1. Introduction
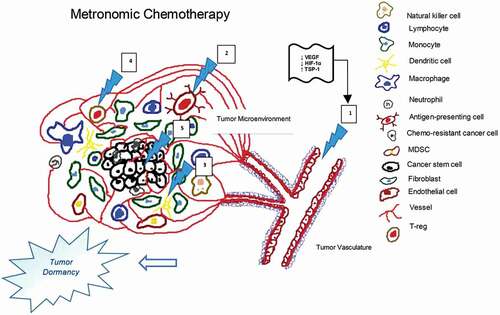
Conventional chemotherapy (CT) is administered at maximum tolerated doses (MTDs) required to attain tumoristatic/tumoricidal effects. Unfortunately, it requires lengthy drug-free breaks due to myelosuppression, which allows time for tumor-bed recolonization by chemo-resistant clones, leading to treatment failure. Metronomic chemotherapy (MTC), on the other hand, is the more frequent administration of chemotherapeutic drugs at less toxic dosages (below MTD) over specific intervals, minimally requiring treatment interruption [Citation1]. Easy access, remarkable tolerability and cost-effectiveness as well as notable activity in resistant tumors are all features that rendered MTC an intriguing research topic in oncology, including gastrointestinal cancers (GI) [Citation2]. MTC is tested in subsequent therapy settings, as maintenance therapy, or in upfront settings in frail CT-ineligible patients. It has a wide spectrum of anticancer effects including anti-angiogenesis, cancer stem-cell reduction, and cellular immune-modulation [Citation2] (). Its direct and indirect effects on tumor cells, immune cells and tumor microenvironment via calibration of circulating regulators such as cytokines, growth factors and angiogenic proteins may explain MTC activity in later lines of resistant tumors [Citation2].
In this editorial, we discuss relevant clinical data of MTC used in variable subtypes of gastrointestinal cancers.
2. MTC by site of primary disease
2.1. Colorectal cancer (CRC)
Capecitabine (Cap), tegafur-uracil (UFUR), doxifluridine (5′-DFUR), and S-1 (tegafur/gimeracil/oteracil) are all oral forms of fluoropyrimidines [Citation3]. Fluoropyrimidines are considered to be the backbone of chemotherapeutic regimens in CRC [Citation3]. Metronomic dosing of these drugs, most commonly Cap (mCap), was evaluated in several trials.
In the phase-III Nordic ACT2 trial, KRAS-mutated mCRC patients with no progression >18 weeks of 1st-line treatment were randomized to receive bevacizumab (BVZ) every 3 weeks or mCap (500 mg bid) in metronomic dosing [Citation4]. The outcomes were relatively similar. 3-month progression-free survival (PFS) rates reached 75% and 66.7% (p = 0.58), and median overall survival (mOS) reached 26.4 and 28 months (p = 0.13) in the BVZ and mCap groups respectively [Citation4]. This established non-inferiority of mCap as maintenance therapy.
In CAIRO-3, 558 mCRC patients without disease progression >6 cycles of first-line CAPOX-BVZ were randomized to receive mCap (625 mg/m2 bid) continuously plus BVZ 7.5 mg/Kg every 3 weeks versus observation [Citation5]. The experimental arm showed higher median PFS1 (mPFS1) and PFS2 (mPFS2) (8.5 vs 4.1 months, p < 0.0001 & 11.7 vs 8.5 months, p < 0.0001 respectively) [Citation5]. A trend toward improved mOS (25.9 months vs 22.4, p = 0.06) was noted without quality of life compromise [Citation5], indicating mCap effectiveness and tolerability in combination with other agents. On the other hand, in MOMA trial (phase-II), the intensification of maintenance MTC with the addition of cyclophosphamide to mCap and BVZ following induction FOLFOXIRI-BVZ showed no advantage [Citation6].
In another retrospective study, UFUR (400 mg bid) maintenance following adjuvant FOLOFOX for stage-III CRC improved 5-year OS rates over observation (86.8% vs 68.5%, p = 0.01 respectively). Patients receiving >5 months of treatment showed the greatest benefit with 5-year OS rate reaching 90.3% [Citation7]. This once more proved MTC efficacy as maintenance therapy.
Metronomic S-1 (mS-1) (80 mg/m2/d on d3-7,10–14,17-21) in combination with weekly intravenous irinotecan (CPT11) (60 mg/m2) was studied as first-line treatment of 45 mCRC patients [Citation8]. The response rate (RR) was 48.9%, mPFS 8.1 months, and mOS 20.9 months. The regimen was well tolerated with mild toxicities [Citation8]. In another study, 5′-DFUR (800 mg/m2/d, 5 days a week) and CPT11 (40 mg/m2 weekly for 3 of 4 weeks) resulted in RR of 36%, mPFS of 187 days, and mOS of 452 days in 45 mCRC patients [Citation9]. In a third study, metronomic CPT11 continuously infused daily for 3 weeks out of 4 (escalated doses in 3 groups: 1.4 mg m−2 day−1/DI = 9.8 mg m−2 week−1; 2.8 mg m−2 day−1/DI = 19.6 mg m−2 week−1; 4.2 mg m−2 day−1/DI = 29.4 mg m−2 week−1) seemed to be well-tolerated and achieved disease control in 20% of mCRC patients refractory to chemotherapy including irinotecan [Citation10]. This indicated potential clinical activity of MTC in resistant tumors despite significantly low therapy-dosing.
2.2. Pancreatic cancer (PC)
In one study, induction S-1 plus metronomic-dose cisplatin (4 mg/m2 on d1-5,8–12,15-19,22–26/28d cycles) followed by S1 maintenance resulted in a RR of 17%, and a disease control-rate (DCR) of 90% (27/30), with no grade 4 toxicities [Citation11]. It proved to be a very tolerable, less toxic regimen in comparison to others in literature [Citation11].
The combination of low-dose weekly nab-paclitaxel (60 mg/m2) and oxaliplatin (50 mg/m2), plus continuous infusion 5FU (180 mg/m2/d, d1–14/28–35d cycle with BVZ (5 mg/m2 d1,15) was retrospectively studied in 65 patients with advanced PC [Citation12]. The regimen proved to be effective and safe with a RR of 49% and a DCR of 81%. mOS was impressive at 19 months (82% living >1 year) [Citation12].
Furthermore, mCap (1500 mg/d) studied in 22 pretreated patients with pancreato-biliary cancer resulted in 33% SD, with higher survival rates in fit patients [Citation13].
All these studies present MTC as a possible tolerable therapeutic option in PC.
2.3. Gastro-esophageal cancer (GEC)
A retrospective series of 47 patients, 25 with progressive GEC receiving mCap (1500 mg/d continuously), proved treatment safety with PR achieved in 1 patient and SD in 6 patients [Citation13]. In another prospective study, mCap (1000 mg/d, d1–28/35) was evaluated in 45 elderly pretreated GEC patients [Citation14]. The RR was 20.9%, the median time to progression (mTTP) was 3.6 months and mOS was 7.6 months [Citation14]. The toxicity profile was acceptable with <10% grade 3 toxicities and no grade 4 toxicities [Citation14].
Another study with metronomic oral etoposide (50 mg/m2) and intravenous 5FU (200 mg/m2) d1-14/28-d cycles in 28 GEC patients resulted in an overall RR of 50%, with an mTTP of 4.5 months and mOS of 9.5 months [Citation15].
All these studies show efficacy and safety of MTC in GEC.
2.4. Hepatocellular carcinoma (HCC)
In one phase-II trial, two cohorts of 59 CT-naïve and 31 pretreated HCC patients received mCap at a dose of 500 mg/m2 bid [Citation16]. PFS reached 6 and 3.3 months, whereas OS was of 14.5 and 9.8 months respectively (16). In another retrospective study, patients failing sorafenib received best supportive care (BSC) (n = 112) or mCap (500 mg bid) (n = 117). In the mCap arm, both median post-sorafenib survival (PSS) and OS from sorafenib initiation were better than BSC (9.9 vs 5.8 months, p = 0.001; and 17.5 vs 12 months, p = 0.034, respectively) [Citation17]. These results were endorsed in a multicentric study reporting better mOS for patients on mCap (500 mg bid) vs BSC after sorafenib discontinuation: 12 vs 9 months; HR 0.45 (p = 0.013) [Citation18].
Thereby, mCap can be a safe and efficacious therapeutic option in HCC patients failing 1st-line sorafenib [Citation18].
In an Egyptian randomized-controlled trial of 40 HCC patients, mCap showed a favorable toxicity profile over doxorubicin in the first-line setting, without a significant difference in TTP (3.4 vs 3.1 months) nor OS (10.2 vs 9.6 months) [Citation19], indicating the possibility of replacing doxorubicin with mCap. Nevertheless, mCap-BVZ combination in the 1st-line setting had limited antitumoral activity (RR 9%, DCR 52%), and efficacy (PFS 2.7 months, OS 5.9 months) [Citation20], decreasing the enthusiasm for its use in 1st-line setting.
In one study, the use of metronomic UFUR in combination with sorafenib in 53 HCC patients showed some antitumoral activity (RR 8%, DCR 57%), with a mPFS of 3.7 months, a mOS of 7.4 months, and a similar toxicity profile versus sorafenib alone [Citation21]. Thalidomide-metronomic UFUR combination was also tested, resulting in a RR of 9%, a DCR of 33%, mPFS of 1.9 months, and mOS of 4.6 months [Citation22].
Therefore, in HCC, MTC is well tolerated and can be effective alone or in combination; however, its activity remains rather limited.
2.5. Bile duct cancer
In a phase-I trial, 6 patients failing 2nd-line CT received weekly low-dose paclitaxel (40 or 50 mg/m2). DCR was 83.3% and mOS from the third-line treatment was 9 months [Citation23], providing a possible treatment choice in this poor-prognosis category of patients.
2.6. Neuroendocrine neoplasms (NEN)
In a study combining metronomic continuous-infusion 5FU (200 mg/m2/day) for ≥6 months with long-acting-release (LAR) octreotide in 29 untreated well-differentiated NEN patients, radiologic RR was 24%, DCR was 93%, biochemical RR was 48% and symptomatic RR was 60%. Therapy was well tolerated [Citation24]. Another phase-II trial studied the XELBEVOCT regimen (mCap 1000 mg x2/d plus biweekly BVZ and octreotide) in 45 patients (32 were CT-naïve). The treatment was well tolerated. Radiologic RR was 17.8% and DCR was 82%, with better response in tumors of pancreatic origin. Biochemical and symptomatic responses were observed in 52.9% and 82.3% of cases respectively [Citation25].
These studies provide insight that even in NEN, MTC can have a role, and is well tolerated.
3. Expert opinion
MTC has proven itself a valid therapeutic option with impressive biologic rationales, good tolerability, and potential clinical benefits. However, available data regarding MTC use in GI malignancies are insufficient to undoubtedly incorporate it in treatment paradigms. Several pitfalls regarding evidence quality exist. Most published data are based on small samples lacking randomization, with the highest-quality evidence coming from few phase-III trials. Furthermore, studied populations are quite heterogeneous, undermining the possibility of generalizable clinical knowledge. Appraisal of MTC in the later lines of treatment by which cancer cells acquired resistance to several previously utilized antineoplastic agents would plunge the likelihood of success of MTC. Besides, multiple questions arise regarding dosing-schedules and variable definitions of MTC regimens. This lack of a unified MTC definition entails the necessity for more pharmacokinetic and pharmacodynamic studies properly defining the low active range of drug concentrations over specific time-intervals that do not increase toxicity nor compromise the quality of life. Some studies have attempted the use of mathematical calculations to establish optimal metronomic dosing; yet, further validation is still required [Citation26].
For mCRC, the role of mCap ± BVZ has shown encouraging results in palliative settings and maintenance therapy for 1st-line responders [Citation27]. In HCC, mCap improved OS compared to BSC beyond sorafenib, with better results in good performance patients (18). Even in PC, GEC and NEN, MTC remains to be a valid therapeutic option [Citation28]. Due to its low toxicity, MTC could be particularly helpful for the elderly and frail [Citation29], accounting for a significant proportion of patients with GI malignancies who are MTD-CT-ineligible. Finally, MTC is a cost-effective therapeutic alternative [Citation30], specifically in low-income countries, given its low cost, easy-access, and less need for infrastructure compared to MTD CT (2).
Efforts should focus on conducting large prospective trials () [Citation31] (ideally phase-III trials) incorporating MTC alone or in combination with synergetic drugs, to attain high-quality evidence supporting its use, specifically in earlier treatment settings [Citation32]. It is important to identify patient-subpopulations for whom MTC would be best and establish the ideal predictive biomarkers that correlate with clinical outcomes [Citation33]. Broader awareness of the potential of MTC should be acknowledged, with more investigations implemented in pursuit of the integration of MTC in cancer treatment algorithms.
Table 1. Active clinical trials with metronomic CT in GI malignancies
Declaration of interest
O Abdel-Rahman has served on an advisory board for Eisai, Eli Lilly and Company and Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect nature reviews. Clin Oncol. 2016;13:659–673.
- Simsek C, Esin E, Yalcin S. Metronomic chemotherapy: a systematic review of the literature and clinical experience J oncology 2019 Mar 20 2019. 2019; 1–31
- García-Alfonso P, Muñoz Martín AJ, Ortega Morán L, et al. Oral drugs in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2021;13:17588359211009001.
- Hagman H, Frödin J, Å B, et al. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: the nordic ACT2 trial. Ann Oncol. 2016 Jan;27(1):140–147.
- Simkens LHJ, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the dutch colorectal cancer group. Lancet. 2015;385(9980):1843–1852.
- Falcone A, Cremolini C, Loupakis F, et al. FOLFOXIRI plus bevacizumab (bev) followed by maintenance with bev alone or bev plus metronomic chemotherapy (metroCT) in metastatic colorectal cancer (mCRC): the phase II randomized MOMA trial. Ann Oncol. 2016 Oct;27(27):vi560.
- Huang W, Ho C, Lee C, et al. Oral tegafur-uracil as metronomic therapy following intravenous FOLFOX for stage III colon cancer. PloS One. 2017;12(3):e0174280.
- Yutaka O, Takaho T, Yoshito A, et al. Multicenter phase II study of a new effective S-1 and irinotecan combination schedule in patients with unresectable metastatic or recurrent colorectal cancer clinical medicine insights. Oncology 2013 Dec 1;2013(7):21–30.
- Yutaka O, Teruo S, Shinjiro M, et al. Significance of thymidine phosphorylase in metronomic chemotherapy using CPT-11 and doxifluridine for advanced colorectal carcinoma. Anticancer Res. 2007 Jul 1;27(4C):2605–2611.
- Allegrini G, Falcone A, Fioravanti A, et al. A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients. Br J Cancer. 2008 Apr 22;98(8):1312–1319.
- Shinomi I, Masaji T, Manabu K, et al. Phase 2 trial of oral S-1 combined with low-dose cisplatin for unresectable advanced pancreatic cancer. Anticancer Res. 2008 Jul 1;28(4C):2373–2377.
- Isacoff WH, Reber HA, Bedford R, et al. Low-dose continuous 5-fluorouracil combined with leucovorin, nab-paclitaxel, oxaliplatin, and bevacizumab for patients with advanced pancreatic cancer: a retrospective analysis. Targ Oncol. 2018 Aug;13(4):461–468.
- Roberto M, Romiti A, Onesti CE, et al. A metronomic schedule as salvage chemotherapy for upper gastrointestinal tract cancer. Anticancer Drugs. 2016 Feb;27(2):106–111.
- He S, He S, Shen J, et al. Capecitabine “metronomic” chemotherapy for palliative treatment of elderly patients with advanced gastric cancer after fluoropyrimidine-based chemotherapy. Med Oncol. 2012 Mar;29(1):100–106.
- Colleoni M, Vicario G, Graiff C, et al. Intermittent continuous infusion of fluorouracil, low-dose oral leucovorin and oral etoposide in advanced gastric cancer. Oncol Rep. 1996 Nov;3(6):1169–1172.
- Brandi G, Rosa F, Agostini V, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist J. 2013 Nov 13,;18(12):1256–1257.
- Trevisani F, Brandi G, Garuti F, et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol. 2018 Feb;144(2):403–414.
- Casadei Gardini A, Foca F, Scartozzi M, et al. Metronomic capecitabine versus best supportive care as second-line treatment in hepatocellular carcinoma: a retrospective study. Sci Rep. 2017 Feb 13;7(1):42499.
- Abd Elatti Khedr G, Elzawawy S, Gowil A, et al. Metronomic capecitabine versus doxorubicin in advanced hepatocellular carcinoma. Korean Clinical Oncology. 2016;12(1):32–40.
- Hsu C, Yang T, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010 Feb 16;102(6):981–986.
- Hsu C, Shen Y, Lin Z, et al. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53(1):126–131.
- Shao Y, Lin Z, Hsu C, et al. Efficacy, safety, and potential biomarkers of thalidomide plus metronomic chemotherapy for advanced hepatocellular carcinoma. Oncology. 2012 Feb;82(1):59–66.
- Tajima H, Ohta T, Shinbashi H, et al. Phase I study of weekly palliative chemotherapy with low-dose third-line paclitaxel for biliary tract cancer. Mol Clin Oncol. 2017 May;6(5):753–757.
- Brizzi MP, Berruti A, Ferrero A, et al. Continuous 5-fluorouracil infusion plus long acting octreotide in advanced well-differentiated neuroendocrine carcinomas A phase II trial of the piemonte oncology network. BMC Cancer. 2009 Nov 3;9(1):388.
- Berruti A, Fazio N, Ferrero A, et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: the xelbevoct study. BMC Cancer. 2014 Mar 14;14(1):184.
- Ciccolini J, Barbolosi D, Meille C et al. Pharmacokinetics and pharmacodynamics-based mathematical modeling identifies an optimal protocol for metronomic chemotherapy. Cancer Res. 2017. September;7717:4723–4733. 17. .
- Geng R, Wang G, Qiu L, et al. Metronomic capecitabine as maintenance treatment after first line induction with XELOX for metastatic colorectal cancer patients. Medicine (Baltimore). 2020;99(51):e23719.
- Cham K, Baker J, Chang C, et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer. 2010;103(1):52–60.
- Perez BL, Trivedi H, Tandon M, et al. Metronomic chemotherapy for elderly patients with advanced gastric cancer: a potentially feasible alternative to therapeutic nihilism. Am J Gastroenterol. 2015;110:S504.
- Chubenko V, Zagorskaya L, Lukyanchikova V, et al. Long-term survival rates in cancer patients achieved with metronomic chemotherapy (cyclophosphamide and methotrexate). J Clin Oncol. 2020;38(15_suppl):e15519.
- ClinicalTrials.Gov [Internet].; cited Last accessed 10 Dec 2020]. Available from: https://www.clinicaltrials.gov/.
- Kwak G, Jo SD, Kim D, et al. Synergistic antitumor effects of combination treatment with metronomic doxorubicin and VEGF-targeting RNAi nanoparticles. J Control Release. 2017;267:203–213.
- Valenzuela P, Parra K, Oaxaca D, et al. Abstract 784: pharmacodynamic biomarkers in metronomic chemotherapy: multiplex cytokine measurements in gastrointestinal cancer patients. Cancer Res. 2017;77:784.