 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Inclacumab is a recombinant, fully human, immunoglobulin IgG4 monoclonal antibody that selectively binds to P-selectin. Initially discovered and developed by Roche through phase 2 clinical studies in peripheral arterial disease and coronary artery disease, inclacumab has been in-licensed by Global Blood Therapeutics (GBT) as a potential treatment to reduce the frequency of vaso-occlusive crises in individuals with sickle cell disease.
Research design and methods
GBT sought to demonstrate the analytical comparability between material produced by Roche and material produced by GBT to ensure that no meaningful differences in identity, safety, purity, potency, or bioavailability exist between the GBT and Roche lots.
Results
Inclacumab samples produced by GBT were found to be comparable to the Roche v0.2 inclacumab samples based on (1) comparable primary and higher-order structures; (2) comparable purity profiles; (3) comparable potency, in vitro functional activities, and in vivo plasma exposures and pharmacokinetic profiles; and (4) comparable degradation patterns and kinetics under forced degradation conditions.
Conclusions
Based on the design of this comparability study and the results obtained, the US Food and Drug Administration approved the changes to the manufacturing process and gave clearance for GBT to proceed with phase 3 clinical trials.
1. Introduction
P-selectin is a cell adhesion molecule expressed on the surface of platelets and endothelial cells that have been activated in response to vascular injury and/or inflammation [Citation1,Citation2]. It plays an essential role in facilitating the multicellular interactions that occur during thrombotic and inflammatory processes and is, therefore, implicated in the pathogenesis of numerous diseases, including coronary artery disease, stroke, diabetes, and malignancy [Citation2,Citation3]. P-selectin is also known to contribute to the occurrence of vaso-occlusive crises (VOCs) in patients with sickle cell disease (SCD) [Citation4,Citation5]. VOCs are thought to be caused by the adhesion of leukocytes, platelets, and sickled red blood cells to the endothelium of blood vessels, resulting in vascular obstruction, tissue ischemia, pain, and in some cases, death [Citation5,Citation6].
Inclacumab is a recombinant, fully human, immunoglobulin G4 (IgG4) monoclonal antibody (mAb) that selectively binds to P-selectin, blocking the interaction with its primary ligand, P-selectin glycoprotein ligand-1 (PSGL-1), and subsequently inhibiting P-selectin–mediated adhesive functions [Citation1,Citation2,Citation7–9]. Proof of concept for the role of P-selectin inhibition in reducing VOCs in patients with SCD has been established by US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval of the humanized IgG2 P-selectin mAb crizanlizumab (Adakveo®) [Citation10,Citation11]. Inclacumab has several potential advantages over crizanlizumab: a distinct epitope that directly overlaps the PSGL-1 binding site; greater maximal platelet-leukocyte aggregate inhibition in response to platelet agonists than the same concentration of crizanlizumab in blood samples from both healthy volunteers and patients with SCD; and clinical properties that support a longer dosing interval compared with crizanlizumab [Citation8–10]. Additionally, two single-point mutations were introduced into the Fc part of inclacumab in order to avoid antibody-dependent cytotoxicity (L235E) and to improve structural stability (S228P) [Citation7]. The L235E point mutation replaces a hydrophobic contact with a highly charged group, reducing undesirable interactions by impairing Fc affinity for Fcγ receptors [Citation12,Citation13].
Inclacumab was discovered and developed by Roche; it was progressed through phase 2 clinical studies in which its safety and pharmacology was well characterized in more than 700 individuals, including both healthy volunteers and patients with cardiovascular disease [Citation14–16]. The program was in-licensed from Roche by Global Blood Therapeutics (GBT) in August 2018 to develop the drug as a potential treatment to reduce the frequency of VOCs in individuals with SCD [Citation17,Citation18]. As is typical for mAbs, process changes in the manufacturing of inclacumab have occurred throughout its lifecycle [Citation19], resulting in the implementation of three distinct manufacturing processes, referred to here as Roche version 0.1 (v0.1), Roche v0.2, and GBT. A summary of the scope of changes introduced among the three different processes is given in .
Table 1. Scope of drug substance manufacturing changes across Roche (v0.1 and v0.2) and GBT processes.
The Roche v0.1 manufacturing process was used to support nonclinical and phase 1/2a clinical studies, with some modifications introduced between the production of nonclinical Roche v0.1 material and clinical Roche v0.1 material due to scale-up requirements and process performance improvements. Later, Roche implemented an improved manufacturing process, designated as Roche v0.2, which offered a higher cell culture product titer, better purification efficiency, and a drug substance formulation composition that conferred greater stability in the final drug product. A comparability assessment of Roche v0.1 and Roche v0.2 drug substance batches was conducted by Roche to support the implementation of the Roche v0.2 process. The assessment included routine analyses, extended characterization of physicochemical properties, in vitro functional assays, in vivo pharmacokinetic (PK) characterization, and a stress stability study. Roche submitted an Investigational New Drug application to the FDA for coronary artery disease in 2012, which included results from the comparability assessment. An overview of the scope of the comparability assessment is outlined in . Taken together, the comparability data demonstrated that the lots produced with the Roche v0.1 and Roche v0.2 processes were comparable, an assessment that was also accepted by the FDA, thus enabling Roche to implement the v0.2 process to supply phase 2b clinical trials.
Table 2. Outline of the scope of the comparability assessments performed for inclacumab to date.
When GBT acquired the rights to develop, manufacture, and commercialize inclacumab worldwide [Citation20], documentation regarding the Roche v0.2 manufacturing process and the analytical procedures and data were transferred to GBT, along with vials of the Roche v0.2 master cell bank and the Roche v0.2 reference standard. Because of the technology transfer, changes made to the v0.2 process to create the GBT process were minimal. The changes that were introduced were needed to replace Roche proprietary technologies that were not transferred to GBT, and the scale of the process was increased to a size suitable for commercial supply. In addition, the production of inclacumab was transferred to a new manufacturing site. Process changes were mostly made within the cell culture and harvest stages of the manufacturing process, whereas the purification steps are based on the Roche v0.2 process, and the GBT formulation is identical to the v0.2 formulation.
A comprehensive comparability study in alignment with regulatory guidelines was undertaken to extensively assess the comparability of inclacumab produced using the Roche and GBT manufacturing processes, ensuring that no meaningful differences in identity, safety, purity, potency, and bioavailability exist between GBT lots to be used in future clinical studies and the lots used in nonclinical safety and early clinical studies conducted by Roche. Based on the design of this comparability study and the results obtained, the FDA approved the changes to the manufacturing process and gave clearance to proceed with phase 3 clinical trials in patients with SCD using the inclacumab produced by GBT. The approval confirms that the GBT inclacumab process development, characterization, and analytical test results are consistent with FDA expectations for a phase 3 mAb therapy. This report presents the results of the aforementioned comparability assessment between inclacumab produced by Roche and inclacumab produced by GBT to demonstrate their comparability and that GBT-produced inclacumab is suitable for use in phase 3 clinical trials.
2. Materials and methods
This comprehensive comparability study was executed in accordance with its protocol, which contained predefined quality and safety criteria, and the following regulatory guidelines: ICH Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process, CPMP/ICH/5721/03 [Citation21]; Guideline on Development, Production, Characterization and Specifications for Monoclonal Antibodies and Related Products, EMA/CHMP/BWP/532517/2008 [Citation22]; and Guideline on Comparability of Biotechnology-derived Medicinal Products after a Change in the Manufacturing Process. Non-clinical and Clinical Issues, EMEA/CHMP/BMWP/101695/2006 [Citation23].
2.1. Inclacumab batches used for comparability assessment
The materials used for the comparability study consisted of two batches of drug product from the Roche v0.1 processes that were produced from independent drug substance batches, one batch each of drug substance and drug product (produced from an independent drug substance batch) from the Roche v0.2 process, and four batches of drug substance from the GBT process. The four batches of Roche material were the only historical batches of inclacumab available to GBT; however, GBT had access to all Roche data (characterization, comparability, release, and stability) from all prior Roche drug substance and drug product lots. These historical data were leveraged to set the comparability acceptance criteria for process variability and product stability (degradation products). Roche v0.1 and v0.2 drug products were stored at 2°C to 8°C since the date of manufacture (duration ~9–12 years), and the Roche v0.2 drug substance was stored at –70°C since 2011 (duration ~9 years).
2.2. Appearance
For color analysis, inclacumab samples were evaluated visually under defined viewing conditions and compared to color references in accordance with European Pharmacopoeia (Ph. Eur.) 2.2.2. For clarity, a quantitative assessment of inclacumab samples was performed using a calibrated turbidimeter, and the results were compared to a set of opalescence references in accordance with Ph. Eur. 2.2.1. Testing was conducted at Eurofins Lancaster Laboratories, Inc. (Lancaster, PA).
2.3. Osmolality
Osmolality of inclacumab samples was determined via measurement with a calibrated osmometer according to Ph. Eur. 2.2.35 and USP <785>. Testing was conducted at Patheon (St. Louis, MO).
2.4. Protein content by UV
Total protein concentration of each inclacumab sample was quantitatively determined via ultraviolet (UV) absorbance using the SoloVPE system. Absorbance at 280 nm was used to calculate the concentration of inclacumab using Beer’s law, A = εlc, where A = absorbance at 280 nm, ε = extinction coefficient of inclacumab (1.54 mL mg–1 cm–1), l = pathlength (variable), and c = concentration of the sample. In this method, the absorbance at 320 nm was subtracted from the absorbance at 280 nm before calculating the concentration. A variable pathlength approach utilized the slope (change of absorbance per change of light pathlength) to calculate the concentration. The absorbance value was directly proportional to the protein concentration. Testing was conducted at Patheon (St. Louis, MO).
2.5. Identity by peptide map
The identity of inclacumab samples was confirmed using gradient reversed-phase high-performance liquid chromatography (HPLC). Each sample was reduced, alkylated, digested with trypsin, and loaded onto an HPLC column. The eluate was then monitored via UV absorbance. The sample chromatogram was compared to the reference material chromatogram for presence of any significant new peak(s) or absence of any characteristic peak(s). A significant peak was defined as one that is ≥10% of the height of a designated peak as identified in the test method. Positive identity was confirmed if the tested material corresponded to the reference material with respect to retention times and relative intensities of the 15 characteristic peaks chosen as per the test method. Testing was conducted at Patheon (St. Louis, MO).
2.6. Purity by SE-UPLC
Size-exclusion ultra-performance liquid chromatography (SE-UPLC) was used to monitor the size heterogeneity of inclacumab samples under native conditions by employing size-exclusion chromatography to separate inclacumab aggregates, intact IgG, and smaller fragments. The assay used an isocratic run with UV detection at 280 nm, allowing for sensitivity to the analytes while reducing interference from the buffer components. The purity of the samples was determined in terms of percentage area of intact IgG, sum of high-molecular-weight (HMW) forms, and sum of low-molecular-weight (LMW) forms. Testing was conducted at Patheon (St. Louis, MO).
2.7. Purity by CE-SDS
Capillary electrophoresis–sodium dodecyl sulfate (CE-SDS) was used to monitor the size heterogeneity of inclacumab samples. Samples were heat denatured with sample buffer containing SDS in the presence of beta-mercaptoethanol (reducing conditions) and in the absence of beta-mercaptoethanol (nonreducing conditions). Samples (reduced and nonreduced) were separated by size in a capillary filled with a polymer matrix in a proprietary buffer that provided a sieving matrix for separation, causing smaller moieties to migrate more quickly through the matrix when voltage was applied. As the molecules moved through the capillary, they were detected by a photodiode array detector at 220 nm. Testing was conducted at Patheon (St. Louis, MO).
For the nonreduced sample, percent of IgG was determined quantitatively in terms of the percent corrected peak area (CPA) of intact IgG. For the reduced sample, percent of IgG was determined quantitatively as percent CPA of heavy chain and light chain.
The CPA calculation for velocity (μV∙s) was performed using 32 Karat software. Peak areas were adjusted for migration times using the formula , where T = migration time (s), LD = capillary length to detector, and A = peak area (μV∙s). Percent CPA was calculated using the formula % CPA = (CPA × 100) ÷ (total CPA).
2.8. Poloxamer 188 content by UPLC with charged aerosol detection
Poloxamer 188 content in inclacumab samples was quantitated using UPLC with a mixed-mode column coupled to a charged aerosol detector (CAD). The mass-sensitive detector responded to essentially all nonvolatile and some semi-volatile compounds eluting from the column. The anion exchange/reversed-phase column exhibited reduced hydrophobic interactions and allowed the polymeric poloxamer 188 to elute in a single peak, improving quantitation while also excluding the overall positively charged protein, thereby minimizing sample interference. The components of the sample not retained by the column were sent to waste, utilizing the low flow diverter on the Corona CAD. The quantitation of poloxamer 188 was performed by comparing the sample peak area response to a standard curve. Testing was conducted at Patheon (St. Louis, MO).
2.9. Potency by binding ELISA
The potency of inclacumab samples was tested using an enzyme-linked immunosorbent assay (ELISA). Samples were incubated with biotinylated P-selectin bound to streptavidin-coated plates, and goat anti-human kappa chain-specific horseradish peroxidase (HRP)-conjugated detection antibody was added subsequently. The associated color change was achieved by addition of the enzyme substrate-chromogen reagent 3,3ʹ,5,5ʹ-tetramethylbenzidine (TMB). The chromogenic signal was proportional to the amount of inclacumab that bound to P-selectin. Potency was determined by comparing the P-selectin binding of inclacumab relative to the reference material. Testing was conducted at Patheon (St. Louis, MO).
2.10. Potency by bioassay
The biological activity of inclacumab samples was tested using an in vitro leukocyte adhesion bioassay. Varying concentrations of samples and inclacumab reference standard were incubated with fluorescence-labeled human PSGL-1-expressing cells in a microtiter plate coated with recombinant human P-selectin. After removal of nonadherent cells, the remaining adherent cells were lysed, and adhesion was quantified in the form of a fluorescence signal. Potency of the samples was measured relative to the reference material. Testing was conducted at Eurofins Lancaster Laboratories, Inc. (Lancaster, PA).
2.11. Host cell protein content
Host cell protein (HCP) content in the inclacumab samples was measured using a Chinese hamster ovary (CHO) HCP ELISA kit from Cygnus Technologies. Samples were mixed with affinity-purified capture anti-CHO antibody immobilized on microtiter strips and an HRP-labeled anti-CHO detection antibody (goat polyclonal). The resulting reaction formed a sandwich complex consisting of solid-phase (capture) antibody, HCP antigen, and enzyme-labeled antibody. After complex formation, the microtiter strips were washed to remove any unbound reactants, and TMB, an HRP-sensitive substrate, was added, with the resulting amount of hydrolyzed TMB being directly proportional to the amount of CHO HCP present. The concentration of HCP in the test sample was calculated from a standard curve generated using a CHO HCP standard. Testing was conducted at Patheon (St. Louis, MO).
2.12. Residual protein A content
Residual protein A content in the inclacumab samples was quantitated using a protein A ELISA kit from RepliGen. Both inclacumab samples and the protein A standards were diluted using sample diluent and incubated with immobilized anti-protein antibodies. Protein A ligand was then detected by the addition of a biotinylated anti-protein A probe. The high substitution of the probe allowed maximum binding of a streptavidin-peroxidase conjugate. The final detection step involved the use of TMB to give a highly sensitive colorimetric reaction. The resulting color intensity was proportional to the amount of protein A ligand present in the sample. The concentration of protein A in the test sample was calculated from a standard curve generated using a protein A standard. Testing was conducted at Patheon (St. Louis, MO).
2.13. Residual host cell DNA content
Residual host cell DNA content in the inclacumab samples was determined using a quantitative polymerase chain reaction (qPCR)-based CHO residual DNA quantitation kit from Life Technologies. This quantitative assay was based on the real-time detection of a fluorescence signal during qPCR, and the amount of signal was directly proportional to the amount of CHO DNA present. Genomic DNA from in-process and bulk drug substance samples was recovered using a nucleic acid extraction kit from Life Technologies. This kit used chemical lysis and magnetic beads to efficiently extract genomic DNA from diverse sample types, including samples that contain high protein and low DNA concentrations. Once DNA was recovered by elution into a matrix ideal for PCR, it was amplified via real-time qPCR. The concentration of DNA in the test sample was calculated by comparing the PCR signal to signals generated from a standard curve prepared using a CHO genomic DNA standard. Testing was conducted at Patheon (St. Louis, MO).
2.14. Extended characterization
Inclacumab (Roche v0.1, v0.2, and GBT samples) were assessed by extended characterization in terms of purity, impurity, and identity. Size variant distribution was further characterized using size-exclusion chromatography with multiangle light scattering (SEC-MALS), and charge heterogeneity was evaluated using ion-exchange HPLC (IE-HPLC). Identity of primary structure was confirmed using electrospray ionization–mass spectrometry (ESI-MS) analysis and peptide mapping. Higher-order structure characterization was performed using Fourier-transform infrared spectroscopy (FTIR), circular dichroism (CD), differential scanning calorimetry (DSC), and disulfide mapping. Post-translational modifications were assessed using peptide map liquid chromatography–mass spectrometry (LCMS), released glycan profiles were analyzed via hydrophilic-interaction liquid chromatography (HILIC)-MS, and sialic acid content was evaluated using UPLC. Testing was conducted at KBI Biopharma, Inc. (Louisville, CO) and Patheon (St. Louis, MO).
2.15. In vitro functional characterization
To assess antigen binding, inclacumab (Roche v0.1, v0.2, and GBT samples) was captured on Biacore chips via anti–human Fc antibodies attached to the chip surface using covalent amine-coupling chemistry. The antigen, P-selectin, was then injected over the chip at various concentrations, and the binding response was measured in terms of resonance units (RU). The contact time (in seconds) with the chip was optimized for each individual inclacumab sample to ensure that the level of inclacumab captured on the chip was comparable across the samples. Binding response difference was measured by comparing the maximum response of each sample. The formula used to calculate the RU maximum difference is as follows: relative RU max difference = [RU max (sample)/RU max (reference standard)] × 100%. Testing was conducted at Sartorius Stedim North America Inc. (Cambridge, MA). To assess neonatal Fc receptor (FcRn) binding, FcRn was directly immobilized onto the Biacore chip surface using covalent amine-coupling chemistry. Test article containing inclacumab (Roche v0.1, v0.2, and GBT samples) was injected over the chip, and binding was measured in RU. Tysabri (natalizumab) was used as the IgG4 control, and a GBT sample lot served as the internal reference standard. The formula used to calculate relative FcRn binding (in terms of RU maximum difference) is as follows: relative RU max difference = [RU max (sample)/RU max (reference standard)] × 100%. Testing was conducted at Sartorius Stedim North America Inc. (Cambridge, MA).
To assess Fc gamma receptor (FcγR) binding, Fc receptors were immobilized on a biosensor, and the association/dissociation rates (koff/kon) of inclacumab samples (Roche v0.1, v0.2, and GBT samples) were measured to determine the binding affinity, which was calculated using an appropriate curve-fitting model. An in-house IgG1 control was confirmed to bind with FcγRI, FcγRIIA, FcγRIIB, FcγRIIIA-F, FcγRIIIA-V, and FcγRIIIB. Testing was conducted at LakePharma, Inc. (San Carlos, CA).
To assess C1q binding, inclacumab (Roche v0.1, v0.2, and GBT samples) was immobilized on the surface of a Series S CM5 Sensor Chip using amine-coupling chemistry and a defined immobilization method. Rituximab (IgG1 isotype) and nivolumab (IgG4 isotype) were also immobilized and served as appropriate positive and negative controls for C1q binding, respectively. C1q was flowed across the biosensor surface to permit binding at various concentrations to determine kon over time; koff was determined upon ceasing the flow. When quantifiable binding was observed, the affinity of C1q to the sample (equilibrium dissociation constant, KD) was calculated from koff and kon. Testing was conducted at Sartorius Stedim North America Inc. (Cambridge, MA).
2.16. In vivo characterization
Inclacumab (Roche v0.1, v0.2, or GBT process batch) was administered as a single intravenous bolus dose of 30 mg/kg to male Wistar rats (n = 12/group). Blood samples for PK evaluation were collected from all animals predose and at 1, 7, 24, 48, 96, 168, 336, 432, 528, and 672 hours postdose. Blood was maintained in chilled cryoracks and centrifuged within 1 hour to obtain plasma. Collected plasma was placed on dry ice prior to storage at approximately – 70°C.
Plasma concentrations of inclacumab were quantified using a validated Meso Scale Discovery electrochemiluminescence immunoassay with a lower limit of quantification of 205 ng/mL. The PK parameters for each group were calculated via noncompartmental analysis using the intravascular input model and linear-up log-down area under the concentration–time curve (AUC) calculation method in Phoenix version 64 (Certara USA, Inc., Princeton, NJ). Plasma sample concentration results below the lower limit of quantitation were reported as such. These values were treated as 0 in PK calculations.
Equivalence analysis was conducted to assess bioequivalence of the GBT material and the Roche v0.2 material in terms of maximum concentration (Cmax) and AUC of inclacumab exposures in plasma (area under the concentration–time profile from time 0 to the last quantifiable concentration [AUClast] and area under the concentration–time profile from time 0 to infinite time [AUC0-∞]). A comparison of Roche v0.1 material to Roche v0.2 material was also performed. To meet acceptance criteria for material comparability, the 90% confidence intervals (CIs) for the ratio of the geometric means for Cmax and AUC were required to fall within the standard bioequivalence limits of 80% to 125% [Citation24]. Inferences assumed log-normal distribution of data. Statistical analysis was implemented via PROC TTEST in SAS v9.4.
In vivo testing was conducted at Covance Laboratories Inc. (Madison, WI) in accordance with their applicable lab standard operating procedures. Plasma sample analysis was conducted at PPD Laboratories (Richmond, VA).
2.17. Thermal stress testing
Stress stability testing of inclacumab samples was performed at 40°C and 75% relative humidity using 2 Roche v0.2 samples and 2 GBT samples. GBT samples were assessed at weeks 0, 2, 4, 6, 8, 12, and 24. Due to limited availability of Roche v0.2 material, Roche v0.2 samples were only tested at weeks 0, 4, 12, and 24. Samples were monitored using 8 test methods: appearance, SE-UPLC, IE-HPLC, CE-SDS (nonreduced/reduced), a cell-based potency assay, protein concentration, and poloxamer 188 concentration. Testing was conducted at Patheon (St. Louis, MO) and Eurofins Lancaster Laboratories, Inc. (Lancaster, PA).
3. Results
3.1. Comparability assessment plan
While the changes that occurred between the v0.1 and v0.2 processes were more extensive compared with those between the v0.2 and GBT processes, the comparability assessment supporting the transition from the v0.2 to GBT process was designed to be more comprehensive, reflecting a scope appropriate for a product advancing to phase 3 studies [Citation19] (). The comparability assessment included inclacumab produced using the GBT process and the Roche v0.1 and v0.2 processes. The comparability assessment was primarily designed to compare GBT batches to Roche v0.2 batches, as the GBT manufacturing process is based on the Roche v0.2 process. Roche v0.1 materials were also included in the comparability assessment, given GBT’s intention to leverage those data to progress to a phase 3 study. Comparability was assessed in terms of general properties, purity and impurity profiles, identity, potency, binding activity, bioavailability, and kinetics of degradation using data from routine release testing, extended characterization, charge isoform characterization, in vitro functional testing, an in vivo PK study, and thermal stress testing. Acceptance criteria for comparability with respect to quality and safety were predefined.
3.2. Routine release testing for comparability assessment
Routine test methods used for the comparability assessment and a summary of results from those tests are shown in . Key assessments are described in more detail in the following sections.
Table 3. Routine testing summary for comparability.
3.2.1. General properties
The composition and strength of drug products and substances were considered obligatory critical quality attributes (CQAs) included in routine release testing. Appearance, pH, osmolality, protein concentration, and poloxamer 188 concentration were comparable for Roche v0.2 and GBT materials. All samples met the set acceptance criteria or the specification set for their respective formulation compositions.
3.2.2. Purity and impurities
3.2.2.1. Size variants
HMW species and LMW species can affect potency and immunogenicity and are thus considered CQAs. The molecular size distribution of the inclacumab samples was assessed using SE-UPLC and capillary electrophoresis–sodium dodecyl sulfate (CE-SDS) analyses, which showed that the main component was the intact IgG. Small changes (<1.2%) in HMW species and LMW species were observed upon long-term storage under non-frozen conditions of both v0.1 samples and one of the v0.2 samples. The Roche v0.2 sample stored at – 70°C and GBT samples had comparable levels of HMW species and LMW species.
3.2.2.2. Process-related impurities
Process-related impurities encompass those that are derived from cell substrates (eg, HCPs, host cell DNA), cell culture (eg, selection agents and other media components), and downstream processing (eg, leached protein A ligand), and are considered CQAs [Citation25]. HCPs, residual protein A, and residual DNA are of particular concern, as they are the most commonly encountered process-related impurities, and they can impact the safety and immunogenicity of an antibody [Citation19]. Assessment of residual HCP content showed fewer and no new HCPs in the GBT samples compared with the Roche samples, demonstrating a more robust clearance of HCPs, attributable to improvements made in the GBT purification process. All GBT and Roche inclacumab samples had residual DNA and protein A below the limit of quantitation of the respective analytical methods.
3.2.3. Potency
Performed as part of routine testing, a cell-based potency assay, exploiting the ability of inclacumab to inhibit binding of human promyeloblast cells (expressing PSGL-1) to immobilized P-selectin, and a biotinylated P-selectin-based antigen-binding ELISA demonstrated that all inclacumab samples produced by GBT were equipotent. The cell-based assay was transferred to GBT from Roche when inclacumab was in-licensed. The ELISA method is more precise and has a tighter acceptance criterion than the cell-based assay. All samples met the set acceptance criteria; however, the relative potencies of the Roche v0.1 samples were lower than those reported by Roche in the original release data. The decrease in potency of the Roche v0.1 samples correlated with the higher oxidation level in the L8 peptide within the antigen-binding region (measured by peptide mapping with LCMS); the higher oxidation level may be attributable to peroxides generated during the extended non-frozen storage.
3.3. Extended characterization for comparability assessment
The extended characterization methods used for the comparability assessment and a summary of results from those tests are shown in . Key assessments are described in more detail in the following sections.
Table 4. Extended characterization summary for comparability.
3.3.1. Purity and impurities
3.3.1.1. Size variants
SEC-MALS analysis was performed to confirm the accuracy of the UV detection used in the routine SE-UPLC method and to characterize the size variant distribution of Roche v0.1, Roche v0.2, and GBT samples. All samples were shown to have comparable intact IgG molecular weights, and the levels of aggregate present in all of the samples were very low and comparable to the levels detected using the UV method.
3.3.1.2. Charge heterogeneity
Like other mAbs, inclacumab is heterogeneous and contains product-related variants that manifest during the manufacturing process [Citation19]. A range of product isoforms is created by varying proportions of post-translational modifications that occur as part of product expression during cell culture production and due to stability and product recovery effects during harvest, purification, formulation, and storage [Citation19]. Some of these modifications alter the charge distribution on the surface of mAbs and result in isoforms with different net surface charges. The net surface charge is used to define isoforms as either acidic, basic, or the main species [Citation19]. The post-translational modifications that create differences in net surface charge can potentially affect the biological activity of an antibody, making the charge isoform profile an important feature to characterize with respect to product quality and comparability [Citation19]. Furthermore, the distribution of charge isoforms can be sensitive to process changes introduced during manufacturing [Citation19]. Based on these considerations, a charge isoform profile is typically designed to be a CQA.
The charge isoform profiles of the inclacumab comparability assessment samples were evaluated using IE-HPLC. All inclacumab samples produced by GBT had a consistent isoform profile, with highly similar peak percentage values. The GBT samples contained no new detectable charge isoforms compared with the Roche samples, though some differences in the proportions of charge isoforms were observed. The historical v0.1 values from original Roche testing showed higher percentages of acidic species than GBT and Roche v0.2 lots, which further increased upon storage under non-frozen conditions. No such change in percentage of acidic species was observed for Roche v0.2 lots, consistent with the expected improvement in the stability of the Roche v0.2 formulation. GBT samples had slightly higher levels of basic species. This may be attributable to a lower proportion of C-terminal lysine clipping of the heavy chain (see the post-translational modification analysis section for further detail).
3.3.2. Identity
Characterization of primary structure along with higher-order structure and post-translational modifications was performed as part of the extended characterization testing.
3.3.2.1. Primary structure
The expected primary structure of inclacumab was confirmed via ESI-MS analysis of intact and reduced inclacumab and via LCMS analysis that employed a trypsin/Lys-C/Glu-C digestion. MS analysis confirmed that the molecular masses of all samples analyzed were consistent with predicted masses deduced from the amino acid sequence of inclacumab, except for 1 Roche v0.1 sample that had a greater mass than predicted (which may have been due to the oxidation of the material under long-term nonfrozen storage). LCMS trypsin/Lys-C/Glu-C peptide mapping confirmed the amino acid sequence for inclacumab with 100% sequence coverage.
3.3.2.2. Higher-order structure
The higher-order structure of inclacumab in the comparability samples was characterized using FTIR and CD to determine the secondary and tertiary structures of the molecule, respectively. DSC was applied to assess the thermal and conformational stability of inclacumab in the comparability samples. Results from these tests showed that all samples had highly similar structures and thermal stability. Disulfide mapping also confirmed that Roche and GBT materials share common patterns of disulfide linkages and unpaired cysteines.
3.3.2.3. Post-translational modifications
Post-translational modifications (PTMs), including methionine oxidation, deamidation of asparagine residues, cyclization of glutamine to form pyroglutamate at the N terminus of the heavy chain, and intact and absent C-terminal lysine, were detected and quantified using peptide mapping analysis with LCMS. Inclacumab present in Roche v0.1, Roche v0.2, and GBT samples had the same types of PTMs at the same sequence locations. The proportions of modifications were comparable across the samples, except increased C-terminal lysine clipping was observed in both Roche v0.1 and v0.2 samples compared with the GBT samples and, while GBT samples contained the same glycan species as Roche v0.1 and v0.2 samples, its glycan profile was between those of the Roche v0.1 and v0.2 samples. Results characterizing the glycan profile of inclacumab samples using a HILIC/fluorescence method coupled with MS detection were generally consistent with the LCMS PTM characterization. This method showed that the relative proportions of glycans were different across different inclacumab sources, with Roche v0.1 samples having the highest levels of galactosylated glycans, the Roche v0.2 samples having the lowest levels of galactosylated glycans, and GBT samples having intermediate levels of galactosylated glycans. Results were consistent across samples from the same source (ie, Roche v0.1, Roche v0.2, or GBT). These differences detected in C-terminal lysine clipping and glycan profiles did not have any effect on the functional activity of GBT samples, which was comparable to that of Roche v0.2 samples based on in vitro and in vivo characterization.
Sialylation is an important characteristic of glycoproteins that can affect their potency, half-life, and immunogenicity by salvaging glycoproteins from degradation [Citation26]. In general, all inclacumab samples contained a low abundance of sialic acid (ie, N-acetylneuraminic acid and N-glycolylneuraminic acid), with low levels assumed to be functionally insignificant in eliciting an immune response [Citation27].
3.4. In vitro and in vivo nonclinical testing for comparability assessment
The scope and results of the studies used to compare in vitro biological functions and in vivo PK are shown in . Overall, the results of the in vitro and in vivo studies demonstrated comparable functional activities, plasma exposures, and PK profiles across GBT and Roche v0.2 samples. Key observations from the comparability assessments are described in more detail in the following sections.
Table 5. In vitro and in vivo testing for comparability.
3.4.1. In vitro functional characterization
Surface plasmon resonance technology (Biacore and Octet) was used to assess antigen and effector function receptor binding affinity. All GBT samples produced similar P-selectin, FcRn, and FcγR binding affinities, and the binding affinities across GBT and Roche v0.2 samples were comparable despite differences observed in the distribution of post-translation isoforms and charge variants. GBT and Roche v0.1 and v0.2 samples had comparable binding to the FcγR panel, though the binding affinity levels for the inclacumab samples were lower than those for a commercial IgG4 molecule (nivolumab) containing the L235E mutation. GBT and Roche samples are highly fucosylated (above 89%), which may further impair binding to FcγRs compared to fucose-deficient antibodies [Citation28]. All inclacumab samples did not bind to complement component 1q, as expected.
3.4.2. In vivo characterization
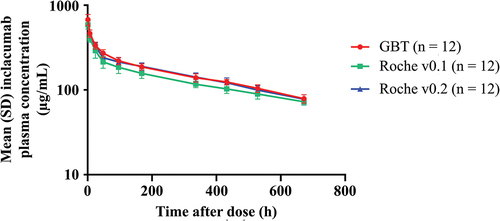
To evaluate any potential effects of the differences observed during the analytical comparability on the relative bioavailability of inclacumab in vivo, a PK study in rats was performed. The mean inclacumab plasma concentration–time profiles in male rats after a single intravenous dose of inclacumab from GBT, Roche v0.1, or Roche v0.2 production processes were closely parallel to each other (). All three manufacturing lots of inclacumab showed similar plasma PK parameter values (Supplemental Table 1). No substantial differences were observed in plasma exposures (90% CIs for Cmax, AUClast, and AUC0-∞ ratios were within the bioequivalence acceptance criteria limits of 80% to 125%) or overall PK profiles for inclacumab derived from the GBT and the Roche v0.2 production processes, demonstrating that the two processes are comparable in this setting. Acceptance criteria for bioequivalence were also met for the comparison of Roche v0.1 material to Roche v0.2 material.
Figure 1. Male rats were administered a single intravenous dose of inclacumab from GBT, Roche v0.1, or Roche v0.2 production processes (30 mg/kg). The plasma concentration of inclacumab in micrograms per milliliter on a logarithmic scale is plotted versus time in hours after dosing. Data points show the mean plasma concentration from 12 samples per group, with error bars representing standard deviation. The lines for all three process materials are closely parallel to each other, decreasing from approximately 600 to 700 μg/mL 1 hour after the dose to approximately 70 to 80 μg/mL 672 hours the after dose. Comparisons of GBT material to Roche v0.2 material and Roche v0.2 material to Roche v0.1 material resulted in 90% CIs of the Cmax, AUClast, and AUC0-∞ ratios within the bioequivalence limits of 80% to 125%. AUC0–∞, area under the concentration–time curve from time 0 to infinity; AUClast, area under the concentration–time curve from time 0 to the time at which the last quantifiable concentration was observed; Cmax, maximum plasma concentration; CI, confidence interval; GBT, Global Blood Therapeutics; SD, standard deviation.
3.5. Thermal stress testing for comparability assessment
Roche v0.2 samples and GBT samples were compared side-by-side in a stress stability study conducted at 40°C and 75% relative humidity. Samples of Roche v0.1 material were not included in this study due to the formulation composition difference and the higher oxidation level observed in the available Roche v0.1 materials. This study was performed to compare the stability and degradation behavior of the samples produced with Roche v0.2 and GBT manufacturing processes after storage for 24 weeks at 40°C. Results of the thermal stress data demonstrated that GBT and Roche v0.2 materials had comparable degradation pathways, rates, and end products, as determined by stability-indicating methods (SE-UPLC, IE-HPLC, CE-SDS [nonreduced/reduced], and a cell-based potency assay).
4. Discussion
Inclacumab samples produced by GBT were found to be comparable to the Roche v0.2 inclacumab samples based on (1) comparable primary and higher-order structures; (2) comparable purity profiles; (3) comparable potency, in vitro functional activities and in vivo plasma exposures and PK profiles; and (4) comparable degradation patterns and kinetics under forced degradation conditions. The study detected several differences between the materials produced by the different manufacturing processes, though the totality of data confirmed that these differences had no effect on product quality or potency of inclacumab produced by GBT and that it is suitable for its intended use in phase 3 clinical trials.
Differences in charge distribution were detected, with GBT material having a lower proportion of acidic charge isoforms than Roche v0.1 material but a comparable proportion to Roche v0.2 material. GBT performed additional charge variant characterization on batches from all three processes for a deeper comparability assessment of charge isoform composition of Roche and GBT materials. The purpose of the additional charge isoform characterization study was to increase the detectability of differences in isoform composition across the samples and to develop an initial understanding of the potential functional implications of the charge isoform profile changes; however, results obtained for the charge variant studies are beyond the scope of this publication.
GBT material also had a slightly higher proportion of basic species due to reduced clipping of C‑terminal lysine; however, these differences did not affect biological activity. Glycan distribution levels and sialic acid in the form of N-acetylneuraminic acid levels for GBT materials fell between those of Roche v0.1 and v0.2 materials, but no new glycoforms were observed, and differences did not affect activity. Roche v0.1 materials stored for more than 10 years at 2°C to 8°C showed oxidation in the L8 (Trp94) peptide that may affect P-selectin binding and oxidation in the H23 (Met256) peptide that may affect FcRn binding.
In addition to demonstrating comparability, this study also contributes to the scientific understanding of quality attributes of inclacumab, as well as of IgG4 antibodies in general. Results from the GBT comparability assessment were submitted in the GBT Investigational New Drug Application for inclacumab, which was approved; GBT is currently enrolling patients with SCD in phase 3 clinical studies of inclacumab [Citation17,Citation18].
5. Conclusions
In summary, GBT designed an extensive analytical comparability plan, in alignment with the regulatory guidelines that rigorously characterized both Roche and GBT batches of inclacumab to demonstrate consistency between the GBT lots and the lots used in preclinical and clinical studies by Roche. Inclacumab samples produced by GBT were found to be comparable to the Roche v0.2 inclacumab samples across all analytical methods used for testing. Based on these results, the FDA approved the changes to the manufacturing process and gave clearance for GBT to proceed with phase 3 clinical trials of inclacumab in patients with SCD.
Declaration of interest
R Mihaila, D Ruhela, L Xu, S Joussef, X Geng, AS Kim, W Yares, and K Furstoss are all employees of Global Blood Therapeutics. J Shi is a former employee of Global Blood Therapeutics. The current affiliation for J Shi is Ideaya Biosciences, South San Francisco, CA, USA. K Iverson is a consultant for Global Blood Therapeutics. Global Blood Therapeutics is a wholly owned subsidiary of Pfizer Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Opinion on Biological Therapy for their review work, but have no other relevant financial relationships to disclose
Author contributions
Conceptualization: K Furstoss, K Iverson; methodology: R Mihaila, X Geng, J Shi; investigation: R Mihaila, L Xu, S Joussef, Xin G, J Shi; data curation: R Mihaila, D Ruhela, L Xu; visualization: R Mihaila, D Ruhela; writing – original draft preparation: R Mihaila, D Ruhela, K Iverson; writing – review and editing: all authors. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
22-10-28_Inclacumab_Comparability_Manuscript_EOBT_Revision_1_Supplement.docx
Download MS Word (47.2 KB)Acknowledgments
We thank Nadine Ritter, PhD, for reviewing the manuscript. Medical writing and editorial assistance were provided by Lindsay Tannenholz, PhD (Healthcare Consultancy Group), funded by Global Blood Therapeutics.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14712598.2022.2143260
Additional information
Funding
References
- Kutlar A, Embury SH. Cellular adhesion and the endothelium: p-selectin. Hematol Oncol Clin North Am. 2014;28(2):323–339.
- André P. P-selectin in haemostasis. Br J Haematol. 2004;126(3):298–306.
- Kappelmayer J, Nagy B Jr., Miszti-Blasius K, et al. The emerging value of P-selectin as a disease marker. Clin Chem Lab Med. 2004;42(5):475–486.
- Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–439.
- Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031.
- Schmitt C, Abt M, Ciorciaro C, et al. First-in-man study with inclacumab, a human monoclonal antibody against P-selectin. J Cardiovasc Pharmacol. 2015;65(6):611–619.
- Geng X, Mihaila R, Yuan Y, et al. Inclacumab, a fully human anti-P-selectin antibody, directly binds to PSGL-1 binding region and demonstrates robust and durable inhibition of cell adhesion. Presented at: American Society of Hematology Annual Meeting & Exposition; 2020 Dec 5–8; virtual. Poster 1707.
- Mayer C, Cooper DS, Redfern A, et al. Preliminary results of a phase 1 study in healthy subjects administered inclacumab, a fully human IgG4 anti-P-selectin monoclonal antibody in development for treatment of sickle cell disease. Presented at: American Society of Hematology Annual Meeting & Exposition; 2021 Dec 11; Atlanta, GA, USA, and virtual. Poster 977.
- Adakveo. Prescribing information. Novartis Pharmaceuticals Corporation. 2021.
- Adakveo. Summary of product characteristics. Novartis Pharmaceuticals Corporation. 2020.
- Hanson QM, Barb AW. A perspective on the structure and receptor binding properties of immunoglobulin G Fc. Biochemistry. 2015;54(19):2931–2942.
- Oganesyan V, Gao C, Shirinian L, et al. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr. 2008;64(Pt 6):700–704.
- GBT provides regulatory and pipeline updates in sickle cell disease (SCD) [Internet]. South San Francisco CA: GBT; 2021 Jul 22 cited Oct 15, 2021]. Available from 2021 Oct 15: https://ir.gbt.com/news-releases/news-release-details/gbt-provides-regulatory-and-pipeline-updates-sickle-cell-disease
- Morrison M, Palermo G, Schmitt C. Lack of ethnic differences in the pharmacokinetics and pharmacodynamics of inclacumab in healthy Japanese and Caucasian subjects. Eur J Clin Pharmacol. 2015;71(11):1365–1374.
- Tardif JC, Tanguay JF, Wright SR, et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J Am Coll Cardiol. 2013;61(20):2048–2055.
- A study of a single dose of inclacumab to reduce re-admission in participants with sickle cell disease and recurrent vaso-occlusive crises: clinicalTrials.gov identifier: NCT04927247; [updated 2021 November 2; cited 2021 December 3]. Available from 2021 Jun 15: https://clinicaltrials.gov/ct2/show/NCT04927247
- A study to assess the safety and efficacy of inclacumab in participants with sickle cell disease experiencing vaso-occlusive crises: clinicalTrials.gov identifier: NCT04935879; [updated 2021 November 5; cited 2021 December 3]. Available from 2021 Jun 23: https://clinicaltrials.gov/ct2/show/NCT04935879
- Ambrogelly A, Gozo S, Katiyar A, et al., Analytical comparability study of recombinant monoclonal antibody therapeutics. MAbs. 2018. 10(4): 513–538.
- GBT expands sickle cell disease pipeline with worldwide licensing agreement for inclacumab for the treatment of vaso-occlusive crisis [Internet]. South San Francisco CA: GBT; 2018 Aug 23 cited 2021 Dec 2]. Available from 2021 Dec 2: https://ir.gbt.com/news-releases/news-release-details/gbt-expands-sickle-cell-disease-pipeline-worldwide-licensing
- ICH Q5E: comparability of biotechnological/biological products subject to changes in their manufacturing process 2005 cited 2021 Dec 2]. Available from 2021 Dec 2: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-5-e-comparability-biotechnological/biological-products-step-5_en.pdf
- Guideline on development, Production, characterisation and specification for monoclonal antibodies and related products; EMA/CHMP/BWP/532517/2008 2016 cited 2021 Dec 2]. Available from 2021 Dec 2: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-development-production-characterisation-specification-monoclonal-antibodies-related_en.pdf
- Guideline on comparability of biotechnology-derived medicinal products after a change in the manufacturing process. Non-clinical and clinical issues; EMEA/CHMP/BMWP/101695/2006 2007 cited 2021 Dec 2]. Available from 2021 Dec 2: https://www.ema.europa.eu/documents/scientific-guideline/guideline-comparability-biotechnology-derived-medicinal-products-after-change-manufacturing-process_en.pdf
- Center for Drug Evaluation and Research. Guidance for Industry. Statistical Approaches to Establishing Bioequivalence 2001 cited 2022 Oct 26]. Available from 2022 Oct 26: https://www.fda.gov/media/70958/download
- ICH Q6B: specifications: test procedures and acceptance criteria for biotechnological/biological products: ICH; 1999 cited 2021 Dec 3]. Available from 2021 Dec 3: https://database.ich.org/sites/default/files/Q6B%20Guideline.pdf
- Bas M, Terrier A, Jacque E, et al. Fc sialylation prolongs serum half-life of therapeutic antibodies. J Immunol. 2019;202(5):1582–1594.
- Ghaderi D, Taylor RE, Padler-Karavani V, et al. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28(8):863–867.
- Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740.
