1. Introduction
Tuberculosis (TB) is a major public health problem, particularly in developing countries [Citation1]. It was only in 2020 that COVID-19 displaced TB as the topmost infectious disease as the cause of mortality globally [Citation2]. An estimated 10 million people suffered from active tuberculosis in 2019, with around 1.4 million deaths due to the disease [Citation2,Citation3]. While developing nations carry the bulk of the disease burden, the problem now extends to developed countries due to global migration and immunosuppression associated with other diseases and therapies [Citation1].
Extrapulmonary TB accounts for 15% of the total TB cases [Citation3,Citation4]. The incidence of Genitourinary TB (GUTB) is variable and may account for 5–30% cases of extrapulmonary TB [Citation4]. Despite this high prevalence of GUTB, male genital TB that includes involvement of epididymis, testis, vas deferens, seminal vesicle, and prostate remains poorly described. Male genital involvement may occur through hematogenous or contiguous spread, sexual transmission, or in patients of non-muscle invasive bladder cancer receiving intravesical BCG.
2. Diagnosis
2.1. Clinical
Clinical manifestations of genital tuberculosis are usually localized to the involved organ. Prostatic involvement may result in lower urinary tract symptoms, hematuria, or hematospermia. Men with epididymo-orchitis may develop a scrotal mass or abscess. Penile disease may present with ulceration of the glans or skin. Examination findings consistent with genital TB include a hard, enlarged epididymis, thickened and beaded vas deferens, and a nodular prostate. Spontaneous fistulae may also be a delayed presenting feature in certain cases [Citation5,Citation6]. An indolent course, poor response to the conventional antibiotic therapy, and a high clinical suspicion suggest genital TB over other common bacterial genital infections.
Genital TB is often misdiagnosed, and patients are treated for other conditions such as bacterial epididymo-orchitis, testicular tumor, penile carcinoma, or benign enlargement of the prostate before the correct diagnosis is made [Citation6]. This results in a delay in treatment and consequent sequelae including abscesses, urethral strictures, fistulae, and infertility.
2.2. Bacteriological
Diagnosis of TB through bacterial culture is difficult as the organism is slow growing and requires rigorous growth environment. In addition, GUTB is paucibacillary and the detection of mycobacteria in biological samples is difficult [Citation7]. Nucleic amplification tests such as polymerase chain reaction (PCR) are increasingly used with sensitivity and specificity of 95% and 90%, respectively. The disadvantage of PCR is its inability to differentiate between active and latent infection and thus the need of therapy in equivocal cases. The recent cassette-based GeneXpert® provides results in 2 hours and identifies multidrug-resistant (MDR) TB by identifying rifampicin-resistant strains. However, in case of genital TB, urine alone may not be a good source of identification and hence semen or expressed prostatic secretion are needed as samples.
2.3. Composite standard
The diagnosis is often based on a composite standard and a high index of clinical suspicion. Supportive evidence in the form of elevated erythrocyte sedimentation rate (ESR), skin hypersensitivity (Mantoux) test, past exposure to TB, and radiological signs can contribute to the diagnosis. Hypoechoic areas on transrectal ultrasound and irregularity on peripheral zone of the prostate may be seen in prostatic tuberculosis. Testicular lesions may appear as heteroechoic mass lesions on ultrasound or diffusely increased vascularity in early phases of inflammation. CT scan may reveal calcifications, necrosis, and caseation. Diffuse, radiating, streaky areas of low signal intensity in the prostate (the watermelon skin sign) on T2-weighted MRI images may be specific for tuberculosis prostatitis [Citation6,Citation7]. All patients with suspected or confirmed male genital TB should be tested for human immunodeficiency virus (HIV). However, elevated ESR is a nonspecific test and the Mantoux test is not useful in high-burden settings. Both tests may also not be useful in HIV patients.
3. Pharmacotherapy for genital TB
Anti-tubercular treatment (ATT) for genital TB is same as that used for pulmonary and other extrapulmonary TB. It includes an intensive phase of four-drug regimen including isoniazid (H), Rifampicin (R), Pyrazinamide (Z), and ethambutol (E). This intensive phase is followed by a continuation phase with H and R for 4 months daily. Alternatively, an alternate-day regimen includes (2(HZRZE)3/4 (HR)3) under the directly observed treatment short course (DOTS) regime [Citation5].
3.1. HIV and other sexually transmitted infections co-infection
Infection with HIV is one of the primary drivers for reactivation of latent TB. ATT in TB/HIV co-infection is usually prolonged beyond the usual 6 months to about 9–12 months [Citation8]. In addition, the use of anti-retroviral therapy adds to the side effects including hepatotoxicity and requires drug and dosage modification. Other sexually transmitted infections and male accessory glandinfections can mimic genital TB and hence the diagnosis may be missed in such cases.
3.2. Misuse of antibiotics
A misdiagnosis of infective epididymo-orchitis or prostatitis is at times treated with a prolonged course of antibiotics. Treatment of unrelated infections with antibiotics that are also used in the treatment of TB (Linezolid, Clindamycin, etc) is common, further complicating the clinical picture and response assessment [Citation9]. The use of fluoroquinolones (FQs) can lead to the suppression of TB in some cases and can also lead to the development of drug-resistant organisms [Citation9]. Thus, judicious use of FQs and second-line antibiotics should be done in cases of suspected genital TB.
3.3. Drug-resistant TB
MDR-TB is defined as strains resistant to H and R, while extended drug-resistant TB is defined as MDR TB strains that are further resistant to FQ and second-line injectable drugs [Citation10]. Both forms of TB require detailed workup and drug testing and are treated in a similar manner irrespective of the site of involvement.
3.4. Difficulty in drug administration and compliance
The DOTS program introduced by the WHO in 1994 is the most commonly used treatment strategy for TB [Citation11]. Despite extensive efforts, compliance with ATT and the penetration of the DOTS program in African and Asian countries are challenging with drop-out rates exceeding 45% [Citation9]. In contrast with pulmonary TB, genital TB has been poorly studied for duration of drug therapy and adherence rates may be even poorer. This increases the risk of drug resistance and disease persistence.
3.5. Intermittent or daily dosing
Most guidelines recommend 6 months of treatment for genital TB [Citation12]. The intermittent dosing schedule was incorporated in the DOTS program to allow for better patient compliance and to balance the side effects, efficacy, and cost-effectiveness [Citation12]. However, many clinicians and institutions preferred daily dosing of combination therapy, especially for GUTB, for better eradication rates of the tubercle bacilli [Citation12]. The duration of treatment and preferred dosing schedule are unclear for genital TB due to unavailability of robust randomized trials. The proposed 6-month duration of therapy is not uniformly followed and longer-duration treatments of 9–12 months are often used at clinician’s discretion [Citation12], particularly as lower cure rates have been reported with intermittent therapy [Citation13].
4. Effectiveness of pharmacotherapy
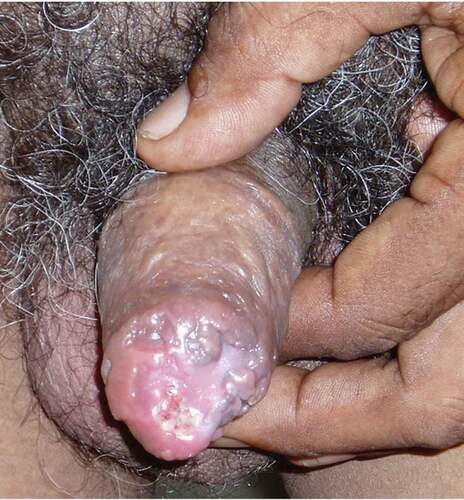
Genital TB does not universally respond to pharmacotherapy and may require additional surgery [Citation14]. There is limited data on the effectiveness of pharmacotherapy in managing infertility caused by genital TB [Citation15]. The sequelae of TB in the genital organs often results from inflammation, scarring, and distortion of the anatomy and may no longer be amenable to medical therapy [Citation16]. Surgical treatments such as vaso-vasostomy, vaso-epididymal anastomosis, and transurethral resection or incision of ejaculatory duct have been described for patients developing male infertility secondary to genital tuberculosis [Citation6]. Structural abnormalities can also cause cosmetic deformities (), functional obstructions, and anatomic obstructions which persist despite adequate pharmacotherapy. Scrotal and prostatic abscesses may require drainage if they do not respond to medical treatment. Cases of urethral strictures requiring urethroplasty have also been reported [Citation17].
4.1. Problems with response assessment and paradoxical response
Assessment of response to pharmacotherapy is also difficult for genital TB. A paradoxical reaction which is a clinical or radiological worsening of the preexisting tubercular lesions or development of new lesions in patients started on ATT after an initial favorable response may also occur. This is a part of immune reconstitution inflammatory syndrome (IRIS) and is more common in patients with concomitant HIV. A clinical examination may be fallacious for response assessment since changes such as an enlarged testis, thickened epididymis, beaded vas deferens, or hard prostate may persist even after successful treatment. A corollary can be drawn from data available on TB lymphadenitis where in a prospective study, re-biopsy in 23 of the 36 patients with post-treatment lymphadenopathy revealed granulomas in 52.2%, positive acid-fast bacilli stain in 17.4%, and positive TB-PCR in 47.8%, but all samples were sterile (no microbiological recurrence) [Citation18]. Most patients improved without further treatment, and it may become difficult to differentiate a paradoxical reaction from an inadequate response to treatment.
4.2. Dose modifications in patients with chronic kidney disease
Patients with genital TB frequently suffer from concomitant urinary tract TB which may give rise to chronic kidney disease [Citation19]. This requires dose modifications for ATT, and certain medications need to be completely omitted from the first-line regimen [Citation20]. The dose and drug therapy modifications may further lead to difficult situations such as development of drug-resistant bacteria.
5. Conclusions
Male genital TB is a difficult problem to diagnose and is often associated with concomitant urinary tract TB. It does not always respond to medical therapy, and surgical intervention is often needed to address deformities and infertility.
6. Expert opinion
HIV and immunodeficiency states have resulted in the resurgence of TB in developed countries, and physicians previously unexposed to this disease will need to be retrained in its diagnosis and management. Genital and urinary TB coexist and have a variable presentation. A high index of clinical suspicion, along with supportive radiological or bacteriological evidence, is required to diagnose TB.
Treatment of male genital TB is multidisciplinary and requires both pharmacotherapy and surgery. Pharmacological management is similar to TB at other sites, but lack of clear end-points of treatment makes it difficult to define the appropriate duration and dosing of therapy. Disfigurement and infertility due to TB sequelae often require surgical intervention. A simple algorithm for the treatment of GUTB has been published earlier [Citation4]. As drug resistance remains a significant problem in the treatment of TB, research is targeted toward the development of newer modalities of diagnosing drug resistance quickly and accurately.
As countries move from ‘control’ toward ‘elimination’ of TB, these rare forms of the disease will become important for recognition of all cases. Early case identification, appropriate drug therapy, drug susceptibility testing, and further therapeutic drug monitoring are the key to providing adequate treatment. Strengthening of public health programs, political will, and systemic reforms for research, similar to the lines of COVID-19 pandemic control, are needed to achieve TB-free world by 2035.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Global Tuberculosis Report 2021. World Health Organization: Geneva. [cited 2021 Nov 27]. Available from www.who.int/publications/i/item/9789240037021
- Chakaya J, Khan M, Ntoumi F, et al. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021 Dec;113(Suppl 1):S7–S12.
- Kulchavenya E. Best practice in the diagnosis and management of urogenital tuberculosis. Ther Adv Urol. 2013;5(3):143–151.
- Kulchavenya E, Kim CS, Bulanova O, et al. Male genital tuberculosis: epidemiology and diagnostic. World J Urol. 2012;30(1):15–21.
- Yadav S, Singh P, Hemal A, et al. Genital tuberculosis: current status of diagnosis and management. Transl Androl Urol. 2017;6(2):222–233.
- Jacob JT, Nguyen TM, Ray SM. Male genital tuberculosis. Lancet Infect Dis. 2008;8(5):335–342.
- Engin G, Acunaş B, Acunaş G, et al. Imaging of extrapulmonary tuberculosis. Radiographics. 2000 Mar-Apr;20(2):471–488.
- Pawlowski A, Jansson M, Sköld M, et al. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8(2):e1002464.
- Chen TC, Lu PL, Lin CY, et al. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis. 2011;15(3):e211–6.
- Migliori GB, Tiberi S, Zumla A, et al.Members of the global tuberculosis network. MDR/XDR-TB management of patients and contacts: challenges facing the new decade. The 2020 clinical update by the global tuberculosis network. Int J Infect Dis. 2020 Mar;92S:S15–S25.
- Sterling TR, Lehmann HP, Frieden TR. Impact of DOTS compared with DOTS-plus on multidrug resistant tuberculosis and tuberculosis deaths: decision analysis. BMJ. 2003;326(7389):574.
- Kadhiravan T, Sharma SK. Medical management of genitourinary tuberculosis. Indian J Urol. 2008;24(3):362–368.
- Menon PR, Lodha R, Sivanandan S, et al. Intermittent or daily short course chemotherapy for tuberculosis in children: meta-analysis of randomized controlled trials. Indian Pediatr. 2010;47(1):67–73.
- Symes JM, Blandy JP. Tuberculosis of the male urethra. Br J Urol. 1973;45(4):432–436.
- Shah RS. Obstructive azoospermia following genital tuberculosis may be reversible with medical therapy. J Urol. 2004;171(4S):422.
- Kumar R. Reproductive tract tuberculosis and male infertility. Indian J Urol. 2008;24(3):392–395.
- Prakash G, Singh V, Sinha RJ, et al. Primary tuberculosis of urethra presenting as stricture urethra and watering can perineum: a rarity. Urol Ann. 2016;8(4):493–495.
- Park KH, Lee MS, Lee SO, et al. Incidence and outcomes of paradoxical lymph node enlargement after anti-tuberculosis therapy in non-HIV patients. J Infect. 2013;67(5):408–415.
- Muneer A, Macrae B, Krishnamoorthy S, et al. Urogenital tuberculosis-epidemiology, pathogenesis and clinical features. Nat Rev Urol. 2019;16(10):573–598.
- Milburn H, Ashman N, Davies P, et al.; British Thoracic Society Standards of Care Committee and Joint Tuberculosis Committee. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;65:557–570.