRs of special interest based on treatment status are shown in Table S5. The proportion was highest for patients with continued treatment status (62.5%), followed by patients with interruption/discontinuation caused by ipragliflozin (37.4%). ADRs leading to study drug interruption/discontinuation reported in more than 50% of patients were skin complications (78.8%), genital infection (61.5%), ketone-body-related events (57.1%) and urinary tract infection (53.5%).
Outcomes of ADRs of special interest are shown in Table S6. Overall, the outcomes of ADRs of special interest were resolved or in remission in more than 80% of events (52.2% resolved, 32.3% in remission). In total, the following 22 ADRs leading to death were observed in 20 patients, with some patients having experienced more than one event contributing to death: pancreatic carcinoma (3 events), myocardial infarction and pneumonia (2 events each), colon cancer, metastases to lymph nodes, pancreatic carcinoma metastatic, lung neoplasm malignant, lung neoplasm, completed suicide, cerebral infarction, acute myocardial infarction, cardiorespiratory arrest, cardiovascular disorder, hypoxia, interstitial lung disease, hepatic cirrhosis, sudden death, and sudden cardiac death (1 event each).
details the risk factors possibly associated with ADRs of special interest according to multivariate logistic regression. Major risk factors with an odds ratio of >2.0 and a p value <0.05 were focused on. Categories with an odds ratio of <0.5 were interpreted as a lower risk compared with the reference group if the p value was <0.05. For any ADRs of special interest, risk factors were moderate renal impairment, absence of concomitant antidiabetic agents throughout the observational period, and increased numbers of concomitant antidiabetic agents at baseline. For hypoglycemia, risk factors were concomitant use of insulin and SU. For genital infection and urinary tract infection, the only risk factor was female sex. Major risk factors for polyuria/pollakiuria were age <65 years, the presence of complications, dose increase (from 25 mg to 50 mg daily) of ipragliflozin during the observation period, and ≥4 concomitant antidiabetic agents at baseline. Risk factors for volume depletion-related events were the presence of complications, moderate renal impairment, moderate hepatic impairment, and concomitant use of insulin and SU. For renal disorder, possible risk factors were mild/moderate renal impairment. Risk factors for malignant tumors were age ≥65 years and a past history of smoking. Risk factors for cardiovascular disease were male sex and concomitant use of insulin and SU, whereas for cerebrovascular disease, risk factors were concomitant use of insulin, SU, diuretics, and agents other than antidiabetic agents and diuretics. For skin complications, risk factors were the presence of complications and ≥4 concomitant antidiabetic agents. Finally, risk factors for hepatic disorder were inpatient treatment status, the presence of complications, mild hepatic impairment and dose increase of ipragliflozin from 50 mg to 100 mg daily.
Table 5. Multivariable regression analyses for factors associated with adverse drug reactions of special interest
Changes in vital signs and laboratory variables among patients in the safety analysis set are shown in Table S7. Throughout all observation periods, statistically significant increases from baseline (P < 0.05) were noted at each observation period for hemoglobin (0.50 ± 1.01 g/dL) and hematocrit (1.98 ± 3.04%), and other parameters (Table S7).
3.4. Effectiveness
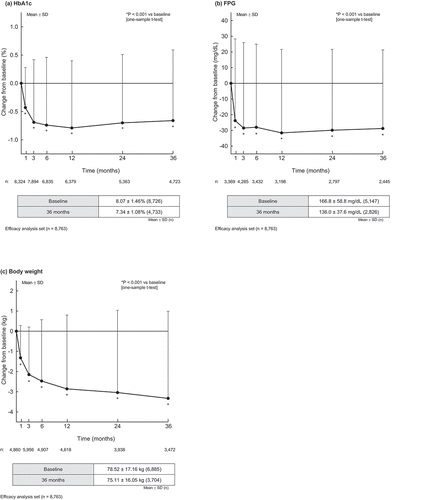
Mean ± SD changes in HbA1c, FPG, and body weight from baseline to 36 months are shown in . These demonstrate a sustained and statistically significant improvement in each parameter at all observation points (P < 0.05 vs baseline). Further, the mean ± SD changes from baseline at 36 months for HbA1c (─0.66 ± 1.25%), FPG (─28.8 ± 50.1 mg/dL) and body weight (─3.33 ± 4.32 kg) were all consistent with sustained improvement across the whole observation period.
Figure 2. Change from baseline in (a) HbA1c, (b) FPG and (c) body weight
The changes in laboratory variables and vital signs during the 3-year observation period are shown in Table S8. Statistically significant decreases were noted throughout all observation periods (P < 0.05 vs baseline) with mean ± SD changes from baseline at 36 months for the following values: fasting insulin (─3.05 ± 8.23 μU/mL), SBP (─4.4 ± 14.7 mmHg), DBP (─2.7 ± 10.1 mmHg), AST (─4.2 ± 15.1 U/L), ALT (─7.6 ± 21.1 U/L), gamma-glutamyl transpeptidase (─12.0 ± 45.6 U/L), LDL cholesterol (─5.5 ± 29.4 mg/dL), triglyceride (─14.4 ± 135.3 mg/dL), uric acid (─0.24 ± 1.01 mg/dL), ALP (─10.8 ± 55.6 U/L) and non-HDL cholesterol (─6.2 ± 30.6 U/L). Further, statistically significant increases from baseline were noted throughout all observation periods for HDL cholesterol (mean ± SD change from baseline at 36 months: 3.2 ± 9.2 mg/dL, P < 0.05).
4. Discussion
These final, 36-month results of the STELLA-LONG TERM study, which represents the largest post-marketing surveillance study of an SGLT2 inhibitor, found no significant increase of ADRs or serious ADRs with long-term ipragliflozin treatment compared with the results based on previously reported interim data at 12 and 24 months [Citation23,Citation25]. Specifically, the overall incidence of ADRs in the present analysis at 36 months was 19.27% compared with 14.6% at 12 months and 17.36% at 24 months with the slight incremental increase potentially related to the greater duration of observation. Importantly, no new safety concerns were observed and, despite the acknowledged difficulties of direct comparisons with trial data, the overall safety profile appears consistent with those of previous clinical trials conducted in Japan [Citation10,Citation11,Citation14,Citation15,Citation17,Citation18] and overseas [Citation30]. Indeed, the incidence of overall ADRs at 36 months in this study was less than that in the pre-approval clinical trials conducted in Japan (32.89%) [Citation10,Citation11,Citation14,Citation15,Citation17,Citation18]; however, the incidence of serious ADRs at a maximum of 52 weeks’ follow-up was 1.90% in this study compared with 0.84% in pre-approval clinical trials. The apparent higher risk of serious ADRs related to malignant tumors, cardiovascular diseases and cerebrovascular diseases in the present analysis compared with previous clinical studies may be considered in light of the difference in observation period (3 years vs 52 weeks) given that these conditions naturally increase in frequency with longer periods of observation. Taking this into account, the incidence of overall and serious ADRs observed in this long-term surveillance study was comparable with previous clinical studies and no new safety issues were found. However, as reported in a previous analysis based on interim data, the mean age of patients at baseline in this study was lower than those of pre-approval clinical trials, which may have also influenced the incidence of ADRs [Citation31].
In terms of ADRs of special interest, polyuria/pollakiuria was the most common ADRs both in this study (5.54%) and in pre-approval clinical trials (10.0%) [Citation10,Citation11,Citation14,Citation15,Citation17,Citation18]. In this study, 38.8% of polyuria/pollakiuria events occurred within 7 days after administration, and most events (89.4%) recovered or were in remission. A relatively high incidence of polyuria/pollakiuria has also been reported in the interim reports of post-marketing surveillance studies of the other SGLT2 inhibitors in Japanese T2DM patients [Citation32,Citation33,Citation34,Citation35]. This ADR may be expected based on the mechanism of action of SGLT2 inhibitors, which have been shown to increase urine volume [Citation36], although the actual change in urine volume was not evaluated in this study.
Volume depletion-related events were also common in the present study, which may be expected as a result of urinary glucose excretion leading to osmotic diuresis, and are likely to be associated with polyuria. In other studies of SGLT2 inhibitors, volume depletion has been shown to be more frequent in elderly patients [Citation24,Citation37]. The associated risks of volume depletion, particularly in the elderly who are more susceptible to postural hypotension effects such as falls [Citation38], highlight the need to consider this ADR in routine clinical practice.
Skin complications were the third most common ADRs associated with ipragliflozin therapy in this study. They tended to occur early in the course of ipragliflozin therapy as previously reported [Citation39], with 38.5% of the events noted within 15 days after initiation of ipragliflozin treatment.
Among particular ADRs of special interest examined in this study, the results of a large, recent meta-analysis using data from over 100 randomized controlled trials of patients with T2DM found no apparent increase in the risk of diabetic ketoacidosis, urinary tract infection and fracture associated with SGLT2 inhibitors [Citation40].
In this study, the incidence rate of particular AEs (malignant tumor, cardiovascular disease and cerebrovascular disease) were evaluated according to definitions used in the J-DOIT3 study. The J-DOIT3 study was an open-label, randomized, parallel-group trial conducted to compare cardiovascular outcomes and mortality between conventional and intensive hypoglycemic, antihypertensive and lipid-lowering therapy in Japanese T2DM patients to help establish effective targets for these risk factors [Citation29]. The incidence per 1,000 person-years (calculated using the median follow-up period of the overall population of 8.5 years) was 12.68 for malignant tumors, 5.09 for cardiovascular disease, and 3.89 for cerebrovascular disease. Although a direct comparison between this study and the J-DOIT3 study is difficult due to differences in certain patient characteristics, such as diabetes duration, BMI, and cholesterol and triglyceride levels, the incidence rate of these events in this study was lower than that in the conventional therapy group of the J-DOIT3 study [Citation29]. These findings suggest that SGLT2 inhibitors do not appear to increase of the risk of these events compared with conventional standard care for T2DM. Due to the aforementioned differences in study design and patient characteristics, and the potential for reporting bias, a direct comparison between this study and the J-DOIT3 study was also difficult when evaluating the incidence of the specific diabetic complications of severe hypoglycemia and diabetic retinopathy as well as renal disorders. However, the incidence of these complications was substantially lower in the present long-term survey than during the intervention period of the J-DOIT3 study in either the intensive or conventional therapy groups [Citation29]. Further, there were no amputations noted in either the present study or the J-DOIT3 study [Citation29].
Various factors (age <65 years, complications, dose increase, concomitant use of multiple antidiabetic drugs, and concomitant use of diuretics) were shown to be risk factors for polyuria/pollakiuria. Elderly patients tend to have lower fluid intake [Citation41] and may also reduce their fluid intake of an evening to help manage nocturia [Citation42], which has the potential to minimize the occurrence of polyuria/pollakiuria compared with younger patients. This study suggested a higher risk of hypoglycemia among patients who also received insulin or SU. A recent report from The Committee on the Proper Use of SGLT2 Inhibitors in Japan highlighted the association between the addition of an SGLT2 inhibitor and severe hypoglycemia in patients receiving insulin, an SU or a rapid-acting insulin secretagogue and suggested that consideration should be given to reducing the dose of agents used in combination with an SGLT2 inhibitor [Citation27]. A higher risk of genital infection and urinary tract infection were shown in female patients. The relatively higher incidences of genital infection and urinary tract infection in woman were also observed in another post-marketing surveillance study of ipragliflozin for elderly patients [Citation43] and in clinical trials of the other SGLT2 inhibitors [Citation44].
In addition to confirming the safety profile, the results of this study demonstrated the long-term effectiveness of ipragliflozin for glycemic control in routine clinical practice. In the present study, the mean change in HbA1c from baseline was ─0.79%, ─0.70% and ─0.66% at 12 months, 24 months and 36 months, respectively. However, the fact that some patients who did not have an improvement in HbA1c discontinued treatment throughout this study and were not considered in this analysis is a limitation that should be acknowledged. A previous summary of clinical trials pointed out that SGLT2 inhibitors appear to show a relatively longer duration of effect on glycemic control (in terms of time to return of HbA1c to baseline at a maximum dose of single oral agent) than other oral antidiabetic drugs, including metformin, SU and DPP-4 inhibitors [Citation45]. Similarly, a recent post hoc analysis suggested a greater durability of glycemic control with the SGLT2 inhibitor dapagliflozin, in patients with T2DM who were inadequately controlled with metformin, compared to that with the DPP-4 inhibitor saxagliptin [Citation46].
Sustained and statistically significant increases in hemoglobin, hematocrit and HDL cholesterol, and decreases in uric acid, LDL cholesterol, triglyceride or non-HDL cholesterol were observed in this study, suggesting that ipragliflozin may reduce cardiovascular risk in Japanese patients, as demonstrated in the global clinical trials of other SGLT2 inhibitors [Citation47]. Previous studies have demonstrated that SGLT2 inhibitors consistently increase hematocrit and decrease uric acid [Citation48,Citation49]. In a post hoc analysis of the EMPA-REG OUTCOME study, changes in hematocrit and hemoglobin were found to mediate 51.8% and 48.9%, respectively, of the effect of empagliflozin vs placebo on the risk of cardiovascular death [Citation50]. Smaller mediation effects (maximum 29.3%) were also observed for uric acid.
Dyslipidemia is one of the well-known complications of T2DM, which is characterized by low HDL cholesterol levels and high levels of triglycerides and LDL cholesterol, and is known to be one of the major risk factors for cardiovascular disease in T2DM [Citation51]. In a preliminary randomized, parallel-group study of Japanese patients with T2DM, patients treated with ipragliflozin experienced a statistically significant reduction from baseline in small dense LDL-C levels and an increase in LDL particle size [Citation52]. In contrast, in a pooled analysis of six randomized, double-blind trials of ipragliflozin, no improvement in LDL-C or non HDL-C levels was found despite improvements in other cardiometabolic risk factors [Citation53]. The results of the present study provide further, real-world, support for improvements in lipid parameters with long-term ipragliflozin therapy.
The proportion of events categorized as interruption/discontinuation among incidents of cardiovascular disease was relatively lower in the present 3-year study report (29.85%) than in previous reports based on interim data of results at 12 months (43.3%) [Citation23] or 24 months (37.5%) [Citation25]. This finding may reflect a change in the usage of SGLT2 inhibitors, including ipragliflozin, in routine clinical practice in light of recent reports related to the clinical benefits of SGLT2 inhibitors for the reduction of cardiovascular risk [Citation4,Citation5,Citation54]. However, it is also possible that patients who experienced an onset of cardiovascular events after long-term use of the study drug may have contributed to a lowered proportion of interruption/discontinuation of therapy given that the characteristics of such patients were not described in this report.
The key strengths of this study were the incorporation of a large sample size and the ability to determine the safety and effectiveness of ipragliflozin in a setting comparable with that used in clinical practice. In contrast, the main limitation of this post-marketing surveillance study is that, being a single-arm, observational study carried out in routine clinical practice, safety and effectiveness may be affected by confounding factors other than ipragliflozin treatment (eg, concomitant antidiabetic drugs). Moreover, as AEs were assessed by physicians and were not confirmed by an independent adjudication committee, possible misclassifications of AEs cannot be ruled out. Although the incidence of cardiovascular events was comparable with pre-approval clinical trials, the lack of a control arm in our survey precludes any definitive assessment being made about cardiovascular outcomes with ipragliflozin therapy, and further study is therefore required. Bias for estimates of possible risk factors may have also remained due to model misspecification or omitted variables in logistic regression. Finally, there was no evaluation of patients after completion or discontinuation of ipragliflozin treatment. As a result, the possible effects of these limitations must be taken into consideration when interpreting these long-term results.
5. Conclusions
The final results of the STELLA-LONG TERM study, which evaluated the safety and effectiveness of ipragliflozin over a period of up to 3 years in more than 11,000 Japanese patients with T2DM demonstrated that ipragliflozin is well tolerated and effective for long-term use in routine clinical practice. Specifically, sustained decreases of HbA1c, FPG and body weight with ipragliflozin were observed for up to 3 years. Further, no new safety concerns were raised, indicating that the safety profile of ipragliflozin was consistent with the known safety profile from previous studies.
Author contributions
Ichiro Nakamura contributed to the study design, study conduct, data collection, data analysis and data interpretation. Hiroshi Maegawa, Kazuyuki Tobe, and Satoshi Uno contributed to the study design, data analysis and data interpretation. All authors contributed to the writing of the manuscript and approved the final draft for submission.
Clinical trial registration
Clinicaltrials.gov identifier: NCT02479399
Declaration of interest
H Maegawa has received lecture fees from Merck Sharp and Dohme (MSD) K.K., Sanofi K.K., Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., Astra Zeneca K.K., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd. and Sumitomo Dainippon Pharma Co. Ltd as well as research support from Astellas Pharma Inc., Astra Zeneca K.K., Nippon Boehringer Ingelheim Co. Ltd., Sunstar Inc., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co. Ltd., Nissan Chemical Corporation and MIKI Corporation. H Maegawa has also received grants from Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Kowa Pharmaceutical Co. Ltd., Taisho Pharma Co. Ltd., Ono Pharmaceutical Co. Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co. Ltd., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Bayer Yakuhin Ltd., Teijin Pharma Limited, Shionogi & Co. Ltd., Novartis Pharma K.K. and Nipro Corporation.
K Tobe received lecture fees from MSD K.K., Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co. Ltd.; grants from Daiichi Sankyo Co. Ltd., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Eli Lilly Japan K.K., Asahi Kasei Pharma Corporation, The Mitsubishi Foundation, and Suntory Global Innovation Center Ltd. Meanwhile, I Nakamura and S Uno are employees of Astellas Pharma Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Data sharing statement
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com.
For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_materials.docx
Download MS Word (95.8 KB)Acknowledgments
The authors thank all the participants in this study. This study was funded by Astellas Pharma Inc. Medical writing support was provided by Atsuko Yamazaki, PhD, and Jordana Campbell of inScience Communications, Springer Healthcare, and was funded by Astellas Pharma Inc.
Supplemental Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e014908.
- Watada H. Current understanding of the effect of sodium–glucose co-transporter-2 inhibitors in Asian patients with diabetes mellitus. Diabetol Int. 2020;11(3):242–244.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New Engl J Med. 2019;380(24):2295–2306.
- Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018;138(5):458–468.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New Engl J Med. 2015;373(22):2117–2128.
- Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2020;41(2):255–323.
- Dennis JM, Henley WE, McGovern AP, et al. Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: a retrospective analysis of primary care data, 2010–2017. Diabetes Obes Metab. 2019;21(7):1576–1584.
- Ito Y, Van Schyndle J, Nishimura T, et al. Characteristics of patients with diabetes initiating sodium glucose co-transporter-2 inhibitors (SGLT2i): real-world results from three administrative databases in Japan. Diabetes Ther. 2019;10(2):549–562.
- Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74(5):611–617.
- Kadokura T, Akiyama N, Kashiwagi A, et al. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106(1):50–56.
- Kashiwagi A, Akiyama N, Shiga T, et al. Efficacy and safety of ipragliflozin as an add-on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo-controlled, double-blind, phase III EMIT study. Diabetol Int. 2015;6(2):125–138.
- Kashiwagi A, Isaka H, Kawano H, et al. Long-term safety, tolerability and efficacy of ipragliflozin in combination with a dipeptidyl peptidase-4 inhibitor in Japanese patients with type 2 diabetes mellitus inadequately controlled with a dipeptidyl peptidase-4 inhibitor alone - DAYLIGHT study. Jpn Pharmacol Ther. 2014;42:941–952.
- Kashiwagi A, Isaka H, Nakahama H, et al. Long-term safety, tolerability and efficacy of ipragliflozin in combination with nateglinide in Japanese patients with type 2 diabetes mellitus inadequately controlled with nateglinide alone - CANDLE study. Jpn Pharmacol Ther. 2014;42:959–975.
- Kashiwagi A, Kazuta K, Goto K, et al. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015;17(3):304–308.
- Kashiwagi A, Kazuta K, Takinami Y, et al. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015;6(1):8–18.
- Kashiwagi A, Kazuta K, Yoshida S, et al. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014;5(4):382–391.
- Kashiwagi A, Shiga T, Akiyama N, et al. Efficacy and safety of ipragliflozin as an add-on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study (the SPOTLIGHT study). Diabetol Int. 2015;6(2):104–116.
- Kashiwagi A, Takahashi H, Ishikawa H, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17(2):152–160.
- Kashiwagi A, Isaka H, Takinami Y, et al. Long-term safety and efficacy of ipragliflozin in combination with an α-glucosidase inhibitor in Japanese patients with type 2 diabetes mellitus inadequately controlled with an α-glucosidase inhibitor alone -AGLOW study. Jpn Pharmacol Ther. 2014;42:923–939.
- Kashiwagi A, Kawano H, Kazuta K, et al. Long-term safety, tolerability and efficacy of ipragliflozin in Japanese patients with type 2 diabetes mellitus -IGNITE study. Jpn Pharmacol Ther. 2015;43:85–100.
- Maegawa H, Tobe K, Tabuchi H, et al. Baseline characteristics and interim (3-month) efficacy and safety data from STELLA-LONG TERM, a long-term post-marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice. Expert Opin Pharmacother. 2016;17(15):1985–1994.
- Tabuchi H, Maegawa H, Tobe K, et al. Effect of ipragliflozin on liver function in Japanese type 2 diabetes mellitus patients: a subgroup analysis of the STELLA-LONG TERM study (3-month interim results). Endocr J. 2019;66(1):31–41.
- Nakamura I, Maegawa H, Tobe K, et al. Safety and effectiveness of ipragliflozin for type 2 diabetes in Japan: 12-month interim results of the STELLA-LONG TERM post-marketing surveillance study. Adv Ther. 2019;36(4):923–949.
- Maegawa H, Tobe K, Nakamura I, et al. Safety and effectiveness of ipragliflozin in elderly versus non-elderly Japanese type 2 diabetes mellitus patients: 12 month interim results of the STELLA-LONG TERM study. Curr Med Res Opin. 2019;35(11):1901–1910.
- Nakamura I, Tobe K, Maegawa H, et al. Safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus: 24‒month interim results of the STELLA-LONG TERM post-marketing surveillance study. Jpn Pharmacol Ther. 2019;47(11):1765–1789.
- Tobe K, Maegawa H, Nakamura I, et al. Safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus stratified by body mass index: a subgroup analysis of 24-month interim results from the STELLA-LONG TERM post-marketing surveillance study. Jpn Pharmacol Ther. 2019;47(11):1791–1805.
- The Committee on the Proper Use of SGLT2 Inhibitors. Recommendations on the proper use of SGLT2 inhibitors. J Diabetes Investig. 2020;11(1):257–261.
- Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. 2020;11(3):165–223.
- Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951–964.
- Kashiwagi A, Shestakova MV, Ito Y, et al. Safety of ipragliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase II/III/IV clinical trials. Diabetes Ther. 2019;10(6):2201–2217.
- Nakamura I, Maegawa H, Tobe K, et al. Safety and efficacy of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice: interim results of the STELLA-LONG TERM post-marketing surveillance study. Expert Opin Pharmacother. 2018;19(3):189–201.
- Inagaki N, Nangaku M, Sakata Y. et al. Safety and efficacy of canagliflozin, an SGLT2 inhibitor, in patients with type 2 diabetes mellitus: an interim analysis of post-marketing surveillance (SAPPHIRE). J New Rem Clin. 2019;68(1):9–37.
- Kaku K, Chin R, Naito Y, et al. Safety and effectiveness of empagliflozin in Japanese patients with type 2 diabetes: interim analysis from a post-marketing surveillance study. Expert Opin Drug Saf. 2020;19(2):211–221.
- Utsunomiya K, Senda M, Kakiuchi S, et al. Safety and efficacy of tofogliflozin in Japanese patients with type 2 diabetes mellitus in real-world clinical practice: results of 3-month interim analysis of a long-term post-marketing surveillance study (J-STEP/LT). J Diabetes Invest. 2019;10(5):1272–1283.
- Yoshida S, Kim H, Takumi Y. Evaluation of safety and efficacy of dapagliflozin in patients with type 2 diabetes: an interim report of a special drug use-results survey for long-term use. Jpn Pharmacol Ther. 2017;45(5):737–762.
- Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20(3):629.
- Kohler S, Zeller C, Iliev H, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. 2017;34(7):1707–1726.
- Mol A, Bui Hoang PTS, Sharmin S, et al. Orthostatic hypotension and falls in older adults: a systematic review and meta-analysis. J Am Med Dir Assoc. 2019;20(5):589–597.e5.
- Yabe D, Nishikino R, Kaneko M, et al. Short-term impacts of sodium/glucose co-transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf. 2015;14(6):795–800.
- Donnan JR, Grandy CA, Chibrikov E, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e022577.
- Schols JM, De Groot CP, van der Cammen TJ, et al. Preventing and treating dehydration in the elderly during periods of illness and warm weather. J Nutr Health Aging. 2009;13(2):150–157.
- Asplund R. Nocturia, nocturnal polyuria, and sleep quality in the elderly. J Psychosom Res. 2004;56(5):517–525.
- Yokote K, Terauchi Y, Nakamura I, et al. Real-world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA-ELDER): final results of a post-marketing surveillance study. Expert Opin Pharmacother. 2016;17(15):1995–2003.
- Geerlings S, Fonseca V, Castro-Diaz D, et al. Genital and urinary tract infections in diabetes: impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103(3):373–381.
- Cherukuri L, Smith MS, Tayek JA. The durability of oral diabetic medications: time to A1c baseline and a review of common oral medications used by the primary care provider. Endocrinol Diabetes Metab J. 2018;2(3):1-4.
- Bailey CJ, Del Prato S, Wei C, et al. Durability of glycaemic control with dapagliflozin, an SGLT2 inhibitor, compared with saxagliptin, a DPP4 inhibitor, in patients with inadequately controlled type 2 diabetes. Diabetes Obes Metab. 2019;21(11):2564–2569.
- Tanaka A, Node K. Promising roles of sodium–glucose cotransporter 2 inhibitors in heart failure prevention and treatment. Diabetol Int. 2020;11(3):252–260.
- Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019;139(17):1985–1987.
- Zhao Y, Xu L, Tian D, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(2):458–462.
- Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356–363.
- Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(3):150–159.
- Bando Y, Tohyama H, Aoki K, et al. Ipragliflozin lowers small, dense low-density lipoprotein cholesterol levels in Japanese patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2016;6:1–7.
- Kashiwagi A, Sakatani T, Nakamura I, et al. Improved cardiometabolic risk factors in Japanese patients with type 2 diabetes treated with ipragliflozin: a pooled analysis of six randomized, placebo-controlled trials. Endocr J. 2018;65(7):693–705.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New Engl J Med. 2018;380(4):347–357.