ABSTRACT
Introduction: Treatment of ulcerative colitis (UC) aims to control symptoms and to suppress intestinal inflammation. Despite considerable advances, a proportion of patients do not respond to currently available drugs. The interleukin (IL)-23 axis plays a significant role in the pathogenesis of UC and has thus become an important target for drug development.
Areas covered: The review briefly summarizes the pathophysiology of the IL-12/23 axis and provides a synopsis of the available evidence for efficacy and safety of ustekinumab, mirikizumab (LY3074828), risankizumab (BI655066/ABBV066), brazikumab (MEDI2070; formerly AMG139) and guselkumab (CNTO1959) in UC. We also provide an overview of ongoing and anticipated trials in this field.
Expert opinion: A Phase 2 trial with mirikizumab and a Phase 3 trial with ustekinumab have demonstrated the efficacy of anti-IL-23 agents in achieving clinical and endoscopic outcomes in UC with a favorable safety profile. Trials of other anti-IL-23 agents in UC are under way and designed to explore head-to-head efficacy with existing biologics, as well as the prospect of combination biological therapy. Apart from data on longer term efficacy and safety, future trials should also explore strategies to inform the positioning of IL-23 antagonists in therapeutic algorithms.
1. Introduction
Ulcerative colitis (UC) is a chronic relapsing disease of unknown cause characterized by continuous mucosal inflammation of the colon leading to substantial morbidity and impaired quality of life [Citation1]. The aim of treatment is to achieve symptom control (clinical remission) and to suppress intestinal inflammation (endoscopic remission) [Citation2].
Current treatment options include 5-aminosalicylates, glucocorticoids, thiopurines, the Janus-associated kinase (JAK) inhibitor tofacitinib and biologics [Citation3]. Tumor necrosis factor (TNF) antagonists effectively induce and maintain disease remission [Citation4], but up to one third of patients do not respond to induction treatment and approximately 40% of patients who initially benefited from this treatment lose response subsequently [Citation5]. Moreover, anti-TNF therapy is associated with potentially serious adverse effects [Citation6]. A new class of biologics, anti-integrin antibodies, reduces lymphocyte trafficking to the intestinal mucosa and represent an attractive alternative to TNF inhibitors with usually a more favorable safety profile [Citation7]. In a recent trial vedolizumab, an α4β7 integrin blocker, was shown to be more effective than the TNF inhibitor adalimumab for moderate-severe UC [Citation8]. Nevertheless, new drugs are eagerly awaited and many are in development.
The interleukin (IL)-23 axis is an emerging therapeutic target in IBD, including UC. Initially approved for psoriasis, ustekinumab, an antibody blocking the p40 subunit of both IL-12 and IL-23, was approved for the treatment of Crohn’s disease (CD) [Citation9] and recently for UC as well. Following tildrakizumab, used in psoriasis, a series of more selective p19 subunit (IL-23) antagonists have been designed, which are currently being developed in clinical trial programmes for UC (). In this article, we intend to review the mechanism of action of anti-IL-23 antibodies and provide a synopsis of existing evidence about their efficacy and safety in UC. We also speculate on the future role of IL-23 blockade in treatment algorithms and identify research priorities.
Table 1. Characteristics and main results from trials of interleukin-23 antagonists in ulcerative colitis.
2. Body of review
2.1. Mechanism of action of IL-23 inhibition
IL-23 is a heterodimeric cytokine composed of a unique p19 subunit and a common p40 subunit which it shares with IL-12 [Citation10]. IL-23 engages with the heterodimeric IL-23 receptor (consisting of an IL-23R chain and an IL-12Rβ1 chain), activates intracellular JAKs (mainly through TYK2 and JAK2) and signal transducer and activator of transcription (STAT) pathways and in turn regulates transcription of downstream genes. IL-23 is mainly synthesized by dendritic cells and macrophages, is one of the key promotors of the T helper 17 (Th17) cell pathway [Citation11] ().
Figure 1. The biology of IL-23 and IL-12. These two cytokines are mainly synthesized by dendritic cells and macrophages. Together with transforming growth factor-β, IL-6 and IL-21, IL-23 promotes differentiation of naïve T helper cells to Th17 cells. IL-23 also stimulates group 3 innate lymphoid cells (ILCs) and invariant NKT cells, which produce similar cytokines to Th17 cells: IL-17, IL-21, IL-22 and IL-26. IL-12 promotes differentiation toward Th1 cells, producing IFN-γ. It also stimulates group 1 ILCs to produce the same cytokine.
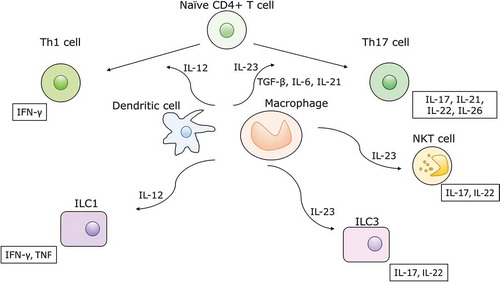
Th17 cells are a subset of effector T helper cells characterized by the production of IL-17, IL-21, IL-22 and IL-26. In health, Th17 cells recruit neutrophils and to a lesser extent monocytes, promote the production of defensins and help maintain the integrity of epithelial barriers, which is critical for the defense against bacterial and fungal infections [Citation12,Citation13]. IL-6 and transforming growth factor-β (TGF-β) in conjunction with T cell receptor activation induce the expression of retinoid-related orphan receptor-γt (ROR-γt), a transcription factor that promotes expression of IL-17 and IL-23 receptors. With the expression of IL-23 receptors on Th17 cells, IL-23 can establish a positive feedback loop leading to the expansion and stabilization of Th17 cells. In addition to Th17 cells, IL-23 also acts on innate lymphoid cells, γδ-T cells, and NKT cells [Citation13]. Sustained expression of IL-22, a Th17 cytokine, has been shown to promote colonic tumorigenesis, which is particularly relevant to UC [Citation14]. Recently, IL-23 blockade has been shown to attenuate the development of intestinal fibrosis in mice [Citation15], which highlights an additional potential therapeutic benefit of this drug class, not only in CD, but also UC [Citation16].
IL-12 promotes the Th1 cell pathway, characterized by the synthesis of interferon-γ (IFN-γ) and TNF. Experimental evidence indicates significant cross-regulation between the IL-23-Th17 and IL-12-Th1 pathway [Citation17,Citation18]. It has been hypothesized that IL-12 mediates more systemic effects, while IL-23 is more specifically implicated in mucosal immunology, but these notions remain unproven in humans [Citation19]. Some preclinical studies showed a higher rate of malignancy with IL-12 blockade compared to IL-23 blockade [Citation20,Citation21], but data are conflicting [Citation22]. Owing to cytokine pleiotropy and the crucial influence of the paracrine milieu, data from experimental models do not permit direct extrapolation to humans.
This caveat is well illustrated by the failed clinical trials of secukinumab (anti-IL-17A) and brodalumab (anti-IL-17RA) in CD [Citation23,Citation24]. Although IL-17 is closely connected to the IL-23 pathway and would therefore represent a rational drug target in inflammatory bowel disease, both studies were terminated early because of disease worsening in the active treatment groups. The IL-17 family consists of six proteins (IL-17A to IL-17F), which have heterogeneous roles with most of them promoting inflammation, but IL-17E being involved in the Th2 response to parasites and the development of allergy [Citation25]. Animal studies following the failed clinical trials have demonstrated an exacerbation of colitis with IL17-A and IL-17RA inhibition owing to a weakened intestinal epithelial barrier [Citation26]. Clinically significant mucocutaneous candidiasis observed in the secukinumab trial also alludes to the potential role of disrupted interactions with the intestinal mycobiome mediated by IL-17.
2.2. Efficacy and safety of anti-IL-23 agents in ulcerative colitis
2.2.1. Ustekinumab
2.2.1.1. Efficacy
Ustekinumab is a human monoclonal antibody against the shared p40 subunit of IL-12 and IL-23, typically administered as an intravenous induction dose, followed by subcutaneous maintenance dosing every 8 or 12 weeks. It has been approved for the treatment of CD, psoriasis and psoriatic arthritis.
In UC, the UNIFI study (NCT 02407236) assessed the efficacy and safety of ustekinumab as induction and maintenance therapy in patients with moderately to severely active UC [Citation27]. In this Phase 3, randomized, double-blind, placebo-controlled study patients with UC who had an inadequate response to or were intolerant of conventional or biologic therapies were randomized 1:1:1 to receive a single intravenous dose of 130 mg or a weight-tiered dose of approximately 6 mg/kg (≤55 kg: 260 mg; 55–85 kg: 390 mg; >85 kg: 520 mg) or placebo. Approximately 50% of the patients had failed prior biologic therapy, 16.6% had failed both vedolizumab and an anti-TNF agent and 51.1% percent were using corticosteroids.
The primary endpoint of UNIFI was clinical remission at week 8 defined as a total Mayo score of ≤2 points with no individual subscore >1, which was attained by 15.6% of the patients in the 130 mg arm, 15.5% in the ~6 mg/kg arm and 5.3% in the placebo arm (P < 0.001). Ustekinumab was also superior to placebo in inducing endoscopic improvement defined as a Mayo endoscopic subscore of 0 or 1 (26.3% vs. 27.0% vs. 13.8%; P < 0.001), histologic improvement defined as the absence of erosion or ulceration, absence of crypt destruction and <5% crypts with neutrophil infiltration (36.8% for combined ustekinumab vs. 21.9% for placebo; P < 0.001) and mucosal healing defined as combined histo-endoscopic remission (19.3% for combined ustekinumab vs. 8.9% for placebo; P < 0.001) [Citation27].
Patients with clinical response (n = 523/961, 54%) to the intravenous induction dose of ustekinumab were rerandomized at week 8 1:1:1 to receiving 90 mg ustekinumab subcutaneously every 8 (q8w) or 12 (q12w) weeks or placebo and were followed up for another 44 weeks. At maintenance baseline, 23.5% of patients were in clinical remission and 37.5% had endoscopic improvement [Citation27].
At week 44 of maintenance treatment (52 weeks after the first induction dose), 43.8% of patients with dosing q8w, 38.4% with dosing q12w and 24.0% of patients receiving placebo were in clinical remission (P < 0.001). Most patients in remission were able to withdraw corticosteroids completely (42.0% with q8w; 37.8% with q12w; 23.4% receiving placebo; P < 0.001). Ustekinumab was superior to placebo in achieving endoscopic improvement (51.1% vs. 43.6% vs. 28.6%; P < 0.001), histologic improvement (59.3% vs. 54.0% vs. 32.9%; P < 0.001) and combined histo-endoscopic mucosal healing (45.9% vs. 38.8% vs. 24.1%; P < 0.001). Among patients who were in clinical remission at maintenance baseline, 65.0% with every 12 weeks dosing (P = 0.011 vs. placebo) and 57.9% with every 8 weeks dosing (P = 0.069 vs. placebo) maintained remission at 44 weeks. Patient-reported outcomes remission, defined as a score of at least 170 on the Inflammatory Bowel Disease Questionnaire (IBDQ), was also significantly higher in patients receiving ustekinumab compared to placebo. Although the study was not powered to compare the two dosing regimens, patients with prior biologic failure were more likely to meet the endpoints during maintenance with dosing every 8 weeks compared to dosing every 12 weeks. In patients without prior biologic failure, outcomes were numerically similar irrespective of dosing regimen.
2.2.1.2. Safety
Ustekinumab had a relatively favorable safety profile in UNIFI. In the induction study, rates of adverse events, serious adverse events (3.7% vs. 3.1% vs. 6.6%), infections (15.9% vs. 15.3% vs. 15.0%) and serious infections (0.6% vs. 0.3% vs. 1.3%) were similar [Citation27]. No malignancies, opportunistic infections or tuberculosis occurred.
In the maintenance study, similar rates of adverse events, serious adverse events (8.5% vs. 7.6% vs. 9.7%), adverse events leading to the discontinuation of the study agent (2.8% vs. 5.2% vs. 11.4%), infections (48.9% vs. 33.7% vs. 46.3%) and serious infections (1.7% vs. 3.5% vs. 2.3%) were reported across treatment arms.
In total, there were three deaths (hemorrhage from esophageal varices, acute respiratory distress syndrome, cardiac arrest in patient with failure to thrive). Seven malignancies were reported in the active treatment group (one each of prostate, colon, renal papillary and rectal; three non-melanoma skin cancers). Four potential opportunistic infections were recorded: cytomegalovirus colitis (two patients), legionella pneumonia, and concurrent ophthalmic and oral herpes simplex virus infection. Three major cardiovascular events occurred (nonfatal cardiac arrest, myocardial infarction, and nonfatal stroke).
Data from registration trials for other indications (CD, psoriasis, psoriatic arthritis) through one year of follow-up with 4521 patient-years for patients treated with ustekinumab, the incidence of infections (125.4 per 100 patient-years vs. 129.4 per 100 patient-years) and serious infections (2.5 per 100 patient-years vs. 4.2 per 100 patient-years) was comparable between ustekinumab and placebo [Citation28]. Among adverse events of particular interest in patients with CD, two serious opportunistic infections (disseminated histoplasmosis and Listeria meningitis) and one presumed case of primary tuberculosis were reported. Despite the favorable safety profile and rarity of serious adverse events, these were more frequent in patients with CD compared to the other two indications, highlighting the potential pitfall of extrapolating safety data across indications.
2.2.2. Mirikizumab
2.2.2.1. Efficacy
Mirikizumab (LY3074828) is a humanized monoclonal antibody against the unique p19 subunit of IL-23, administered as three intravenous doses during a 12-week induction period followed by subcutaneous maintenance dosing every 4 (q4w) or 12 weeks (q12w). Trials in UC, CD (NCT03926130, VIVID-1; NCT02891226, SERENITY) and psoriasis (NCT03482011, NCT03718884, NCT03535194, NCT01947933, NCT02899988, NCT03556202) are ongoing or have recently been completed, but this agent has not yet been approved for any indication.
In a Phase 2, double-blind, randomized, placebo-controlled trial, patients with moderate to severe UC (Mayo score of 6–12, with an endoscopic score of at least 2) who had failed at least one conventional therapy (n = 186) were randomized 1:1:1:1 to receive placebo, mirikizumab 50 mg, mirikizumab 200 mg (both arms with exposure-based dosing with doses increased on day 29 or 57 if mirikizumab serum concentrations were below 0.5 µg/mL in the 50 mg arm or below 2.0 µg/mL in the 200 mg arm) or a fixed dose of mirikizumab 600 mg intravenously at weeks 0, 4 and 8 (NCT02589665, AMAC) [Citation29].
Most patients (63%) were biologic-exposed and 47.3% were using corticosteroids at baseline. After 12 weeks, clinical remission rates defined as a modified Mayo score (excluding physician global assessment) with subscores of 0 for rectal bleeding, 0 or 1 for stool frequency and 0 or 1 for endoscopy in patients treated were higher with 200 mg mirikizumab compared to placebo (22.6% vs. 4.8%, P < 0.01). The clinical remission rates in the other two treatment arms were not significantly different from placebo (15.9% in the 50 mg arm and 11.5% in the 600 mg arm). The rate of endoscopic improvement was higher in the 50 mg arm (23.8%) and 200 mg arm (30.6%) compared to placebo (6.3%; P < 0.05 and P < 0.001 respectively), but not in the 600 mg arm (13.1%). Although the exposure increased with dose, the pharmacodynamic differences between the active treatment arms are difficult to interpret at present, possibly due to the small sample sizes and the refractoriness of the population with the majority having been treated with two or more biologics.
In the AMAC trial, clinical responders to induction treatment were rerandomized to receive 200 mg mirikizumab subcutaneously q12w or q4w as a purely observational maintenance experiment. Eighty-six of the ninety-three randomized patients (92%) completed the study through week 52, when clinical remission was attained in 46.8% of patients with q4w dosing and 37.0% of patients with q12w. Interestingly, there was no difference in remission rates by prior biologic exposure. Among patients who only achieved clinical response without remission by week 12, 37.9% with q4w dosing and 36.4% with q12w dosing reached clinical remission at week 52. Week 12 non-responders received open-label mirikizumab with an additional 12 weeks of ‘extended induction’ (initially 600 mg IV q4w [n = 20], increased to 1000 mg IV q4wfollowing a protocol amendment [n = 64]) [Citation30]. Patients with clinical response at 24 weeks after extended induction received maintenance treatment at 200 mg mirikizumab subcutaneously q4w. Following extended induction 15.0% of the patients with 600 mg dosing and 9.4% of those with 1000 mg dosing achieved clinical remission at week 24.
Phase 3 randomized, double-blind, placebo-controlled studies for induction (NCT03518086, LUCENT 1) and maintenance (NCT03524092, LUCENT 2) treatment with mirikizumab in UC are ongoing and so is an open-label extension program (NCT03519945, LUCENT 3).
2.2.2.2. Safety
Although the study population was relatively small, mirikizumab appeared to have a favorable safety profile during induction with 54.1% of treatment-emergent adverse events across all treatment arms compared to 50.8% in the placebo arm. Serious adverse events were rare across all treatment groups and adverse events leading to discontinuation occurred in <5% [Citation29]. In the maintenance phase, there were no concerning or unexpected safety signals either. The commonest treatment-emergent adverse events (defined by a rate of at least 10% in any of the two groups) were nasopharyngitis, worsening of UC, headache, upper respiratory tract infections and arthralgia. The induction Phase 2 study in CD [Citation31] showed no additional safety concerns.
A Phase 2 study in psoriasis identified some additional isolated adverse events not seen in patients with inflammatory bowel disease, namely hypertension, suicidal ideation in two patients with known psychiatric illness and elevated liver enzymes in a patient with a history of alcohol abuse and hypercholesterolemia [Citation32].
2.2.3. Other IL-23 antagonists
2.2.3.1. Risankizumab
Risankizumab (BI655066/ABBV066) is a humanized monoclonal antibody against the p19 subunit of IL-23. It has been approved for moderate to severe plaque psoriasis. A Phase 2 study with open-label extension has been completed in CD [Citation33,Citation34], studies are ongoing or have recently been completed for psoriatic arthritis (NCT02719171), hidradenitis suppurativa (NCT03926169), ankylosing spondylitis (NCT02047110), asthma (NCT02443298) and atopic dermatitis (NCT03706040).
A Phase 2/3 randomized, double-blind, placebo-controlled trial for induction treatment (NCT03398148) and a Phase 3 randomized, double-blind, placebo-controlled trial for maintenance treatment (NCT03398135) are ongoing in moderate to severe UC.
Safety data are only available for the CD phase 2 study, in which the rate of adverse events was similar between patients treated with risankizumab and those receiving placebo [Citation33]. Infusion/injection reactions were rare, and were mild or moderate. Three serious infections were observed in the active treatment arms (pneumonia, osteomyelitis, anal abscess). The commonest adverse events in the open-label maintenance study were arthralgia, headache, abdominal pain, nasopharyngitis, nausea, and pyrexia [Citation34].
2.2.3.2. Brazikumab
Brazikumab (MEDI2070, previously known as AMG 139) is a human monoclonal antibody against the p19 subunit of IL-23. It was studied in a Phase 2a study in CD [Citation35], a Phase 2/3 study is ongoing (NCT03759288). In moderate to severe UC, two randomized, double-blind, double-dummy, placebo and active-controlled Phase 2 studies are ongoing (NCT03616821, EXPEDITION), one of them with vedolizumab as active comparator.
There are no data on safety for brazikumab in UC. In CD, the drug’s safety profile was similar to placebo with adverse events reported in 67.8% patients in the brazikumab arm and 68.3% in the placebo arm during the double-blind period of the study [Citation35]. The commonest adverse event was headache, occurring in 16.9% of patients treated with brazikumab. The rate of serious adverse events was also similar between the two arms (8.5% vs. 8.3%). Significant infections occurred in four patients treated with brazikumab (bronchitis, cellulitis, gastroenteritis and subcutaneous abscess.
2.2.3.3. Guselkumab
Guselkumab (CNTO 1959) is yet another human monoclonal antibody against the p19 subunit of IL-23. It has been approved for moderate to severe plaque psoriasis. A Phase 2/3 study is recruiting in CD (NCT03466411, GALAXI) and other studies are ongoing in UC, adenomatous polyposis coli (NCT03649971) and psoriatic arthritis (NCT03796858).
A Phase 2a randomized, double-blind, active-controlled study is evaluating the efficacy and safety of combination treatment with guselkumab and golimumab in moderate so severe UC (NCT03662542, VEGA). This represents the first effort to enhance efficacy by combining antibodies with distinct targets. A Phase 2b/3 randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of guselkumab monotherapy in patients with moderate to severe UC started in September 2019 (NCT04033445, QUASAR).
No safety data for guselkumab in inflammatory bowel disease is currently available. Studies in psoriasis and psoriatic arthritis showed no major safety concerns [Citation36,Citation37].
3. Conclusion
Agents targeting IL-23, either specifically (anti-p19) or in conjunction with IL-12 (anti-p40) have shown promising efficacy in Phase 2 and 3 trials in UC. They were effective in achieving both clinical and endoscopic outcomes in biologic naïve patients as well as in the more challenging population of patients who had failed one or more biologics. Their safety profile in inflammatory bowel disease and other indications is favorable with rare serious adverse events and a comparable rate of adverse events to placebo, although longer term data are still lacking. It appears, without head-to-head comparisons being available, that antibodies targeting IL-23 do not have side effects associated with immunosuppression that are commonly seen with TNF antagonists.
Phase 2 and 3 trials of other anti-p19 agents (risankizumab, brazikumab, guselkumab) are ongoing and will provide further information not only on their efficacy and safety per se, but also on head-to-head efficacy compared to existing biologics and on the evolving therapeutic concept of combination treatment with multiple biologics.
4. Expert opinion
Anti-TNF agents have improved the management of UC, with the intravenous agent infliximab still being the most potent available agent for severe UC. Subcutaneous anti-TNF agents (adalimumab, golimumab) have only moderate efficacy in ulcerative colitis and recently the anti-integrin vedolizumab was found to be more effective than adalimumab [Citation8].
The recent approval of the oral JAK inhibitor tofacitinib has provided an additional treatment opportunity for patients who had either failed previous treatment or suffered serious side effects, although safety concerns related to thromboembolic side effects need to be taken into account [Citation38]. The position that sphingosine-1-phosphate receptor modulators, such as ozanimod and etrasimod, will eventually take depends on the outcome of several ongoing phase 3 trials.
Successful management of patients with moderate-severe, corticosteroid-dependent and refractory UC is a formidable challenge, among others because the efficacy of the second drug is usually considerably lower than in bio-naïve patients [Citation39,Citation40]. This observation was not made in the phase 2 mirikizumab trial, however. The development of anti-IL-23 agents reflects the progress in our understanding of disease pathophysiology and will probably mean a significant add-on in the therapeutic armamentarium in inflammatory bowel disease.
Taking all data together, however, it is uncertain that IL-23 blockade will lead to superior outcomes than TNF-blockade. The most significant gain appears to lie in the very reassuring safety profile of IL-23 inhibitors, which creates the opportunity to combine these agents with drugs that target different mechanisms. The most appealing combination from a safety standpoint is anti-IL-23 with anti-integrin antibodies. From a pathophysiological standpoint it may be the combination of anti-IL-23 and anti-TNF, since some evidence suggests that refractoriness to anti-TNF may be triggered by upregulation of the IL-23 pathway. This question is being answered in the guselkumab-golimumab combination trial.
The pharmacokinetics of IL-23 antibodies remain poorly understood. An exposure-response relationship was demonstrated for ustekinumab in CD [Citation41] and it appears that doses higher than those currently approved may be necessary to achieve serum concentrations sufficient for endoscopic remission [Citation42]. Accumulating data suggest that both intravenous reinduction [Citation43] and dosing interval shortening can be successful in recapturing clinical response [Citation44], but target drug concentrations remain unknown and the pharmacodynamics effects may appear in a time- rather than dose-dependent fashion. In UC, outcomes during maintenance treatment were numerically better with q8w than q12w dosing, suggesting an exposure-response relationship in UC, too. Currently available pharmacokinetic data for induction treatment with mirikizumab are difficult to interpret as efficacy did not appear to mirror serum concentrations.
It remains unresolved, whether blocking IL-23 alone is more effective than blocking both IL-12 and IL-23. Cytokine biology is complex and the results of interfering with a particular signaling pathway are consequently unpredictable, as illustrated by the failure of anti-IL-17 agents in CD despite sound preclinical data [Citation23,Citation24]. Based on the limited available data in UC, only cautious indirect comparisons can be made with numerically similar outcomes for anti-p19 (mirikizumab) and anti-p40 agents (ustekinumab) despite a higher percentage of patients who had failed biologic therapy in the former study. While extrapolation across indications can be misleading, two intriguing insights can be drawn from trials in psoriasis. Selective IL-23 blockade with risankizumab was more effective than nonselective p40 blockade with ustekinumab [Citation45,Citation46] and a substantial proportion of non-responders to ustekinumab subsequently benefited from selective p19 inhibition with guselkumab [Citation47]. Despite reassuring available data, only long-term safety studies may give an answer to the theoretical concerns about the safety of concomitant IL-12 inhibition in terms of malignancy. The evolution of clinical trial design in anti-IL-23 will address two poorly studied concepts in inflammatory bowel disease: combination biological therapy and head-to-head comparisons of biological agents.
Choosing the right biologic drug for the right patient at the right time remains an imperfect practice. Our choices are based on the perceived safety profile and risks, comorbidities, disease phenotype and route of administration, which are, admittedly, superficial and fail to capture the full complexity of inflammatory bowel disease. One obvious subgroup of patients where anti-IL-23 agents could be useful are those who developed paradoxical psoriasis with anti-TNF therapy. Increasingly specific immunosuppression holds promise for disease-specific action with minimal adverse effects. It remains to be seen if anti-IL-23 antibodies can fulfil these expectations. Should this be the case, it is plausible that they could become first-line biologics in UC.
In summary, initial data on anti-IL-23 agents in UC are promising and provide a solid foundation for the further development of this drug class. Nevertheless, future trials will also need to focus on issues beyond efficacy and safety in other to help clinicians navigate an increasingly complex therapeutic landscape riddled with uncertainty regarding optimum drug selection.
Article Highlights
The IL-12/23 axis is an important emerging therapeutic target in ulcerative colitis
Several monoclonal antibodies targeting the p19 subunit of IL-23 are advancing through clinical testing
A Phase 2 trial in mirikizumab and a Phase 3 trial in ustekinumab have demonstrated efficacy of these agents in moderate-to-severe ulcerative colitis with a reassuring safety profile
Data on other agents, as well as long-term efficacy and safety studies are anticipated
This box summarizes key points contained in the article.
Declaration of interest
J Hanžel has received speaker fees from Biogen, Janssen and Takeda. GR D’Haens has served as advisor for Abbvie, Ablynx, Allergan, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meiers Squibb, Boerhinger Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Covidien/Medtronics, Ferring, DrFALK Pharma, Eli Lilly, Engene, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Hospira/Pfizer, Immunic, Johnson and Johnson, Lycera, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prometheus laboratories/Nestle, Progenity, Protagonist, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor; received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
Additional information
Funding
References
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389:1756–1770.
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338.
- Hindryckx P, Vande Casteele N, Novak G, et al. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drug[s] for our patients? J Crohns Colitis. 2018;12:105–119.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
- Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135.
- Singh J, Wells G, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2:CD008794.
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710.
- Sands BE, Peyrin-Biroulet L, Loftus EV, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381:1215–1226.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960.
- Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725.
- Patel DD, Kuchroo VK. Th17 cell pathway in human immunity: lessons from genetics and therapeutic interventions. Immunity. 2015;43:1040–1051.
- Gaffen SL, Jain R, Garg AV, et al. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600.
- Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16:185–196.
- Huber S, Gagliani N, Zenewicz LA, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263.
- Mathur R, Alam MM, Zhao X-F, et al. Induction of autophagy in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol. 2019;12:612–623.
- Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. 2019;65:100–109.
- Becker C, Dornhoff H, Neufert C, et al. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–2764.
- O’Connor Jr W Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell–mediated intestinal inflammation. Nat Immunol. 2009;10:603–609.
- Uhlig HH, McKenzie BS, Hue S, et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318.
- Airoldi I, Di Carlo E, Cocco C, et al. Lack of IL12rb2 signaling predisposes to spontaneous autoimmunity and malignancy. Blood. 2005;106:3846–3853.
- Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465.
- Street SEA, Trapani JA, MacGregor D, et al. Suppression of lymphoma and epithelial malignancies effected by interferon γ. J Exp Med. 2002;196:129–134.
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700.
- Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2016;111:1599–1607.
- Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: from discovery to targeting. Trends Mol Med. 2016;22:230–241.
- Maxwell JR, Zhang Y, Brown WA, et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity. 2015;43:739–750.
- Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214.
- Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf. 2019;42:751–768.
- Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2019; published ahead of print.
- Sandborn WJ, Ferrante M, Bhandari BR, et al. Extended treatment with mirikizumab in patients with moderately-to-severely active ulcerative colitis: results from a phase 2 trial. Gastroenterology. 2019;156:S1094.
- Sands BE, Sandborn WJ, Peyrin-Biroulet L, et al. Efficacy and safety of Mirikizumab (LY3074828) in a phase 2 study of patients with Crohn’s disease. Gastroenterology. 2019;156:S216.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY 3074828) in the treatment of moderate‐to‐severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88–95.
- Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–1709.
- Feagan BG, Panés J, Ferrante M, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3:671–680.
- Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153:77–86.
- Deodhar A, Gottlieb AB, Boehncke W-H, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213–2224.
- Reich K, Papp K, Armstrong A, et al. Safety of guselkumab in patients with moderate‐to‐severe psoriasis treated through 100 weeks: a pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2019;180:1039–1049.
- FDA. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) [Internet]. 2019. Available from: Last accessed 2019 Sept 6. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and
- Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease - a prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44:1199–1212.
- Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY consortium. Am J Gastroenterol. 2018;113:1345.
- Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology. 2018;154:1660–1671.
- Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn’s disease only in part explains limited endoscopic remission rates. J Crohns Colitis. 2019;13:864–872.
- Park S, Evans E, Sandborn WJ, et al. Ustekinumab IV 6 mg/kg loading dose re-induction improves clinical and endoscopic response in Crohnʼs disease: a case series. Am J Gastroenterol. 2018;113:627–629.
- Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohnʼs disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis. 2017;23:833–839.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus Ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551–1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–661.
- Langley RG, Tsai T‐F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double‐blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114–123.
