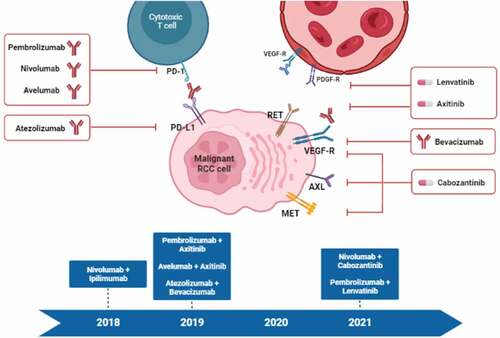
In the last few years, kidney cancer has undergone one of the most revolutionary progresses in terms of ever-growing treatment options. The clear cell renal cell carcinoma (ccRCC) therapeutic scenario can be described as increasingly fervent, both considering the novelties in the locoregional disease stage, with the introduction of adjuvant immunotherapy after nephrectomy, and the abundance of combination strategies now available in the first-line setting () () [Citation1–12]. The management of metastatic RCC (mRCC) was firstly renovated thanks to the introduction of targeted therapies against vascular endothelial growth factor receptor (VEGFR) or mammalian target of rapamycin (mTOR), which rapidly replaced the dated and unsatisfactory ‘dark age’ of cytokines [Citation3]. Another turning point that marked the end of the so-called ‘modern age’ ruled by tyrosine kinase inhibitors (TKIs), was the approval in the second-line setting of nivolumab, a programmed death-1 (PD-1) immune checkpoint inhibitor (ICI) [Citation4]. Consequently, the focus of the scientific community was promptly moved to the investigation of agents able to replace the host immune response against malignant cells, thanks to the blockade of critical immune checkpoints, giving birth to the ‘golden age’ of advanced RCC treatment [Citation5]. In very recent years, the therapeutic armamentarium has additionally been enlarged thanks to the introduction of doublets composed of an ICI and a TKI or a second ICI targeting the cytotoxic T-lymphocyte–associated Protein 4 (CTLA-4) [Citation2–11]. These immunocombinations will promptly lead RCC treatment to the revolutionary ‘diamond age,’ with the ambitious aim to further improve outcomes in the advanced disease setting [Citation7].
Table 1. Randomized phase III clinical trials testing immunocombinations in ccRCC.
The ever-changing expansion of RCC therapeutic scenario is progressively improving survival outcomes and quality of life of patients affected by this disease, mainly as a result of ICI-based combinations’ approval. However, real-world data is showing that ‘one-size does not fit all,’ unveiling the physicians’ need for discerning between patients who may benefit most from novel immunocombinations. It should be also understood how to match the most adequate ICI-based regimen (among the several ones approved) with each mRCC patient with his distinct characteristics [Citation16]. The direct comparison between immunocombinations’ landmark trials is not easily feasible, due to many differences between planned end points, enrolled patients, time of follow-up, and risk classes. To the current day, the validation of predictive biomarkers for RCC is still an unmet clinical need, and, consequently, international guidelines only suggest guiding therapeutic decision-making on the basis of the single patient’s clinical risk. Furthermore, risk stratification tools were developed before the beginning of the immunotherapy-era and they currently seem to be less accurate in differentiating responsiveness to novel combinations while apparently maintaining a certain prognostic value.
When opting for an ICI-doublet or ICI/TKI regimen, we should take into account the symptomatic or broad disease burden. Given their pharmacodynamic profiles, ICI/TKI combinations may be considered for patients with symptomatic and high disease burden in which a fast tumor shrinkage is required, as these regimens have been characterized by the higher ORR over the ICI-doublet combination, regardless of International Metastatic RCC Database Consortium (IMDC) criteria [Citation16]. Conversely, for asymptomatic patients in which a fast tumor-size decrease is not needed, the ICI/ICI combination may be favored, in the light of its durable response along with the longer treatment-free interval [Citation16]. It is also important to keep in mind that mRCC patients with sarcomatoid features are shown to benefit the most from ICI-based combinations, mainly from the nivolumab plus ipilimumab regimen, due to the immuno-inflamed phenotype that characterized this form of dedifferentiation.
Anti-VEGF targeted therapy still represents a valid approach in some untreated mRCC patients. Firstly, IMDC good risk patients can gain benefit from TKI monotherapy, especially when characterized by a low tumor burden and an indolent course of disease. The subgroup analysis of CheckMate 214 trial showed that survival benefits tend to be greater with sunitinib than with nivolumab/ipilimumab in these patients [Citation17]. Focusing on ICI/TKI combinations, survival benefits of good risk patients appeared inferior or comparable to those of the unselected population. Consequently, using the immunocombinations in these patients may represent an overtreatment, hence exposing them to increased toxicity risk and eliciting avoidable resistance.
TKI monotherapy could also be particularly helpful in patients whose RCC has a defined angiogenic signature, even though gene expression profiles (GEPs) have not yet been validated as predictive biomarkers in mRCC, thus hampering the identification of these patients in everyday clinical practice [Citation18]. Nevertheless, the phase II BIONIKK trial has been the first study to prospectively assess personalized therapies taking into account GEPs in mRCC and dividing patients into separate cohorts based on distinct molecular subtypes. Each cohort received a single-agent TKI, an ICI monotherapy or an ICI-doublet combination, with recent data suggesting that a biomarker-driven decision-making process is feasible in mRCC management, especially by identifying TKI monotherapy-sensitive angiogenesis-rich patients [Citation19].
Moreover, TKI could be administered in patients who cannot tolerate the combination-related toxicities (i.e. unfit and/or elderly people) or are ineligible for ICI-based regimensfor example, due to significant comorbidities (i.e. severe autoimmune diseases) [Citation16].
Immunotherapy seems to be a promising approach even in non-clear cell RCC (nccRCC), although final results from KEYNOTE-427 are still awaited and other trials testing immunotherapy alone or in combination with targeted therapy are ongoing [Citation20]. A recent breakthrough in the treatment of papillary RCC (pRCC) is represented by cabozantinib, tested in the phase II PAPMET trial, that really appears to be the most effective therapy in this setting [Citation21]. Great interest is addressed to PAPMET2 (NCT05411081), the ongoing trial designed to define the potential additional effect of atezolizumab in metastatic pRCC patients receiving cabozantinib.
As the number of available systemic treatments increases, establishing the most accurate therapeutic iter based on particular and validated biomarkers is one of the most critical purposes for future researchers. A plethora of prognostic and predictive factors have been tested, with most of the data deriving from studies on metastatic patients with ccRCC. Biomarkers such as programmed death ligand-1 (PD-L1) or tumor mutational burden (TMB) failed as potential drivers for therapeutic process in kidney cancer while having a confirmed role in other malignancies (i.e. non-small cell lung cancer). Conversely, other markers are progressively affirming their prognostic or predictive value in this setting, including PBRM1, SETD2, BAP1 mutations, or tumor microenvironment features. Probably, the integration of different biomarkers will help improve the predictive ability to detect responders to ICI-based therapies. Furthermore, omics-based approaches are emerging even more in this disease, in order to highlight biologically driven patient subgroups. The above-mentioned BIONIKK study recently pointed out a noteworthy overlap between certain GEPs and IMDC risk groups [Citation19]. The integration of this biomarker selection approach, alongside IMDC classes, surely deserves future attention. However, further extensive and prospective trials are required for validation.
Another expanding area of research is one of the metabolic reprogramming, that represents a cornerstone of carcinogenesis and typically characterizes RCC, so that this tumor has been defined as a ‘metabolic disease.’ Indeed, this type of tumor presents mutations in genes involved in metabolic pathways, such as VHL (implicated in cellular oxygen detection) and PI3K/Akt pathway (correlated to aerobic glycolysis or ‘Warburg effect,’ which is maximally expressed in ccRCC) [Citation22]. Metabolic alterations found in RCC result in increased glycolysis, pentose phosphate pathway, and glutamine uptake and downregulation of tricarboxylic acid cycle. All these metabolic modifications induce the production of metabolites that regulate epigenetic factors, correlated to tumor development.
Metabolic factors could also interact with and influence the immune response, thus possibly affect response to ICIs. Several studies pointed out that RCC could be divided into immune infiltrated or non-infiltrated tumors. ccRCC with upregulated immune markers was shown to be associated with increased metabolic processes, underlying the important crosstalk between these two pathways [Citation23]. Furthermore, the study by Wang Y et al. divided ccRCC in four subtypes on the basis of the interaction between the immune and metabolic activity: M1 low metabolic activity (but higher than M3) and low immune infiltration (but higher than M2), M2 with the highest metabolic activity and the lowest immune infiltration, M3 with the lowest metabolic activity and highest immune infiltration, M4 with high metabolic activity (but lower than M2) and high immune infiltration (but lower that M3). These subgroups presented different prognoses, with M1 and especially M2 being the groups with worse prognosis. Moreover, taking into account a cohort of ccRCC treated with ICIs, the M3 subtype resulted to be associated with higher response rates [Citation24]. This balance between the immune and metabolic processes suggests that a tumor microenvironment enriched with metabolic activity could suppress the immune response, thus supporting tumor growth.
Another major issue to be addressed is defining the most adequate sequence of therapeutic strategies for each mRCC patient. To date, only few prospective data assessing what to do after failure to first-line ICI-based combinations are available, although several prospective studies are ongoing. This available data as well as the international guidelines recommend the use of not previously exploited anti-VEGF TKIs as second-line therapy. Post-hoc exploratory analyses of landmark KEYNOTE-426 and CLEAR trials, presented during the American Society of Clinical Oncology (ASCO) 2022 Annual Meeting, described the anti-VEGF TKI as the most common subsequent therapy for patients treated with both first-line immunocombinations, whereas in patients treated with sunitinib the second line was more evenly distributed between TKI and ICI [Citation25,Citation26].
Nowadays, current evidence seems to encourage the use of cabozantinib in patients who progressed after nivolumab/ipilimumab or combinations of an ICI plus a TKI other from cabozantinib and in primary refractory patients after first-line therapy [Citation27]. However, defining the subsequent treatment for mRCC patients progressed on first-line nivolumab/cabozantinib combination is a problem that needs to be overwhelmed as soon as possible, as the aforementioned progression would preclude the use of two milestones in the second-line therapy of mRCC. Hopefully, several new compounds are under investigation (alone or in combination) even in pretreated patients, such as belzutifan (NCT04586231, NCT04195750), batiraxcept (NCT04300140), telanaglestat (NCT01038778), and others.
Another unsolved question is what to administer at relapse in patients subjected to adjuvant pembrolizumab. At the ASCO 2022 Annual Meeting were presented data of the expanded efficacy analysis of the KEYNOTE-564, in which adjuvant pembrolizumab was associated with reduced risk for time to first subsequent therapy (TFST) and better progression-free survival 2 (PFS2) compared with placebo and in which was highlighted that patients who received at least one line of subsequent therapy (90.0% in the pembrolizumab group and 85.9% in the placebo one) received a VEGF/VEGFR-targeted therapy [Citation28]. Of the 40 PFS2 events in the pembrolizumab group, 12 were deaths and 28 progressions. The choice of subsequent treatment should also take into account that 61.1% of patients in the pembrolizumab arm completed the full 17 cycles of trial treatment, while in 21.3% of cases treatment was discontinued due to adverse events. In this latter group, another immunotherapy treatment may not be feasible due to cross toxicities. Another compelling factor to underline considering the population enrolled in the KEYNOTE-564 is that 5,8% of patients presented radically resected metastatic disease (M1 no evidence of disease – NED). In this subgroup, pembrolizumab confirmed to be associated with an improved disease-free survival even though this subset of patients should be considered at higher risk of relapse. The treatment choice in patients with M1 NED that completed adjuvant pembrolizumab could be challenging, considering that these patients already received immunotherapy for a long period of time.
So far, great work has been done, but extra efforts are definitely needed to resolve all these unanswered issues, thus helping clinicians to select the most effective therapy in every setting of the disease, possibly relying on validated biomarkers that may contribute to address the best therapy to the patient who may benefit the most from it.
Declaration of Interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385(8):683–694.
- Motzer RJ, Powles T, Atkins MB, et al. Final overall survival and molecular analysis in IMmotion151, a phase 3 trial comparing atezolizumab plus bevacizumab vs sunitinib in patients with previously untreated metastatic renal cell carcinoma. JAMA Oncol. 2022;8(2):275–280.
- Iacovelli R, Massari F, Albiges L, et al. Evidence and clinical relevance of tumor flare in patients who discontinue tyrosine kinase inhibitors for treatment of metastatic renal cell carcinoma. Eur Urol. 2015;68(1):154–160.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813.
- Massari F, Nunno VD, Mollica V, et al. Immunotherapy in renal cell carcinoma from poverty to the spoiled of choice. Immunotherapy. 2019;11(17):1507–1521.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290.
- Massari F, Rizzo A, Mollica V, et al. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer. 2021;154:120–127.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–841.
- Motzer RJ, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(7):888–898.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127.
- Powles T, Plimack ER, Soulieres D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020 Dec;21(12):1563–1573.
- Motzer R, Alekseev B, Rha S-Y, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–1300.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115.
- Choueiri TK, Motzer RJ, Rini BI, et al. Updated efficacy results from the JAVELIN renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–1039.
- Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415.
- Rizzo A, Rosellini M, Marchetti A, et al. Determinants of treatment for first-line immune-based combinations in metastatic renal cell carcinoma: a critical overview of recent evidence. Immunotherapy. 2021;13(8):685–692.
- Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079.
- Verbiest A, Renders I, Caruso S, et al. Clear-cell renal cell carcinoma: molecular characterization of IMDC risk groups and sarcomatoid tumors. Clin Genitourin Cancer. 2019;17(5):e981–e994.
- Vano YA, Elaidi R, Bennamoun M, et al. Nivolumab, nivolumab-ipilimumab, and VEGFR-tyrosine kinase inhibitors as first-line treatment for metastatic clear-cell renal cell carcinoma (BIONIKK): a biomarker-driven, open-label, non-comparative, randomised, phase 2 trial. Lancet Oncol. 2022;23(5):612–624.
- McDermott DF, Lee JL, Ziobro M, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021;39(9):1029–1039.
- Pal SK, Tangen C, Thompson IMsJr, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. 2021;397(10275):695–703.
- Lucarelli G, Loizzo D, Franzin R, et al. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev Mol Diagn. 2019;19(5):397–407.
- Wang Y, Zheng XD, Zhu GQ, et al. Crosstalk between metabolism and immune activity reveals four subtypes with therapeutic implications in clear cell renal cell carcinoma. Front Immunol. 2022;13:861328.
- Braun DA, Hou Y, Bakouny Z, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26(6):909–918.
- Powles T, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): analysis of progression after first subsequent therapy in KEYNOTE-426. J Clin Oncol. 2022;40:4513.
- Voss MH, Powles T, McGregor BA, et al. Impact of subsequent therapies in patients (pts) with advanced renal cell carcinoma (aRCC) receiving lenvatinib plus pembrolizumab (LEN + PEMBRO) or sunitinib (SUN) in the CLEAR study. J Clin Oncol. 2022;40(16_suppl):4514.
- Santoni M, Massari F, Bracarda S, et al. Cabozantinib in patients with advanced renal cell carcinoma primary refractory to first-line immunocombinations or tyrosine kinase inhibitors. Eur Urol Focus. 2022;S2405-4569(22):00049–9. DOI:10.1016/j.euf.2022.02.004.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab for postnephrectomy renal cell carcinoma (RCC): expanded efficacy analyses from KEYNOTE-564. J Clin Oncol. 2022;40(16_suppl):4512.