ABSTRACT
Introduction: The development of novel complex biotherapeutics led to new challenges in biopharmaceutical industry. The potential of these particles has been demonstrated by the approval of several products, in the different fields of gene therapy, oncolytic therapy, and tumor vaccines. However, their manufacturing still presents challenges related to the high dosages and purity required.
Areas covered: The main challenges that biopharmaceutical industry faces today and the most recent developments in the manufacturing of different biotherapeutic particles are reported here. Several unit operations and downstream trains to purify virus, virus-like particles and extracellular vesicles are described. Innovations on the different purification steps are also highlighted with an eye on the implementation of continuous and integrated processes.
Expert opinion: Manufacturing platforms that consist of a low number of unit operations, with higher-yielding processes and reduced costs will be highly appreciated by the industry. The pipeline of complex therapeutic particles is expanding and there is a clear need for advanced tools and manufacturing capacity. The use of single-use technologies, as well as continuous integrated operations, are gaining ground in the biopharmaceutical industry and should be supported by more accurate and faster analytical methods.
1. Introduction
Biotherapeutic particles are playing an increasingly important role in the different fields of vaccination, gene therapy, and cancer treatment. Virus-based particles and extracellular vesicles (EVs) are examples of these very large and complex molecules, that have been successfully applied in these different areas [Citation1]. Although these particles are different, they present some similarities besides their size range. Extracellular vesicles are surrounded by a lipid membrane that also contains cell membrane proteins very similar to an enveloped virus. Moreover, comparable to virus, EVs can bind to the plasma membrane of other cells and enter them by fusion or endocytosis [Citation2].
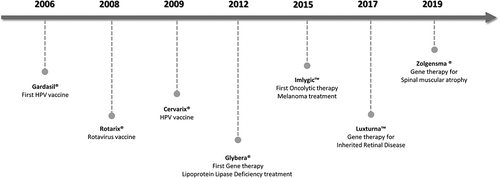
Vaccination remains the most effective way to prevent viral diseases, with the smallpox eradication in 1979 being a successful example of it. Besides vaccination, viral particles such as adenovirus, adeno-associated virus (AAVs), and lentivirus have been used as viral vectors in gene therapy treatments to deliver a specific genetic material into the target cells, or for oncolytic treatment, being extensively used in clinical trials with promising results [Citation3]. Extracellular vesicles, namely exosomes, have been evaluated as drug delivery vehicles, as biomarkers for cancer diagnostic and in cancer treatment with decreased tumor cell invasion, migration, and proliferation [Citation4]. shows important landmarks in the biotherapeutic particles history, with the approval of the first cancer vaccine, first gene therapy and the first oncolytic virotherapy product.
These classes of biologics have an increased complexity, where factors such as the cell line, the particle stability, the analytical methods, and the scale-up technologies may have a negative impact on the time to market of the desired therapy. The manufacturing of these biotherapeutic particles includes its production followed by its purification in order to meet the requirements of its final use. A series of unit operations that goes from the upstream processing until the final sterile filtration, including the analytical methods for process monitoring and control, is required for their manufacturing. Positive data in clinical trials led to the enthusiasm of the biopharmaceutical industries to enter in this field of biotherapeutic particles manufacturing [Citation5]. However, there are still unmet challenges to be overcome either in product generation or in its purification and final characterization when moving from product development toward product in the clinic, which will be reviewed in this article.
2. Complex biotherapeutic particles – the main challenges
The inherent complexity of this class of biologics is strongly related to the physical and chemical properties displayed. The size of these particles can range from 20 nm in the case of AAVs to 1000 nm for the case of measles virus. Furthermore, their shape attributes can provide additional complexity. Adenovirus and AAVs have an icosahedral geometry while Influenza VLPs and measles virus have a more pleomorphic shape. Overall, these biotherapeutic particles are complex structures increasing the complexity of the purification strategies. Their intricate nature has several implications on the downstream processing concerning purity, potency, and quality of the final product [Citation6]. The particle structure and integrity must be maintained across the purification process, as well as, the proper post-translational modifications of the exposed proteins that are critical for the desired immune responses, in the case of viral particles used for vaccination. Furthermore, viral vectors should maintain their infectivity across the entire purification process, which in some purification techniques could be a challenge. Moreover, the size and pleomorphic nature of some viruses (e.g. measles) avoid a final sterile filtration step, requiring additional efforts during the purification processes such as, aseptic conditions that should be maintained through the entire purification process. This will lead to higher costs in manufacturing of these particles. Thus, purification processes must be designed in accordance with these particles’ properties. Before starting the development of a purification process, it is crucial to collect all available information related to the particle properties, to design an approach that will fit the final requirements. Impurities should also be characterized in the initial material since there are process and product-related impurities that should be removed during DSP. Host cell protein (HCP) and host cell DNA (HCD), as well as cell culture media components, are the typical process-related impurities that should be below a certain limit, in the final product. Product-related impurities can be particle aggregates, noninfectious particles or empty capsids that in the case of AAV vectors are difficult to be removed [Citation7]. Concerning the purity levels, the guidelines for biological products of the regulatory agencies [Citation8] – US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) – must be satisfied; and they will keep progressing as the field evolves.
There are no general purification processes for these complex particles. The diversity of production conditions of the different particles increases the complexity of having a purification process platform. There are, however, common steps and unit operations that provide a good starting point (). Although the purification challenges are different, and there isn’t a one-fits-all solution, the DSP should have similar characteristics: – Reduced complexity of the DSP train; – Reduced number of operations (recovery yield decreases with the increase of processing steps); – Increased process efficiency; -Scalable processes; – Processes that meet the standards of current Good Manufacturing Practices (GMPs).
Figure 2. Flow chart of the main manufacturing steps of biotherapeutic particles – cell culture, purification and quality control, and its final usage in the different field of gene therapy, vaccination, oncolytic therapy and tumor vaccines. During the manufacturing platform, the main goals are to reduce the process time and cost as well as increase the recovery yields and product quality.
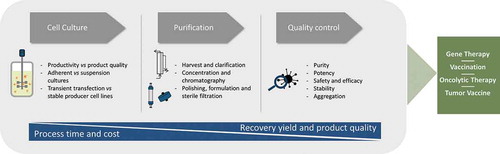
Moreover, robust purification methods capable of providing a balance between recovery yields and impurities removal, in a cost-effective manner also very appreciated. Noteworthy, the development of efficient processes that fulfill these requirements is one of the major challenges in biotherapeutic particles manufacturing [Citation9].
3. Upstream processing – new developments
The generation of these biotherapeutic particles relies predominantly on the mammalian and insect cells cultured either in adherent or suspension conditions (). Most of the cell lines grow naturally in adherent conditions, with some exceptions for some insect cell lines, cells derived from the blood system as well as tumor cells [Citation10]. Mammalian cell lines can generate viral vectors using stable producer cell lines, transient systems or by infection. Transient transfection of mammalian cells is the most widely used methodology for viral vector production and is easy to perform at lab scale. On the other hand, when moving to large scale production, the variability that is observed in transfection efficiency can be critical in vector production as well as the high costs of the high-quality DNA and transfection reagents that are required. This leads to the evaluation of stable producer cell lines as an alternative for viral vectors production [Citation11], that is also challenging since the virus themselves are toxic to the producer cells. Tomás et al recently developed a new lentivirus stable producer cell line – LentiPro26-A59, that constitutively produces titres above 106 TU.mL−1.day−1, aiming for clinical gene therapy applications [Citation12].
The use of adherent cultures come with the challenge of scaling up. This can be performed by using a scale-out approach, adding more production units to achieve the desired culture area. Among the different adherent culture systems, cell factories, cell stacks, HYPERStacks, and roller bottles are widely used. The manipulation of these culture systems is more challenging, increasing the potential risk of contamination. Monitoring and controlling of cell culture conditions (dissolved oxygen, pH, etc.) in these systems is also difficult and limited. The recent innovations in adherent cultures led to the development of single-use fixed-bed bioreactors. Disposable iCEllis fixed-bed bioreactor was developed to increase the surface area available to grow adherent cells in a scalable and efficient way, reducing the footprint. This bioreactor has been used for the production of lentiviral vectors with transient transfection in adherent HEK 293T cells [Citation13], γ-retroviral vectors for phase I/II trials [Citation14] and adenovirus serotype 5 [Citation15]. More recently, Univercells presented Scale-X, a new fixed-bed bioreactor system for intensified viral production. Adherent HEK293 cells have been cultured in this single-use bioreactor for the production of adenovirus [Citation16] in alternative to better-known microcarrier processes have been used to cultivate these anchorage-dependent cells for the manufacturing of virus particles and exosomes in bioreactors [Citation17,Citation18].
New improvements in cell line development allowed the adaptation of adherent cells to suspension conditions. Several cell lines have been adapted to suspension conditions, and have been used for the production of different biotherapeutic particles [Citation11,Citation19,Citation20]. However, the adaptation of animal cells to suspension conditions is time-consuming, delaying the process development. Moreover, not all producer cell lines can be adapted while maintaining the same productivities. Manufacturing of these complex particles using suspension cell lines can be performed using the traditional stirred tank reactors (STR) or the most recent disposable STR such as the Xcellerex™ XDR, the Mobius® CellReady, the Allegro™ STR and the BIOSTAT STR® [Citation21]. Other disposables bioreactors have gained some importance such as the case of the now classical Wave bioreactors that have been used for the production of lentiviral vectors, AAVs and γ-retroviral vectors [Citation22–Citation24]. For process development purposes, ambr® systems are a high throughput tool, automated bioreactors that can be used as scale-down models for evaluation of upstream optimizations [Citation25]. Perfusion systems can be coupled with the majority of these bioreactors configurations where suspension cells or microcarriers are retained and the cell culture media is removed while fresh media is added to keep the cells viable and procuding the desired product. These perfusion-based strategies have been used for the production of lentiviral vectors, improving the titre to 3.2 × 107 TU/mL and cumulative functional LV titres by up to 15-fold when compared to bacth mode [Citation26]. Hybrid systems including fed-batch and perfusion strategies have also been used for the production of Modified Vaccinia Ankara (MVA) virus and Influenza A virus where, comparable or even higher cell-specific virus yields and columetric productivities were obtained [Citation27], contributing to the development of new approaches for viral vaccine production.
Regarding insect cells (IC), Baculovirus expression vector system (BEVS) is the major technology platform being mainly used in the production of Adeno-Associated Virus (AAVs) and different VLPs [Citation28,Citation29]. Both High Five and Sf9 cells have been used with BEVS for the production of clinical biologicals such as Cervarix®, a GSK vaccine against human papillomavirus and Flublock®, an Influenza vaccine [Citation30,Citation31]. Regarding cultivation technologies, since IC grow in suspension conditions, all the bioreactors detailed before are suitable for its cultivation. It is also worth noticing that although fixed-bed bioreactors such as the iCEllis, have been designed to cultivate adherent cells, they can also be used for the cultivation of insect cells [Citation32].
4. Traditional purification methods
In the early days of vaccine manufacturing, the purification strategies were based on virus size and density properties. Ultracentrifugation and density gradient centrifugation, in combination with filtration techniques, were the first methods to be used for the purification of these complex particles [Citation33–Citation35]. These gradients were used for the purification of adenoviral vectors [Citation36–Citation38], AAVs [Citation39,Citation40], influenza [Citation41], lentivirus [Citation42] and other viral vaccines [Citation43,Citation44]. More recently, and due to the similarities of extracellular vesicles and virus particles, ultracentrifugation was also the first methods to be applied for these vesicles’ purification. However, CsCl leads to great losses in viral infectivity, low recoveries and high ratios of total: infectious particles. For EVs, it has been demonstrated that ultracentrifugation techniques may yield aggregated EVs after pelleting and also results in low and operator-dependent yields, damaging the EVs due to shearing forces obtained at higher centrifugation speeds [Citation45]. Furthermore, CsCl and sucrose reagents need to be removed using a desalting step, increasing the number of unit operations thus decreasing the yield. Owing to the time required for the viral vectors to reach the equilibrium, this method is also very time-consuming. Segura et al. used a simpler strategy using iodixanol (OptiPrep™), which is also a density gradient medium, for the retrovirus purification. This method requires fewer manipulations, leads to higher titres and allows lower processing times due to the lower viscosity of iodixanol. Since it is a nontoxic substance to the cells, there is no need for its removal.
Regarding large scale production, continuous flow ultracentrifugation is also becoming a well-established approach to concentrate and purify the virus in one step, as it allows for processing of large volumes [Citation46–Citation48]. Nevertheless, these ultracentrifugation techniques are poorly scalable, difficult to maintain, due to the size and price of the equipment, and allow for the co-purification of some impurities [Citation49]. Recently, a new CsCl density gradient method using general centrifugation (40,000 x g) was described, instead of the conventional method (100,000 x g), for virus purification [Citation50]. The use of a common centrifuge instead of ultracentrifuges reduces the cost and becomes a more accessible virus purification method. Besides the disadvantages of ultracentrifugation, this strategy is still used to produce small quantities of the highly purified virus at lab scale or even for phase I clinical trials. However, in the past few years, the industry is trying to move away from these centrifugation processes and focus on chromatography and membrane-based separation techniques.
5. Downstream processing – purification strategies for complex biopharmaceuticals
5.1. Harvest and clarification
Cell harvest is usually the interface between up- and downstream processes. The time of harvest (TOH) should be determined to take into consideration different parameters like product quality and not only productivity. The product stability, as well as the cell density and cell viability, also have an enormous impact on the purification steps (). Higher culture times will lead to lower cell viabilities that consequently will end in higher particle production as well as an increase in host cell proteins, host cell DNA and cell debris that will burden the purification process [Citation51]. However, there is no ideal TOH and a balance between these parameters should be taken into account when developing a manufacturing process for these particles.
Figure 3. Effect of the different upstream processing parameters and culture options in the downstream processing.

The release of viral particles by infected cells is dependent upon the virus cycle. Enveloped particles are released to the extracellular medium by budding from the cell membrane while naked viruses are assembled intracellularly. However, although at the TOH there is a high percentage of viruses in the extracellular medium, it could be advantageous to lyse the host cells to maximize virus yield [Citation52]. Freeze-thaw cycles, homogenization, sonication or chemical lysis by the addition of detergents are techniques that can be applied for cell lysis [Citation53]. At large scale production, chemical lysis is the strategy of choice, as opposed to homogenization, and is usually done by the addition of low concentrations of surfactants (0.1% Triton X-100 or NP-40) [Citation52–Citation54]. More recently, Triton X-100 was included on the list (Annex XIV) of Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH), prohibiting its use from 4 January 2021, leading to the evaluation of different cell lysis detergents [Citation55]. However, when adding surfactants, or even other reagents to the culture, they should be removed or reduced later to decrease the impact on the performance of the next unit operations. This should be taken into consideration when the virus particles are also released to the extracellular medium, the increase of virus yield obtained with the addition of a cell lysis step should compensate the extra efforts needed to remove the lysis reagents that were added.
After cell lysis, there is an accumulation of host cell proteins, host cell DNA and cell debris that are partially removed by centrifugation and/or filtration techniques such as depth-filtration or microfiltration [Citation54,Citation56]. Usually, a combination of both techniques is used to remove first, the larger particles by centrifugation – cells, cell debris (primary clarification) and afterward, the removal of low molecular particles through filtration (secondary clarification) [Citation54]. Disk-stack centrifuges can be used for processing of large volumes of culture broth, in batch or continuous mode. Still, the capital expenses should be considered since these costs tend to dominate at lower scales [Citation57].
Unlike centrifugation, filtration techniques simplify the cleaning and validation by the use of disposable material, that is easily scalable and provide easier control of shear stress during filtration [Citation56,Citation58,Citation59]. Filtration techniques, using either dead-end and depth filters or membrane devices with pore sizes ranging typically from 0.2 to 3 µm, have been incorporated into the manufacturing processes of viral particles [Citation60–Citation63] and extracellular vesicles [Citation64]. Depth-filtration has been used for clarification of Influenza virus-like particles, Hepatitis C virus-like particles, and rota virus-like particles, produced using insect cells [Citation53,Citation65,Citation66]. Human Influenza A virus have also been clarified directly from the culture broth with HA recovery yields ranging from 85–93% for depth filtration and microfiltration, respectively [Citation60]. Normal flow filtration (NFF) using depth filters can increase the removal of HCPs and DNA by its absorption in the membranes [Citation67]. However, these filters have a limited capacity that is highly correlated with the culture broth conditions (cell viability and cell density) [Citation68]. Turbidity is an important parameter that should be monitored during the clarification process. Clarification efficiency can be assessed by measuring the turbidity reduction; the turbidity breakthrough is an indication of the filter capacity [Citation59]. However, when the cell culture supernatant is processed, the retentate containing the cells and debris is concentrated and can form a concentration polarization layer on the filter surface. This gel polarization layer or fouling can decrease the filtrate flux and the recovery yield and, increase the overall processing time. Increase the filter area, the TMP or the cross-feed flow are some approaches that could lead to a reduction of this fouling effect.
Nuclease treatment is commonly used to reduce DNA size and decrease bulk viscosity [Citation69]. During biotherapeutic particles manufacturing, and in order to achieve the HC DNA levels imposed by regulatory authorities, the nuclease is usually used before the clarification step since it is a process additive that should be removed during the next purification processes [Citation70]. However, this also increases the manufacturing costs since this enzyme suitable for biopharmaceutical manufacturing is expensive. To overcome or reduce the impact of nuclease costs, some manufacturers perform this treatment in a more concentrated stage of the process where the volume of product is lower. Recently, Oxford Biomedica developed a new strategy – SecNunc ™, which is a highly efficient alternative approach to bypass the nuclease step by using secreted nucleases in co-production with viral vector manufacturing [Citation71]. Still, removal of cell debris and DNA early in the process will prevent the formation of complexes with virus particles [Citation72] since the strong negative charge of DNA promotes the interaction with viral particles [Citation73], and can compensate the high costs of nuclease in the clarification stage.
5.2. Intermediate purification – concentration and chromatography
The following steps after clarification usually include a concentration technique using ultra or microfiltration, and/or a chromatography method [Citation74–Citation78]. Indeed, filtration and chromatography have become the pillars for downstream processing of biological particles.
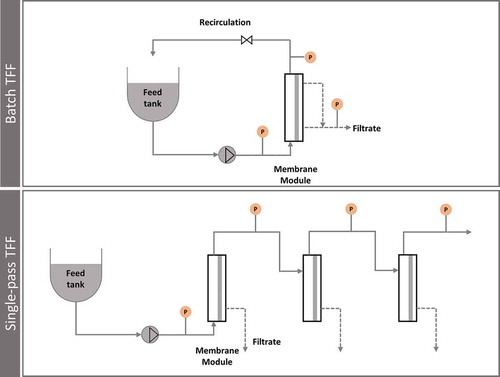
Ultrafiltration is the method of choice for concentration and diafiltration (buffer exchange) of these particles while removing low molecular weight impurities and reducing the handled volume [Citation63,Citation79–Citation84]. In this membrane filtration technique, hydrostatic pressure forces the liquid (water and low molecular weight solutes) to pass through a semipermeable membrane (permeate), while the suspended solids or solutes with high molecular weight are retained and recirculated for reprocessing. Ultrafiltration is a robust, relatively low cost and scalable technique usually with moderate to high recovery yields for viral particles and extracellular vesicles [Citation78,Citation85,Citation86]. Hollow-fibres or flat sheet membranes (membrane cassettes) are the most common devices used in this operation with typical molecular weight cutoffs between 100–1000 kDa [Citation78,Citation80,Citation83]. Ultrafiltration membranes are available in different materials: regenerated cellulose (RC), polysulfone (PS), polyethersulfone (PES) and polyvinylidene fluoride (PVDF). PES Membrane cassettes showed a better performance for Influenza VLPs when comparing with RC material, not only regarding permeate fluxes but also in terms of impurities (DNA and proteins) clearance [Citation78]. PES membrane cassettes of 100 or 300 kDa have also been used for lentiviral vectors concentration allowing 66-fold concentration with complete vector recovery [Citation83]. The use of Hollow-fibres devices can result in lower shear rates when compared with membrane cassette devices and have been used in the manufacturing of several viral particles – Adenovirus, Influenza virus, rotavirus like particles and HIV VLPs [Citation53,Citation55,Citation60,Citation63]. Regarding extracellular vesicles, PES or RC membrane devices with 100 kDa have been used, but their behavior in the purification of these particle has not been fully explored yet [Citation76,Citation84,Citation86]. Overall, ultrafiltration is a powerful technique and its use can go beyond concentration followed by a chromatographic step and can move to an entire membrane process as described recently by Carvalho et al for Influenza VLPs [Citation78]. Nevertheless, when developing a manufacturing process for these complex particles, a study of the ultrafiltration step should be performed to ensure that the most suitable operating parameters are being used: transmembrane pressure (TMP), cross-flow, retentate and permeate fluxes, concentration factor, and diafiltration volumes. The membrane material and membrane area that is used is also extremely important for the different particles. A different operation mode of ultrafiltration, presented in , is the single-pass TFF (SPTFF), where the conventional recirculation loop is eliminated and a staged flow-path design is used, allowing for concentrations of 2-30x in a single pass, to be achieved continuously [Citation87,Citation88].
Figure 4. Comparison of traditional configuration batch TFF with single-pass TFF, where the recirculation loop is eliminated.
Chromatography can also be used as an intermediate purification step. Chromatography has been used in the bio separations field for decades for the purification of small proteins and antibodies. This technique is based on the different partitioning between the mobile and stationary phases. There are different types of chromatography that are related to the interaction between the component to be purified and the stationary phase. In the biopharmaceutical industry, chromatography continues to be the gold standard for purification as it can be used at different stages with distinct purposes.
Different chromatographic modes can be applied for the purification of these particles, where affinity chromatography have recently gained more importance in biotherapeutics manufacturing field due to their unique selectivity capacity [Citation89–Citation93]. The principle is based in highly specific and reversible interactions between the particles and the ligand bound on the matrix, offering a reduction in the number of purification steps due to the high selectivity [Citation33]. Affinity resins for AAVs have been fully explored as this viral vector is one of the most actively investigated for gene therapy purposes [Citation94]. AVB Sepharose is an immunoaffinity media where the matrix is cross-linked agarose and was design to purify AAV serotype 1, 2, 3 and 5. However, purifications of AAV serotype 1 and 5 have also been performed using this resin [Citation95]. Besides AVB, CaptureSelect AAV8 and AAV9 resins have also been developed to purify these AAVs serotypes, using the POROS™ bead technology, which presents large throughpores of the beads leading to higher capacity for the resins. More recently, a new resin, POROS™ CaptureSelect™ AAVX was developed to purify a wide range of AAV serotypes, from AAV 1–9 as well as synthetic AAV serotypes [Citation93]. Besides the development of new affinity resins, biotherapeutic particles can be modified to have a specific affinity for a target molecule. Lentiviral vectors (LV) have been modified to express cyclical biotin mimetope on their surface, allowing these LV particles to be captured on streptavidin [Citation96]. However, the use of affinity chromatography in large-scale processes is still reduced due to the high economic challenges of ligand design and manufacturing, to obtain high-productivity chromatography media [Citation93].
Ion-exchange chromatography (IEC) is one of the most widely used chromatographic techniques for purification of large particles which exploits the differences in particle’s charge [Citation97]. Anion or cation exchange chromatography techniques can be applied depending on the net charge of the particle to be purified. The net charge at neutral pH will depend on the particle surface composition which differs from each viral particle and serotype. Most of the viral particles have an isoelectric point below 7.4, being negatively charged at physiological pH. Adenovirus, AAV, retrovirus, lentivirus and baculovirus are some examples of viral particles that have been successfully purified using anion exchange chromatography [Citation35].
Hydrophobic interaction chromatography (HIC) has been used for the purification of vaccinia virus, foot-and-mouth disease virus and influenza virus, although its use is far lower than IEC [Citation89,Citation98–Citation100]. Moreover, the high salt concentrations used in HIC can damage the integrity of the particles, being one of the reasons why this chromatographic technique is less employed [Citation101].
Mixed-mode chromatography (MMC) is based on a combination of different binding mechanisms such as, for example, ligands that combine electrostatic and hydrophobic interactions [Citation101]. Hydroxyapatite is one of the examples of MMC where the resins bind both positively charged functional groups and negatively charged phosphate groups, which have been used for the purification of adenovirus, dengue virus and retrovirus [Citation73,Citation102,Citation103]. Besides Hydroxyapatite, another example of MMC is the Capto™ Core 700 and Capto™ Core 400 resins from GE Healthcare which have a ligand-activated core and an inactive shell, presenting both size exclusion and binding properties. These resins are suitable for flow-through mode chromatography since they can efficiently bind the impurities while the target particles pass through the resin and are collected in the flow-through. Several purification processes for viral particles and extracellular vesicles have used this technology [Citation104–Citation107].
The development of new materials such as adsorptive membranes, monolithic supports, and nonporous media are interesting alternatives to the traditional porous resins that can be used for biotherapeutic particles purification [Citation74,Citation108–Citation110]. Recently, sulfated cellulose membrane absorbers (SCMA) were developed for the purification of Influenza virus and virus-like particles, with recovery yields around 80% [Citation77,Citation111,Citation112]. These membranes take advantage of the affinity properties of sulfated cellulose and the convective flow of membrane absorbers. Monolithic supports are characterized by its very high porosity, with channels size mostly ranging between 1–5 µm, high binding capacity, and dominantly convective mass transport, providing high flow rates, reducing the processing time [Citation113,Citation114]. Hereby, monoliths are of huge interest in this field, being used for the purification of several particles [Citation108,Citation115]. Recently, a CiMmultus™ QA anion exchange monolith column was used to isolate 2.4 × 1011 of the EVs present in a litre of conditioned media in fewer than 3 hours, showing that these devices are therefore a step forward in terms of processing time [Citation64]. New adsorption designs have also been evaluated for the negative chromatography purification of hepatitis B virus-like particles [Citation116,Citation117]. In this operation mode, as described before, the target particles are collected in the flow-though, eliminating the elution step and reducing the risk of VLPs disassembly [Citation118,Citation119]. Other development in the chromatographic field is the cellulose nanofibers, a new adsorbent design material that has an open structure large enough to be accessible to viral vectors, allowing mass transfer based on convection. Adenovirus type 5 and Lentiviral particles have been recently purified using this nanofiber-based ion-exchange chromatography [Citation120,Citation121]. The most recent purification processes for different biotherapeutic particles and their respective production system, purification steps and recovery yields are present in .
Table 1. Examples of recent purification processes for different biotherapeutic particles. The production system, as well as the purification steps and the recovery yields, are described.
5.3. Polishing, formulation and sterile filtration
The last steps in biotherapeutic particles manufacturing usually include an important polishing step that can be associated with the final particles’ formulation. This purification step is critical due to the guidelines for purity and quality/potency of the final product that should be as high as possible and feasible. The remaining impurities are usually related to the product and more difficult to remove, which should be eliminated in this step. Size exclusion chromatography is still one of the most used techniques for polishing, to remove low molecular weight impurities and for buffer exchange (final formulation) [Citation51,Citation126]. However, due to some SEC limitations regarding low capacity and product dilution, flow-through chromatographic separation has become an important step for polishing purposes. Resins such as Capto Core 700, and different membrane adsorbers have been used in a negative mode for polishing of biotherapeutic particles [Citation52,Citation77,Citation127–Citation129]. Besides chromatography, UF/DF can also be used as an alternative step for polishing which allows simultaneously purification and exchange into the final formulation buffer [Citation56,Citation78].
Additionally, not only the final product purity is important but also its final formulation, that must be able to elicit protective immune responses [Citation130]. Formulation and stabilization of the different biotherapeutic particles is an extensive topic that will not be covered in this manuscript. Formulation of the final product is intended to be not only the maintenance of the native particle structure and function over time, but also improve immune responses by the use of adjuvants [Citation130]. Sucrose, trehalose, and dextrose are examples of stabilizer adjuvants used in vaccine formulations [Citation52]. Moreover, in exosome-based candidate therapies, trehalose has also been used to overcome the exosome aggregation effect [Citation131].
Following a biopharmaceutical process structure, sterile filtration is usually the latest process before vialing. This step is extremely desirable from a safety and regulatory perspective and is highly dependent on the particle size, where particles larger than 200 nm cannot be filtered, such as the case of poxvirus and vaccinia virus. The membrane material is a key parameter in sterile filtration that should be optimized to minimize the particles’ losses due to nonspecific binding and membrane fouling [Citation33,Citation132]. Shoaebargh et al recently evaluated several sterile filters (0.2 and 0.22 µm) for oncolytic adenovirus filtration where the two-layered sterile filters, Fluorodyne EX EDF, and MiniSart Plus, demonstrated slower transmembrane pressure (TMP) increase along with a higher filtered viral volume [Citation132]. Among the filters tested, the total virus recovery did not exceed 25%. This value is quite low and represents a particular concern that affects the large-scale manufacturing of several therapeutic particles. Furthermore, and as described by Shoaebargh et al, the presence of aggregated virus particles may cause partial blockage of the pore of the membranes [Citation132]. Due to the challenges and difficulties observed with sterile filtration, there are some manufacturing processes that instead of performing this step take every precaution to ensure product sterility at all steps of the downstream.
6. Innovations in purification technologies
The increase in efficiency of the existing downstream processes and the development of new unit operations and integrated processes is the way to go for the manufacturing of the different biotherapeutic particles. Innovative manufacturing processes for cost-effective production of these particles are needed in the industry, and in fact, new concepts and new modes of operation have been tested.
Continuous processes have been employed in pharmaceutical industries as the need for high throughput downstream processes has increased [Citation133]. Continuous chromatography has been developed for the purification of antibodies [Citation57,Citation134] and more recently, for different particles such as adenovirus [Citation135–Citation137], influenza [Citation138,Citation139] and extracellular vesicles [Citation84]. Periodic countercurrent chromatography (PCC) and countercurrent simulated moving bed (SMB) are the most advanced systems used in biopharmaceutical industry [Citation138,Citation140]. The shift to continuous processing is usually associated with higher purity and productivity, and lower buffer consumption [Citation137], reducing the overall process costs. Nevertheless, this mode of operation is often correlated with a complex and robust purification set-up system.
Besides continuous chromatography, steric exclusion chromatography (SXC) has also been proposed for purification of Influenza virus and Bacteriophages [Citation141,Citation142]. The principle of this technique is the capturing of the target particles based on the mutual steric exclusion of polyethylene glycol (PEG) between the product and a non-reactive hydrophilic surface. In SXC, there is the mixing of the feedstock material, previously clarified, with polyethylene glycol (PEG) at a defined concentration and molecular weight (MW), and the respective loading into the non-reactive hydrophilic matrix. Influenza viruses were purified with SXC with recoveries above 95% and depletion of host cell DNA and total protein above 99% and 92%, respectively [Citation142].
A new area that started to be exploited in the last couple of years, especially in the separation field, is additive manufacturing or 3D printing [Citation143]. Simon et al, have recently described a 3D printed fully functional stationary phase for protein separations [Citation144]. In this work, 3D printed anion exchange membrane adsorbers were evaluated for adsorption of bovine serum albumin and c-phycocyanin, showing adsorption characteristics in line with the membrane adsorbers commercially available. This field of 3D printing technologies is continuously growing with the design of new solid-phase geometry structures, that can increase process efficiency as well as the opportunity to customize our own porous bed for particular applications [Citation145].
Aqueous two-phase systems (ATPs) continue to be a promising tool in downstream processing due to their main characteristics of scalability, label-free process and cost-effective methodology [Citation146]. Polymer-polymer ATPS are the most widely used, in particular, PEG-dextran systems. This liquid-liquid extraction technique has been exploited for the purification of extracellular vesicles, providing high recovery efficiency (~70-100%) in a short processing time [Citation147,Citation148]. Furthermore, for human B19 parvovirus-like particles purification, ATPS systems of PEG and salt, resulted in recoveries between 40–100% depending on the operation mode: single-stage or multi-stage ATPS and host cell impurities clearance [Citation149]. Jacinto et al also purified human immunodeficiency virus (HIV) VLPs using the PEG/salt system to recover the VLPs from the Chinese hamster ovary (CHO) cell supernatant and evaluated this extraction using a microfluidic setting [Citation150]. In addition, Marchel et al evaluated ionic liquids (ILs) as adjuvants in aqueous biphasic systems, for purification of enveloped Hepatitis C VLP [Citation151]. However, although the applicability of ATPs has been already demonstrated, protein solubility of some ATPS is still an issue. Furthermore, the lack of industry knowledge on installation, validation, and operation of such systems hampered the adoption of this technique by the industry [Citation152,Citation153].
7. Analytics in process development
Analytical tools are critical in process development and play a crucial role in up and downstream process monitoring, being also fundamental to ensure the final product characterization regarding its quality and safety (). This characterization is usually obtained through the combination of different analytical methods [Citation97]. Furthermore, the development of fast and more accurate analytical assays is always a need since some of the existing methods are time-consuming, presenting a significant bottleneck in the development of new products. shows the most commonly used analytical assays for virus-based therapeutics in comparison with mAbs. In virus-based biologics quantification, different analytical methods should be addressed. For genome particles quantification, real time polymerase chain reaction (qPCR) is usually the method of choice. Droplet digital polymerase chain reaction (ddPCR) has also been recently used for quantifying viral genome, without the need for a standard curve [Citation154,Citation155]. Depending on the final use of virus-based particles, and different from mAbs, infectivity can be a critical parameter. Thus, infectious particles quantification is usually performed by plaque assay or tissue culture infective dose assay (TCID50). Besides genome particles quantification, there are other techniques that quantify the total physical particles. Due to the nanosize of these biotherapeutics, nanoparticle tracking analysis (NTA) and tunable resistive pulse sensing (TRPS) are techniques can be used not only for particles quantification but also characterization regarding size distribution [Citation156,Citation157]. NTA has also been used for stability studies of exosomes particles during storage at different temperatures [Citation158]. To confirm the virus presence, morphology and integrity, transmission or scanning electron microscopy are useful techniques to complement the different analytical assays. Commercially available enzyme-linked immunosorbent assay (ELISA) is also a widely used technique to quantify different virus antigens. Besides these techniques, western blotting for protein identity matching, size exclusion chromatography HPLC, capillary zone electrophoresis, Asymmetrical-Flow Field-Flow Fractionation (A4F) and surface plasmon resonance are assays that can be used to complement biotherapeutic particles characterization [Citation159–Citation161]. Analytical ultracentrifugation (AUC) has been the method of choice for investigation of the empty capsids content [Citation162]. This technique distinguishes and quantifies the different species (empty and full particles) by either mass or density without the need for standard material for quantification comparison. Detection and quantification of biotherapeutic particles can also be performed by multi-angle light scattering (MALS) detectors coupled with size exclusion HPLC or asymmetrical field flow fractionation [Citation163,Citation164]. This quantification method does not require a calibration sample since the particle concentration can be directly deduced from the scattered light.
Table 2. Specific analytical assays used in virus-based therapeutics and protein therapeutics (mAbs) for final product, categorized by identity, potency, quantity, purity and safety.
For final product characterization, and as well as for mAbs, quantification of residual DNA from the producing cell line is always required for clinical lot release due to the stringent guidelines for this impurity (100 pg/dose < DNA < 10 ng/dose) [Citation165]. Among the different methods used for host cell DNA quantification PicoGreen® assay, hybridization, Threshold™ technology, and qPCR, are the most used [Citation165–Citation167]. From the methods presented, Threshold™ (1–3 pg rDNA) and qPCR are the most sensitives. Even though Threshold™ has relatively high cost and low throughput it is one of the most used in the biotechnology industry. Picogreen® assay is a relatively low-cost method and easy to use, however, with few sensitivity ranges. Lastly, in hybridization methods, besides the quantification of host cell DNA, the size of the DNA can also be estimated. Since this method presents long running times it is no longer widely used in the biotechnology industry [Citation165].
Host cell proteins (HCP) are an impurity that must be reduced during the manufacturing process to low levels to ensure clinical safety. The levels of HCP in biotherapeutic particles are reviewed case-by-case but the levels range from 1–100 ppm of HCP [Citation168]. Quantification of HCP is commonly performed by ELISA technique, using either commercially available or customized assays. This is largely due to the relative ease and good precision of this method [Citation169]. Mass spectrometry-based methods can also be used for protein identity matching, recognizing HCP present in the final product formulation [Citation168,Citation170].
Lastly, there is definitely room for further analytical improvement, as recent areas like robotics and microfluidics are progressing, giving rise to the development of automatic processes; and these require fast if possible, in-line, methodologies.
8. Conclusion
The development of emerging therapies and the demand for new biotherapeutic particles challenged the biopharmaceutical industry. These new therapeutic modalities are complex vehicles that present its own unique production and purification challenges. Purification processes and, in particular, chromatography operations continue to be widely used; however, available technologies tend to not fit the desired needs. Downstream processes for these complex particles are far from being mature, as new resins, matrices and operation modes are being developed aiming for better purification processes with high particles yield, reducing the overall purification costs and time. Finally, more robust, faster and better analytical methods are needed not only for the final characterization of the product but also for the development of purification processes, as this greatly impacts the time of process development
9. Expert opinion
Complex biotherapeutic particles such as virus-based particles, extracellular vesicles, and viruses are experiencing a rapid growth in the number of their applications. These therapies range from gene therapy, vaccination, drug delivery, to oncolytic virotherapy [Citation5,Citation171–Citation173]. The recent approvals of gene therapeutics such as Luxturna™ – voretigene neparvovec-rzyl (AAV2) and, more recently, Zolgensma® – Onasemnogene abeparvovec (AAV9), in addition to the approved oncolytic virotherapy Imlygic – talimogene laherparepvec (HSV-1), underlines the quick expansion of the field. On another note, the number of advanced clinical trials using complex biotherapeutic particles, such as virus-like particles, adenovirus, lentivirus, or exosomes follow the same trend. Although the success rate of these programs is often low, the large number of studies provides a clear vision of what to expect in the next decade.
These biotherapeutic particles have complex physicochemical properties that strongly compromises the establishment of platforms (). Not only are these biologicals significantly larger than mAbs, but their more complex surface properties can also further change within the same group. For the particular case of AAV, a change in the serotype may be sufficient to substantially alter the purification behavior. Additionally, biotherapeutic particles are often labile/fragile products even more so when enveloped (e.g. lentivirus and retrovirus), thus requiring major improvements in their biological knowledge exploitation supporting upstream and downstream processing to achieve quality, purity, dose, and formulation facilitating their clinical utilization.
Figure 5. Schematic comparison of a common mAbs purification process vs virus-based therapeutics purification alternatives. MAbs purification processes are already well established in opposition to virus-based particles purification, where several combinations and different techniques (e.g. chromatography operations) can be used. Sterile filtration step cannot be performed for particles larger than 0.2µm.
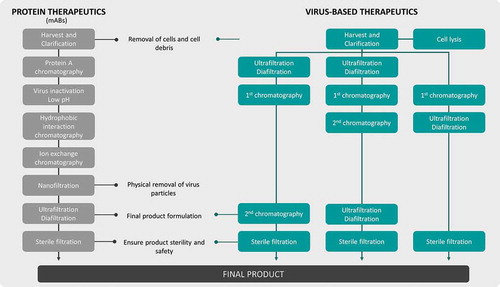
Despite the large expansion of discoveries in these new modalities, much has yet to be developed on their manufacturing counterpart. Comparing these classes of biologics against mAbs, large differences are clear. One key aspect for the success and widespread use of mAbs is the existence of solid manufacturing platforms. The use of well-developed mammalian cell culture expression system, with the development of stable cell lines in a fast, standardized way, coupled with robust fed-batch culture processes has driven substantial titre increases. Furthermore, the use of template purification processes, supported by an efficient affinity capture step facilitates process development and reduces complexity.
For these new complex particles production challenges are commonly associated with their target-specific low productivities, lack of stable producers and absence of suspension cell lines. The development of these cell lines is far from being mature when compared with the success of CHO cells; once available they will facilitate the establishment of fully integrated manufacturing processes. Moreover, when producing these particles, other challenges arise: co-production of empty capsids, viral particles that encapsulate a different genetic material, broken/disassemble particles, virus-DNA aggregates and in the case of viral products, extracellular vesicles can also be an impurity [Citation174]. Thus, tailored purification processes should be designed taking into account the specific properties of each product.
Although there isn’t a ‘one type fits all’ manufacturing platform for the different biotherapeutic particles, as shown in , there still are some similarities across the processes. For the downstream steps, the ultimate goal is to develop purification processes that work as robustly as those used nowadays for mAbs. Purification platforms consisting of low number of purification steps will produce a higher overall process yield, which increases production rates and lowers cost, to facilitate faster dissemination of these novel and promising modalities. One unmet need is the lack of affinity resins like protein A for mAbs. Only very few options exist and they are not yet cost-effective to be made single-use. Thus, there is a major opportunity to develop new matrices and new materials for these large biotherapeutic particles as the field grows. Concomitantly, the diversity and level of implementation of single-use options is becoming obvious and ubiquitous and will keep expanding. The use of these materials in both production and purification processes is gaining traction as the need to clean, sterilize and validate the equipment/material is eliminated [Citation153].
The lack of efficient process monitoring analytical tools is critical for bioprocess development as well as proper characterization and robust quantification methods to cope with regulatory demands. Stringent guidelines from the regulatory authorities become obsolete almost immediately after long delays in approval and implementations, as the field identifies newer analytical solutions. New analytical tools are needed for measuring, monitoring, and process modeling but are also critical for process control and product qualification (often more costly than production itself). Finally, such monitoring tools are a cornerstone for the development and future use of continuous and integrated operations.
While the pipeline of complex therapeutic particles expands there is a clear need for advanced tools, as well as manufacturing capacity, still a rare resource. Moreover, the biopharmaceutical industry will continue to face pressure from the healthcare environment toward lower-cost products and faster times to market. One way of overcoming these challenges is through the collaboration between small and large industry, academia, and regulators. In fact, due to the lack of internal innovation and research capabilities faced by many of the medium-to-large size biopharmaceutical companies, there is a growth in the outsourcing of research activities to academics and private contract research organizations (CROs) leading to increased competitiveness and flexibility. As large companies start to invest hundreds of millions of dollars for manufacturing plants, one should expect that a somewhat similar trend in increased robustness and decreased costs to what has been seen in mAbs in the last two decades will also take place in advancing these new particulate modalities of biopharmaceuticals.
Article highlights
Biotherapeutic particles are very complex structures which have been successfully applied in the fields of vaccination, gene therapy, drug delivery and oncolytic virotherapy.
The complexity can be associated not only with the particle structure but also with the diversity of production cell lines, particle stability, purification technologies, and analytical methods.
The most recent developments in up and downstream steps are highlighted.
Insights are given for future improvements of manufacturing platforms for these complex particles.
This box summarizes key points contained in the article.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Additional information
Funding
References
- Effio CL, Hubbuch J. Next generation vaccines and vectors: designing downstream processes for recombinant protein-based virus-like particles. Biotechnol J. 2015;10:715–727.
- Nolte-‘t Hoen E, Cremer T, Gallo RC, et al. Extracellular vesicles and viruses: are they close relatives? Proc. Natl Acad Sci. 2016;113:9155–9161.
- Merten O-W, Schweizer M, Chahal P, et al. Manufacturing of viral vectors: part II. Downstream processing and safety aspects. Pharm Bioprocess. 2014;2:237–251.
- Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208.
- Keeler AM, ElMallah MK, Flotte TR. Gene therapy 2017: progress and future directions. Clin Transl Sci. 2017;10:242–248.
- Vicente T, Mota JPB, Peixoto C, et al. Rational design and optimization of downstream processes of virus particles for biopharmaceutical applications: current advances. Biotechnol Adv. 2011;29:869–878.
- Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15:840–848.
- FDA. Guidance for industry: characterization and qualification of cell substrates and other biological materials used in the production of viral vaccines for infectious disease indications additional. 2010.
- Morenweiser R. Downstream processing of viral vectors and vaccines. Gene Ther. 2005;12:S103–S110.
- Merten O-W, Schweizer M, Chahal P, et al. Manufacturing of viral vectors for gene therapy: part I. Upstream processing. Pharm Bioprocess. 2014;2:183–203.
- van der Loo JCM, Wright JF. Progress and challenges in viral vector manufacturing. Hum Mol Genet. 2016;25:R42–R52.
- Tomás HA, Rodrigues AF, Carrondo MJT, et al. LentiPro26: novel stable cell lines for constitutive lentiviral vector production. Sci Rep. 2018;8:1–11.
- Valkama AJ, Leinonen HM, Lipponen EM, et al. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Ther. 2018;25:39–46.
- Wang X, Olszewska M, Qu J, et al. Large-scale clinical-grade retroviral vector production in a fixed-bed bioreactor. J Immunother. 2015;38:127–135.
- Lesch HP, Heikkilä KM, Lipponen EM, et al. Process development of adenoviral vector production in fixed bed bioreactor: from bench to commercial scale. Hum Gene Ther. 2015;26:560–571.
- Dohogne Y, Collignon F, Drugmand J, et al. scale-XTM bioreactor for viral vectors production: proof of concept for scalable HEK293 cell growth & adenovirus production (Application note). Univercells. p. 1–4.
- Reiter M, Mundt W. Method for large scale production of virus antigen. United States patent US 6,951,752 B2; 2005.
- Colao IL, Corteling R, Bracewell D, et al. Manufacturing exosomes: a promising therapeutic platform. Trends Mol Med. 2018;24:242–256.
- Côté J, Garnier A, Massie B, et al. Serum-free production of recombinant proteins and adenoviral vectors by 293SF-3F6 cells. Biotechnol Bioeng. 1998;59:567–575.
- Ansorge S, Lanthier S, Transfiguracion J, et al. Development of a scalable process for high-yield lentiviral vector production by transient transfection of HEK293 suspension cultures. J Gene Med. 2009;11:868–876.
- Larroche C, Sanromán AM, Du G, et al. Current developments in biotechnology and bioengineering: bioprocesses, bioreactors and controls. Amsterdam: Elsevier; 2017.
- Grieger JC, Soltys SM, Samulski RJ. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol Ther. 2016;24:287–297.
- Van Der Loo JCM, Swaney WP, Grassman E, et al. Scale-up and manufacturing of clinical-grade self-inactivating γ-retroviral vectors by transient transfection. Gene Ther. 2012;19:246–254.
- Greene MR, Lockey T, Mehta PK, et al. Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum Gene Ther Methods. 2012;23:297–308.
- Baumann P, Hubbuch J. Downstream process development strategies for effective bioprocesses: trends, progress, and combinatorial approaches. Eng Life Sci. 2017;17:1142–1158.
- Manceur AP, Kim H, Misic V, et al. Scalable lentiviral vector production using stable HEK293SF producer cell lines. Hum Gene Ther Methods. 2017;28:330–339.
- Vázquez-Ramírez D, Jordan I, Sandig V, et al. High titer MVA and influenza A virus production using a hybrid fed-batch/perfusion strategy with an ATF system. Appl Microbiol Biotechnol. 2019;103:3025–3035.
- Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943.
- Fernandes F, Teixeira AP, Carinhas N, et al. Insect cells as a production platform of complex virus-like particles. Expert Rev Vaccines. 2013;12:225–236.
- Kwang TW, Zeng X, Wang S. Manufacturing of AcMNPV baculovirus vectors to enable gene therapy trials. Mol Ther - Methods Clin Dev. 2016;3:15050.
- Felberbaum RS. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol J. 2015;10:702–714.
- Kamilla S, Virgínia PC. Recombinant Glycoprotein Production: Methods and Protocols. New York: Springer; 2018.
- Segura MM, Mangion M, Gaillet B, et al. New developments in lentiviral vector design, production and purification. Expert Opin Biol Ther. 2013;13:987–1011.
- Coroadinha AS, Gama-Norton L, Amaral AI, et al. Production of retroviral vectors: review. Curr Gene Ther. 2010;10:456–473.
- Otto-Wilhelm M, Al-Rubeai M. Viral vectors for gene therapy. London: Humana Press; 2011.
- Ugai H, Yamasaki T, Hirose M, et al. Purification of infectious adenovirus in two hours by ultracentrifugation and tangential flow filtration. Biochem Biophys Res Commun. 2005;331:1053–1060.
- Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Japanese J Med Sci Biol. 1994;47:157–166.
- Curiel D. Adenoviral vectors for gene therapy. London: Academic Press; 2002.
- Zolotukhin S, Byrne BJ, Mason E, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985.
- Hermens WTJMC, Ter BO, Dijkhuizen PA, et al. Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum Gene Ther. 1999;10:1885–1891.
- Reimer CB, Baker RS, Newlin TE, et al. Influenza virus purification with the zonal ultracentrifuge. Am Assoc Adv Sci. 1966;152:1379–1381.
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245.
- Pyke AT, Phillips DA, Chuan TF, et al. Sucrose density gradient centrifugation and cross-flow filtration methods for the production of arbovirus antigens inactivated by binary ethylenimine. BMC Microbiol. 2004;4:1–8.
- Guo Y, Cheng A, Wang M, et al. Purification of anatid herpesvirus 1 particles by tangential-flow ultrafiltration and sucrose gradient ultracentrifugation. J Virol Methods. 2009;161:1–6.
- Mol EA, Goumans MJ, Doevendans PA, et al. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine Nanotechnology, Biol Med. 2017;13:2061–2065.
- Morita M, Aizawa M, Toi H, et al. Continuous flow ultracentrifuge system for production of infection prevention vaccines. Hitachi Rev. 2011;60:257–261.
- Chen H, Marino S, Ho CY. 97. Large scale purification of AAV with continuous flow ultracentrifugation. Mol Ther. 2016;24:S42.
- Meriño S, Glanzman S. Continuous flow centrifugation: importance in vector scale up. Cell Gene Ther Insights. 2016;2:577–582.
- Seppen J, Barry S, Lam GM, et al. Retroviral preparations derived from PA317 packaging cells contain inhibitors that copurify with viral particles and are devoid of viral vector RNA. Hum Gene Ther. 2000;11:771–775.
- Nasukawa T, Uchiyama J, Taharaguchi S, et al. Virus purification by CsCl density gradient using general centrifugation. Arch Virol. 2017;162:3523–3528.
- Nestola P, Peixoto C, Silva RRJS, et al. Improved virus purification processes for vaccines and gene therapy. Biotechnol Bioeng. 2015;112:843–857.
- Wen Emily P, Ronald Ellis, Narahari S Pujar. Vaccine development and manufacturing. New Jersey: Wiley & Sons; 2015.
- Peixoto C, Sousa MFQ, Silva AC, et al. Downstream processing of triple layered rotavirus like particles. J Biotechnol. 2007;127:452–461.
- Besnard L, Fabre V, Fettig M, et al. Clarification of vaccines: an overview of filter based technology trends and best practices. Biotechnol Adv. 2016;34:1–13.
- Moleirinho MG, Rosa S, Carrondo MJT, et al. Clinical-grade oncolytic adenovirus purification using polysorbate 20 as an alternative for cell lysis. Curr Gene Ther. 2018;18:366–374.
- Vicente T, Roldão A, Peixoto C, et al. Large-scale production and purification of VLP-based vaccines. J Invertebr Pathol. 2011;107:S42–S48.
- Xenopoulos A. A new, integrated, continuous purification process template for monoclonal antibodies: process modeling and cost of goods studies. J Biotechnol. 2015;213:42–53.
- Reeves L, Cornetta K. Clinical retroviral vector production: step filtration using clinically approved filters improves titers. Gene Ther. 2000;7:1993–1998.
- Cherradi Y, Le MS, Sim L, et al. Filter-based clarification of viral vaccines and vectors. Bioprocess Int. 2018;16(4):48–53.
- Kalbfuss B, Genze Y, Wolff MW, et al. Harvesting and concentration of human influenza a virus produced in serum-free mammalian cell culture for the production of vaccines. Biotechnol Bioeng. 2006;97:73–85.
- Thomassen YE, Van ’t Oever AG, Van Oijen MGCT, et al. Next generation inactivated polio vaccine manufacturing to support post polio-eradication biosafety goals. PLoS One. 2013;8:1–12.
- Rodrigues T, Carrondo MJT, Alves PM, et al. Purification of retroviral vectors for clinical application: biological implications and technological challenges. J Biotechnol. 2007;127:520–541.
- Negrete A, Pai A, Shiloach J. Use of hollow fiber tangential flow filtration for the recovery and concentration of HIV virus-like particles produced in insect cells. J Virol Methods. 2014;195:240–246.
- Heath N, Grant L, De Oliveira TM, et al. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci Rep. 2018;8:1–12.
- Xenopoulos A. Production and purification of hepatitis c virus-like particles [Webinar]. EMD Millipore Webinar Ser. 2015.
- Carvalho SB, Silva RJS, Moreira AS, et al. Efficient filtration strategies for the clarification of influenza virus-like particles derived from insect cells. Sep Purif Technol. 2019;218:81–88.
- Yigzaw Y, Piper R, Tran M, et al. Exploitation of the adsorptive properties of depth filters for host cell protein removal during monoclonal antibody purification. Biotechnol Prog. 2006;22:288–296.
- Iammarino M, Nti-gyabaah J, Chandler M, et al. Impact of cell density and viability. Bioprocess Int. 2007;38–50.
- Gousseinov E, Kools W, Pattnaik P. Nucleic acid impurity reduction in viral vaccine manufacturing. Bioprocess Int. 2014;12:59–68.
- Wolf MW, Reichl U. Downstream processing of cell culture-derived virus particles. Expert Rev Vaccines. 2011;10:1451–1475.
- Oxford Biomedica plc. SecNunc™ for AAVs, lentiviral vectors and adenovirus (Application Note), 2019, p. 1–2.
- Hughes K, Zachertowska A, Wan S, et al. Yield increases in intact influenza vaccine virus from chicken allantoic fluid through isolation from insoluble allantoic debris. Vaccine. 2007;25:4456–4463.
- Konz JO, Lee AL, Lewis JA, et al. Development of a purification process for adenovirus: controlling virus aggregation to improve the clearance of host cell DNA. Biotechnol Prog. 2005;21:466–472.
- Vicente T, Sousa MFQ, Peixoto C, et al. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J Memb Sci. 2008;311:270–283.
- Hu J, Ni Y, Dryman BA, et al. Purification of porcine reproductive and respiratory syndrome virus from cell culture using ultrafiltration and heparin affinity chromatography. J Chromatogr A. 2010;1217:3489–3493.
- Nordin JZ, Lee Y, Vader P, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed Nanotechnol Biol Med. 2015;11:879–883.
- Weigel T, Solomaier T, Wehmeyer S, et al. A membrane-based purification process for cell culture-derived influenza A virus. J Biotechnol. 2016;220:12–20.
- Carvalho SB, Silva RJS, Moleirinho MG, et al. Membrane‐based approach for the downstream processing of influenza virus‐like particles. Biotechnol J. 2019;14:1800570.
- Grzenia DL, Wickramasinghe SR, Carlson JO. Ultrafiltration of parvovirus. Sep Sci Technol. 2007;42:2387–2403.
- Grzenia DL, Carlson JO, Czermak P, et al. Purification of densonucleosis virus by tangential flow ultrafiltration. Biotechnol Prog. 2006;22:1346–1353.
- Healthcare GE, Sciences L. Concentration and diafiltration of cell-derived, live influenza virus using 750 C hollow fiber filter cartridge (Application note 29-0928-26 AA). 2014.
- Wickramasinghe SR, Kalbfuß B, Zimmermann A, et al. Tangential flow microfiltration and ultrafiltration for human influenza A virus concentration and purification. Biotechnol Bioeng. 2005;92:199–208.
- Geraerts M, Micheils M, Baekelandt V, et al. Upscaling of lentiviral vector production by tangential flow filtration. J Gene Med. 2005;7:1299–1310.
- Moleirinho MG, Silva RJS, Carrondo MJT, et al. Exosome-based therapeutics: purification using semi-continuous multi-column chromatography. Sep Purif Technol. 2019;224:515–523.
- Benedikter BJ, Bouwman FG, Vajen T, et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci Rep. 2017;7:1–13.
- Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:1–11.
- Marino K, Levison P. Achieving process intensification with single-pass TFF. Genet Eng Biotechnol News. 2017;37:30–31.
- Dizon-Maspat J, Bourret J, D’Agostini A, et al. Single pass tangential flow filtration to debottleneck downstream processing for therapeutic antibody production. Biotechnol Bioeng. 2012;109:962–970.
- Wolff MW, Siewert C, Hansen SP, et al. Purification of cell culture-derived modified vaccinia ankara virus by pseudo-affinity membrane adsorbers and hydrophobic interaction chromatography. Biotechnol Bioeng. 2010;107:312–320.
- Opitz L, Hohlweg J, Reichl U, et al. Purification of cell culture-derived influenza virus A/Puerto Rico/8/34 by membrane-based immobilized metal affinity chromatography. J Virol Methods. 2009;161:312–316.
- Ohtaki N, Takahashi H, Kaneko K, et al. Purification and concentration of non-infectious West Nile virus-like particles and infectious virions using a pseudo-affinity cellufine sulfate column. J Virol Methods. 2011;174:131–135.
- Chand K, Biswas SK, Mondal B. Purification of infective bluetongue virus particles by immuno-affinity chromatography using anti-core antibody. VirusDisease. 2016;27:98–101.
- Zhao M, Vandersluis M, Stout J, et al. Affinity chromatography for vaccines manufacturing: finally ready for prime time?. Vaccine. 2019;37:5491–5503.
- Naso MF, Tomkowicz B, Perry WL, et al. Adeno-Associated Virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317–334.
- Mietzsch M, Grasse S, Zurawski C, et al. OneBac: platform for scalable and high-titer production of AAV serotype 1-12 vectors for gene therapy. Human. 2015;25:212–222.
- Mekkaoui L, Parekh F, Kotsopoulou E, et al. Lentiviral vector purification using genetically encoded biotin mimic in packaging cell. Mol Ther - Methods Clin Dev. 2018;11:155–165.
- Kramberger P, Urbas L, Štrancar A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum Vaccines Immunother. 2015;11:1010–1021.
- Hansen SP, Faber R, Reich M, et al. Purification of vaccinia viruses using hydrophobic interaction chromatography. United States patent US 9,109,201 B2. 2015.
- Weigel T, Soliman R, Wolff MW, et al. Hydrophobic-interaction chromatography for purification of influenza A and B virus. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1117:103–117.
- Li H, Yang Y, Zhang Y, et al. A hydrophobic interaction chromatography strategy for purification of inactivated foot-and-mouth disease virus. Protein Expr Purif. 2015;113:23–29.
- Gagnon P. Chromatographic purification of virus particles. Encycl Ind Biotechnol. 2009; p. 1-21.
- Kuiper M, Sanches RM, Walford JA, et al. Purification of a functional gene therapy vector derived from moloney murine leukaemia virus using membrane filtration and ceramic hydroxyapatite chromatography. Biotechnol Bioeng. 2002;80:445–453.
- Kurosawa Y, Saito M, Kobayashi S, et al. Purification of dengue virus particles by one-step ceramic hydroxyapatite chromatography. World J Vaccines. 2012;02:155–160.
- Tseng YF, Weng TC, Lai CC, et al. A fast and efficient purification platform for cell-based influenza viruses by flow-through chromatography. Vaccine. 2018;36:3146–3152.
- Weigel T, Solomaier T, Peuker A, et al. A flow-through chromatography process for influenza A and B virus purification. J Virol Methods. 2014;207:45–53.
- Corso G, Mäger I, Lee Y, et al. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci Rep. 2017;7:1–10.
- Shen CF, Jacob D, Zhu T, et al. Optimization and scale-up of cell culture and purification processes for production of an adenovirus-vectored tuberculosis vaccine candidate. Vaccine. 2016;34:3381–3387.
- Oksanen HM, Domanska A, Bamford DH. Monolithic ion exchange chromatographic methods for virus purification. Virology. 2012;434:271–277.
- Orr V, Zhong L, Moo-Young M, et al. Recent advances in bioprocessing application of membrane chromatography. Biotechnol Adv. 2013;31:450–465.
- McNally DJ, Darling D, Farzaneh F, et al. Optimised concentration and purification of retroviruses using membrane chromatography. J Chromatogr A. 2014;1340:24–32.
- Opitz L, Lehmann S, Reichl U, et al. Sulfated membrane adsorbers for economic pseudo-affinity capture of influenza virus particles. Biotechnol Bioeng. 2009;103:1144–1154.
- Carvalho SB, Fortuna AR, Wolff MW, et al. Purification of influenza virus-like particles using sulfated cellulose membrane adsorbers. J Chem Technol Biotechnol. 2018;93:1988–1996.
- Krajacic M, Ravnikar M, Štrancar A, et al. Application of monolithic chromatographic supports in virus research. Electrophoresis. 2017;38:2827–2836.
- Gagnon P. The emerging generation of chromatography tools for virus purification. BioProcess Int. 2008;6:24–30.
- Lesch HP, Laitinen A, Peixoto C, et al. Production and purification of lentiviral vectors generated in 293T suspension cells with baculoviral vectors. Gene Ther. 2011;18:531–538.
- Lee MFX, Chan ES, Tan WS, et al. Negative chromatography purification of hepatitis B virus-like particles using poly(oligo(ethylene glycol) methacrylate) grafted cationic adsorbent. J Chromatogr A. 2015;1415:161–165.
- Lee MFX, Chan ES, Tan WS, et al. Negative chromatography of hepatitis B virus-like particle: comparative study of different adsorbent designs. J Chromatogr A. 2016;1445:1–9.
- Yu M, Zhang S, Zhang Y, et al. Microcalorimetric study of adsorption and disassembling of virus-like particles on anion exchange chromatography media. J Chromatogr A. 2015;1388:195–206.
- Yang Y, Yu M, Zhang S, et al. Adsorption of virus-like particles on ion exchange surface: conformational changes at different ph detected bydual polarization interferometry. J Chromatogr A. 2015;1408:161–168.
- Ruscic J, Perry C, Mukhopadhyay T, et al. Lentiviral vector purification using nanofiber ion exchange chromatography. Mol Ther Methods Clin Dev. 2019;15:52–62.
- Turnbull J, Wright B, Green NK, et al. Adenovirus 5 recovery using nanofiber ion-exchange adsorbents. Biotechnol Bioeng. 2019;116:1698–1709.
- Lima TM, Souza MO, Castilho LR. Purification of flavivirus VLPs by a two-step chomatographic process. Vaccine. 2019;37:7061–7069.
- Lin S, Chiu H, Chiang B, et al. Development of EV71 virus-like particle purification processes. Vaccine. 2015;33:5966–5973.
- Tomono T, Hirai Y, Okada H, et al. Ultracentrifugation-free chromatography-mediated large-scale purification of recombinant adeno-associated virus serotype 1 (rAAV1). Mol Ther - Methods Clin Dev. 2016;3:15058.
- Tomono T, Hirai Y, Okada H, et al. Highly efficient ultracentrifugation-free chromatographic purification of recombinant AAV Serotype 9. Mol Ther Methods Clin Dev. 2018;11:180–190.
- Weggeman M, Van Corven EJJ Virus purification methods. United States patent US 2009/0017523 A1; 2009.
- Fernandes P, Peixoto C, Santiago VM, et al. Bioprocess development for canine adenovirus type 2 vectors. Gene Ther. 2013;20:1–8.
- Iyer G, Ramaswamy S, Asher D, et al. Reduced surface area chromatography for flow-through purification of viruses and virus like particles. J Chromatogr A. 2011;1218:3973–3981.
- Lee DS, Kim BM, Seol DW. Improved purification of recombinant adenoviral vector by metal affinity membrane chromatography. Biochem Biophys Res Commun. 2009;378:640–644.
- Jain NK, Sahni N, Kumru OS, et al. Formulation and stabilization of recombinant protein based virus-like particle vaccines. Adv Drug Deliv Rev. 2015;93:42–55.
- Bosch S, De Beaurepaire L, Allard M, et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep. 2016;6:1–11.
- Shoaebargh S, Gough I, Fe M, et al. Sterile filtration of oncolytic viruses : an analysis of effects of membrane morphology on fouling and product recovery. J Memb Sci. 2018;548:239–246.
- Jungbauer A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013;31:479–492.
- Ötes O, Flato H, Ramirez VD, et al. Scale-up of continuous multicolumn chromatography for the protein a capture step: from bench to clinical manufacturing. J Biotechnol. 2018;281:168–174.
- Nestola P, Silva RJS, Peixoto C, et al. Adenovirus purification by two-column, size-exclusion, simulated countercurrent chromatography. J Chromatogr A. 2014;1347:111–121.
- Nestola P, Silva RJS, Peixoto C, et al. Robust design of adenovirus purification by two-column, simulated moving-bed, size-exclusion chromatography. J Biotechnol. 2015;213:109–119.
- Silva RJS, Mota JP, Peixoto C, et al. Improving the downstream processing of vaccine and gene therapy vectors with continuous chromatography. Pharm Bioprocess. 2015;3:489–505.
- Kröber T, Wolff MW, Hundt B, et al. Continuous purification of influenza virus using simulated moving bed chromatography. J Chromatogr A. 2013;1307:99–110.
- Fischer LM, Wolff MW, Reichl U. Purification of cell culture-derived influenza A virus via continuous anion exchange chromatography on monoliths. Vaccine. 2018;36:3153–3160.
- Godawat R, Brower K, Jain S, et al. Periodic counter-current chromatography - design and operational considerations for integrated and continuous purification of proteins. Biotechnol J. 2012;7:1496–1508.
- Lee J, Gan HT, Latiff SMA, et al. Principles and applications of steric exclusion chromatography. J Chromatogr A. 2012;1270:162–170.
- Marichal-Gallardo P, Pieler MM, Wolff MW, et al. Steric exclusion chromatography for purification of cell culture-derived influenza A virus using regenerated cellulose membranes and polyethylene glycol. J Chromatogr A. 2017;1483:110–119.
- Kalsoom U, Nesterenko PN, Paull B. Current and future impact of 3D printing on the separation sciences. TrAC - Trends Anal Chem. 2018;105:492–502.
- Simon U, Dimartino S. Direct 3D printing of monolithic ion exchange adsorbers. J Chromatogr A. 2018;1587:119–128.
- Fee C. 3D-printed porous bed structures. Curr Opin Chem Eng. 2017;18:10–15.
- Rito-palomares M. Aqueous two-phase systems for bioprocess development for the recovery of biological products. Cham: Springer International Publishing; 2017.
- Shin H, Han C, Labuz JM, et al. High-yield isolation of extracellular vesicles using aqueous two-phase system. Sci Rep. 2015;5:1–11.
- Shin H, Park YH, Kim YG, et al. Aqueous two-phase system to isolate extracellular vesicles from urine for prostate cancer diagnosis. PLoS One. 2018;13:1–15.
- Ladd Effio C, Wenger L, Ötes O, et al. Downstream processing of virus-like particles: single-stage and multi-stage aqueous two-phase extraction. J Chromatogr A. 2015;1383:35–46.
- Jacinto MJ, Soares RRG, Azevedo AM, et al. Optimization and miniaturization of aqueous two phase systems for the purification of recombinant human immunodeficiency virus-like particles from a CHO cell supernatant. Sep Purif Technol. 2015;154:27–35.
- Marchel M, Soares HR, Hubbuch J, et al. High-throughput screening of aqueous biphasic systems with ionic liquids as additives for extraction and purification of enveloped virus-like particles. Engineering reports. 2019;1:1–16.
- Torres-Acosta MA, Mayolo-Deloisa K, González-Valdez J, et al. Aqueous two-phase systems at large scale: challenges and opportunities. Biotechnol J. 2019;14:1–12.
- Jagschies G, Lindskog E, Lacki K, et al. Biopharmaceutical processing - development, design, and implementation of manufacturing processes. Amsterdam: Elsevier; 2018.
- Abachin E, Convers S, Falque S, et al. Comparison of reverse-transcriptase qPCR and droplet digital PCR for the quantification of dengue virus nucleic acid. Biologicals. 2018;52:49–54.
- Americo LJ, Earl LP, Moss B. Droplet digital PCR for rapid enumeration of viral genomes and particles from cells and animal infected with orthopoxviruses. Virology. 2017;511:19–22.
- Yang L, Yamamoto T. Quantification of virus particles using nanopore-based resistive-pulse sensing techniques. Front Microbiol. 2016;7:1-7.
- Steppert P, Burgstaller D, Klausberger M, et al. Quantification and characterization of virus-like particles by size-exclusion chromatography and nanoparticle tracking analysis. J Chromatogr A. 2016;1487:89–99.
- Sokolova V, Ludwig AK, Hornung S, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87:146–150.
- Bettonville V, Nicol JTJ, Furst T, et al. Quantitation and biospecific identification of virus-like particles of human papillomavirus by capillary electrophoresis. Talanta. 2017;175:325–330.
- Yang Y, Li H, Li Z, et al. Size-exclusion HPLC provides a simple, rapid, and versatile alternative method for quality control of vaccines by characterizing the assembly of antigens. Vaccine. 2015;33:1143–1150.
- Sitar S, Kejžar A, Pahovnik D, et al. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Anal Chem. 2015;87:9225–9233.
- Burnham B, Nass S, Kong E, et al. Analytical ultracentrifugation as an approach to characterize recombinant adeno-associated viral vectors. Hum Gene Ther Methods. 2015;26:228–242.
- Bousse T, Shore DA, Goldsmith CS, et al. Quantitation of influenza virus using field flow fractionation and multi-angle light scattering for quantifying influenza A particles. J Virol Methods. 2013;193:589–596.
- Kondylis P, Schlicksup CJ, Zlotnick A, et al. Analytical techniques to characterize the structure, properties, and assembly of virus capsids. Anal Chem. 2019;91:622–636.
- Wang X, Morgan DM, Wang G, et al. Residual DNA analysis in biologics development: review of measurement and quantitation technologies and future directions. Biotechnol Bioeng. 2012;109:307–317.
- Gijsbers L, Koel B, Weggeman M, et al. Quantification of residual host cell DNA in adenoviral vectors produced on PER. C6® Cells Hum Gene Ther. 2005;16:393–398.
- Wang Y, Cooper R, Kiladjian A, et al. A digestion-free method for quantification of residual host cell DNA in rAAV gene therapy products. Mol Ther - Methods Clin Dev. 2019;13:526–531.
- Reiter K, Suzuki M, Olano LR, et al. Host cell protein quantification of an optimized purification method by mass spectrometry. J Pharm Biomed Anal. 2019;174:650–654.
- Wang X, Hunter AK, Mozier NM. Host cell proteins in biologics development: identification, quantitation and risk assessment. Biotechnol Bioeng. 2009;103:446–458.
- Farrell A, Mittermayr S, Morrissey B, et al. Quantitative host cell protein analysis using two dimensional data independent LC-MSE. Anal Chem. 2015;87:9186–9193.
- Conry RM, Westbrook B, McKee S, et al. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccines Immunother. 2018;14:839–846.
- Kingstad-Bakke BA, Osoria JE. Zika virus vaccines using virus-like particles. United States patent US 2018/0028643 A1, 2018.
- Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51:1–12.
- Steppert P, Burgstaller D, Klausberger M, et al. Separation of HIV-1 gag virus-like particles from vesicular particles impurities by hydroxyl-functionalized monoliths. J Sep Sci. 2017;40:979–990.