ABSTRACT
Introduction
It is established that the exon-skipping approach can restore dystrophin in Duchenne muscular dystrophy (DMD) patients. However, dystrophin restoration levels are low, and the field is evolving to provide solutions for improved exon skipping. DMD is a neuromuscular disorder associated with chronic muscle tissue loss attributed to the lack of dystrophin, which causes muscle inflammation, fibrosis formation, and impaired regeneration. Currently, four antisense oligonucleotides (AONs) based on phosphorodiamidate morpholino oligomer (PMO) chemistry are approved by US Food and Drug Administration for exon skipping therapy of eligible DMD patients.
Areas covered
This review describes a preclinical and clinical experience with approved and newly developed AONs for DMD, outlines efforts that have been done to enhance AON efficiency, reviews challenges of clinical trials, and summarizes the current state of the exon skipping approach in the DMD field.
Expert opinion
The exon skipping approach for DMD is under development, and several chemical modifications with improved properties are under (pre)-clinical investigation. Despite existing advantages of these modifications, their safety and effectiveness have to be examined in clinical trials, which are planned or ongoing. Furthermore, we propose clinical settings using natural history controls to facilitate studying the functional effect of the therapy.
1. Introduction
Duchenne muscular dystrophy (DMD) is an X-linked inherited muscle disorder, caused by an absence of a functional dystrophin protein. Dystrophin is a crucial protein for the maintenance of muscle fiber stability. It links the intracellular actin cytoskeleton and extracellular matrix through N-terminal and C-terminal domains, respectively, thus forming the dystrophin protein complex (DPC). The DPC functions as a structural complex to mechanically stabilize the muscle membrane, and as a signaling complex. Due to the absence of dystrophin, the DPC cannot properly form. Consequently, muscle fibers of DMD patients are susceptible to damage during contraction, leading to chronic inflammation, impaired regeneration, and fibrosis formation [Citation1]. DMD is primarily diagnosed in early childhood when clinical signs and symptoms such as muscle weakness, clumsiness, and difficulties with climbing and walking occur. Further testing for serum creatine kinase level, which is increased in DMD patients, and testing for DMD gene mutations confirm a suspected diagnosis of DMD. The disease progresses over the lifespan gradually leading at first to skeletal muscle dysfunction and loss of ambulation, next to the loss of arm function, dysfunction of respiratory and cardiac muscles, and death usually in the 3rd to 4th decade [Citation2].
The dystrophin protein is encoded by the DMD gene, which is the largest gene in the human genome. A broad range of DMD gene mutations is associated with either DMD or Becker muscular dystrophy (BMD), which is a milder version of DMD, with a later age of onset and a slower disease progression. In BMD, mutations maintain the messenger RNA (mRNA) reading frame of the dystrophin transcripts and allow the production of internally deleted semi-functional dystrophin. By contrast, in DMD, mutations cause disruption of the reading frame or introduce a premature stop codon that prematurely truncates translation of the functional dystrophin, resulting in nonfunctional proteins.
There is an unmet medical need for DMD as treatment is only symptomatic to slow down disease progression by reducing muscle inflammation with glucocorticoids. In an effort to develop a targeted therapy, academia and industry are focusing on approaches to restore functional dystrophin. Exon skipping is the most promising and advanced approach for the restoration of dystrophin production. Recently, the US Food and Drug Administration (FDA) has granted accelerated approval for four exon skipping drugs, albeit based on low levels of dystrophin restoration [Citation3–6].
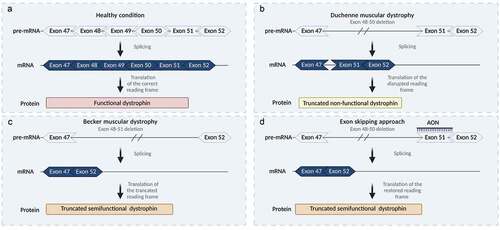
The exon skipping therapy is based on the ability of antisense oligonucleotides (AONs) to specifically target an exon during the pre-mRNA splicing process so that it is not included in the mRNA. This will induce restoration of the dystrophin transcript reading frame, allowing the production of internally deleted dystrophins similar to those expressed by BMD patients () [Citation7].
Figure 1. Representation of the DMD gene transcript splicing and dystrophin protein translation at different conditions. (A) In a healthy condition, functional dystrophin is translated from the mRNA with the correct reading frame. (B) In Duchenne muscular dystrophy, an out-of-frame exon 48–50 deletion results in splicing of exon 47 to 51 and translation of a prematurely truncated nonfunctional dystrophin. (C) In Becker muscular dystrophy, an in-frame exon 48–51 deletion results in splicing of exon 47 to 52 and translating of an internally truncated semi-functional dystrophin. (D) The exon skipping approach applied for an out-of-frame exon 48–50 deletion uses antisense oligonucleotides (AONs) that target exon 51 and hide it from the splicing machinery. This results in exon 51 exclusion, thus restoring the reading frame and allowing translation of a semi-functional BMD-like dystrophin. Created with BioRender.com.
AONs are short single-stranded RNA and DNA analogs that are chemically modified to provide them with druglike properties. Various chemical modifications have been evaluated for DMD exon skipping in preclinical and clinical trials [Citation8]. The chemistries that have been most advanced in clinical development are the 2ʹ-O-methyl phosphorothioate (2OMePS) and phosphorodiamidate morpholino oligomers (PMOs). The 2OMePS AON clinical development was stopped after a phase 3 clinical trial failed to meet the primary endpoint and the FDA denied approval. Currently, 4 PMO AONs have been approved by FDA. However, these PMOs were shown to induce the restoration of low levels of dystrophin, and their clinical benefits are still to be established. Further clinical trials of approved AONs are currently ongoing, aiming to demonstrate the functional effect of exon skipping therapy on DMD patients. Moreover, additional AONs with novel chemical modifications are in or close to clinical trials based on promising results in preclinical studies.
In this scientific review, we summarize ongoing studies of exon skipping AONs, with a focus on those that aim to improve the efficiency and applicability of AONs for DMD, and that are in, or close to evaluation in clinical trials. We will outline efforts and challenges in ongoing research and discuss future directions in the development and clinical implementation of the exon skipping approach for DMD patients.
2. AON modifications assessed in clinical trials
Both 2OMePS and PMO backbone modifications have been shown to be able to induce exon skipping and dystrophin restoration in DMD animal models and patients. 2OMePS is a negatively charged oligonucleotide in which an O-methyl modification replaces oxygen at the 2’ position of the ribose, while for the phosphorothioate backbone (PS) sulfur replaces the oxygen normally present in a phosphodiester linkage (PO). Due to the O-methyl chemistry, 2OMePS AONs have an enhanced affinity to target transcripts and are able to inhibit its translation by steric blockade of the ribosome, whereas the PS backbone increases AONs resistance to nucleases, thus improving AON stability [Citation9]. Moreover, the PS backbone enhances AONs bioavailability, due to high-affinity binding to serum proteins causing reduced renal clearance of AONs [Citation10,Citation11]. PMO is an uncharged oligonucleotide, where the ribose is replaced by a morpholine moiety, while the PO linkage is replaced by a phosphorodiamidate linkage. The PMO chemistry improves the AONs resistance to nucleases and proteases and increases tissue concentrations as the uncharged character imposes low affinity to cellular components [Citation12]. The effectiveness of 2OMePS and PMO AON treatment in DMD was tested in preclinical studies in muscle cell lines, mouse, and dog disease models (requiring skipping of 2 exons), as well as non-human primates. Both 2OMePS and PMO AONs were shown to be taken up more efficiently by dystrophic muscles than healthy muscles. Furthermore, both were able to rescue dystrophin expression, were stable and safe, and had favorable pharmacokinetics [Citation13–17].
2.1. 2OMePS
2.1.1. Drisapersen and suvodirsen
Drisapersen was the first 2OMePS-modified AONs tested for DMD in clinical trials () and was developed to skip exon 51. The first clinical trial was by conducted by Prosensa Therapeutics to study drisapersen in four DMD patients, who received a local injection dose of 0.8 mg [Citation18]. In a follow-up dose-finding study (0.5, 2.0, 4.0, and 6.0 mg/kg) drisapersen was delivered systemically by subcutaneous injection in 12 patients. This demonstrated that drisapersen is well tolerated but can cause some adverse effects such as injection site reactions and proteinuria [Citation19]. A phase 2 placebo-controlled trial tested two regimens of drisapersen administration where 18 DMD patients received continuous subcutaneous injections of 6 mg/kg dose and 17 patients received intermittent injections [Citation20] (https://clinicaltrials.gov/ct2/show/NCT01153932). In parallel phase 2 trials, two doses of 3 and 6 mg/kg were tested in a placebo-controlled trial in which 35 DMD patients received subcutaneous injections for 24 weeks [Citation21] (https://clinicaltrials.gov/ct2/show/NCT01462292). A phase 3 placebo-controlled study of drisapersen tested subcutaneous weekly injections of 6 mg/kg in 125 patients vs 61 placebo-treated patients in order to show a statistically significant effect of drisapersen treatment on disease progression [Citation22] (https://clinicaltrials.gov/ct2/show/NCT01254019). Overall, these studies demonstrated that drisapersen is well tolerated but causes injection-site irritation, subclinical proteinuria in all patients, and thrombocytopenia in a few patients. Even though in both phase 2 clinical trials, 6 mg/kg drisapersen treatment improved the distance walked during the 6-min walk test (6MWT) compared to placebo, the phase 3 trial did not demonstrate a significant difference in this test. Post-hoc analysis revealed that the DMD patients in the phase 3 trials had more advanced disease and that a treatment effect could be discerned in the subset of participants who met the inclusion criteria for the phase 2 trials. However, faced with this evidence, FDA did not approve drisapersen, also in light of the side effects observed. The marketing authorization application to the European Medicines Agency (EMA) was withdrawn before the committee for human medicinal products gave an opinion, and the clinical development of drisapersen and other 2OMePS AONs for exon 44, 45, 53 were stopped as the sponsor (BioMarin) deemed the 2OMePS AONs to have an insufficient efficacy and safety profile [Citation23,Citation24]
Table 1. Overview of exon skipping therapeutics under development for DMD therapy.
Suvordirsen is a stereopure 2OMePS and 2’fluoro(2‘ F)-modified AONs developed by Wave Life Sciences for DMD patients with mutations amenable to exon 51 skipping. Normally, PS modified 20-mer oligonucleotides will have 19 PS bonds that all have a chiral center in either an Sp or an Rp stereochemical configuration, giving 219 of stereochemical variations to 20-mer AONs that all have slightly different pharmacological properties. Suvodirsen was developed to have a defined chirality in each 19 PS linkage and induced more effective exon skipping in cultured cells. In a phase 1 dose-escalating clinical trial, it was established that suvodirsen is safe and well-tolerated (https://clinicaltrials.gov/ct2/show/NCT03508947). However, in an extension study, weekly doses of 3.5 and 5 mg/kg of suvodirsen for 12 or 22 weeks failed to induce dystrophin protein production, and Wave Life Sciences terminated suvodirsen development [Citation25] (https://clinicaltrials.gov/ct2/show/NCT03907072).
2.1.2. PN backbone chemistry modification
Wave Life Sciences in collaboration with Matthew Wood’s research group continues to investigate the effectiveness of the stereopure PS backbone. They synthesized and tested chimeric stereopure AONs with various 2OMe and 2’ F modification patterns. For the splice modulation approach for spinal muscular atrophy, it was shown that 2’ F-modified AONs forming a duplex with target pre-mRNA recruit interleukin enhancer-binding factors 2 and 3 (ILF2/3 proteins) and enhance alternative splicing of target transcripts [Citation26]. Previously 2’ F technology was shown to enhance the effectiveness of AONs in DMD exon skipping in vitro, while AONs in vivo tolerability depended on the position of the 2’ F modification [Citation27,Citation28]. In addition to the 2’ F chemistry, the impact of phosphoryl guanidine (PN) linkages on the effectiveness of AONs with stereopure PS backbone was tested. Compared to PS linkages, neutral PN linkages reduce the overall charge of an oligonucleotide and are more resistant to the nuclease degradation [Citation29]. Experiments showed that oligonucleotides comprising defined 2OMe and 2’ F patterns were well tolerated and more efficient compared to AONs with 2OMe modification only. While the focus of Wave Life Sciences is on their stereopure technology, it is likely that the increased efficiency they see in vitro and in vivo is also or primarily due to the 2’ F and PN modifications. AONs with PN linkages in clinically relevant doses increased dystrophin protein production in the diaphragm and heart and improved survival and respiratory functions in mdx/utrophin double knockout (dKO) mice with severe phenotype. In addition, exon skipping activity was improved in AONs with PN and stereopure PS compared to PN and stereorandom PS, suggesting that controlling stereochemistry allows to improve AONs effectiveness [Citation29]. In September 2021 Wave Life Sciences initiated phase 1b/2a clinical trial of a stereopure oligonucleotide with PN backbone chemistry modification for exon 53 skipping (WVE-531) (https://clinicaltrials.gov/ct2/show/NCT04906460). The trial will evaluate the safety and tolerability of four escalating doses of WVE-531 [Citation30].
2.1.3 2OMePS/ENA
Another chemically modified nucleic acid that was shown to be effective for exon skipping therapy is the 2′-O, 4′-C-ethylene-bridged nucleic acid (ENA). The ethylene linkage between 2’-O and 4’-C of ribose imparts a high binding affinity for (pre-)mRNA and high nuclease resistance [Citation31]. As ENAs have a very high binding affinity, they are used in a chimeric fashion, usually in combination with 2ʹOMe. This allows improved target binding compared to 2ʹOMe modification only, while preventing self-structuring and/or impaired mismatch discrimination of a fully modified ENA AON. In the preclinical study, 2ʹOMe and ENA-modified chimera oligonucleotides in combination with PO backbone were shown to induce exon skipping in vitro and to be tolerated by mdx mice [Citation32], while in another study ENA chimera on a PS backbone were shown to induce exon skipping in the diaphragm, cardiac and skeletal muscles and to be more active than 2OMePS and PMO modified AONs with the same target [Citation33]. Renadirsen is a 2OMe and ENA chimera on a PS backbone (2′OMePS/ENA) developed by Daiichi Sankyo (). In the first safety clinical trial of subcutaneous administration, renadirsen was well tolerated and induced the production of exon 45 skipped mRNA in all DMD patients, while expression of restored dystrophin was observed in several patients [Citation34] (https://clinicaltrials.gov/ct2/show/NCT02667483). Since renadirsen was safe for patients and skipped exon 45 in all patients, in 2021 Daiichi Sankyo continued the investigation and initiated a phase 2 extension study (https://clinicaltrials.gov/ct2/show/NCT04433234).
2.2. PMO
2.2.1. Eteplirsen
Eteplirsen, the first PMO AONs tested in clinical trials (), was developed by Sarepta Therapeutics to target exon 51. The first clinical study of eteplirsen was performed in seven 10–17 years old DMD boys where local injections of 0.09 or 0.9 mg doses to the extensor digitorum brevis muscle were tested. The dose of 0.9 mg/kg induced local exon 51 skipping and expression of restored dystrophin [Citation35] (https://clinicaltrials.gov/ct2/show/NCT00159250). In the next open-label clinical trial, six escalating doses (0.5, 1.0, 2.0, 4.0, 10.0, and 20.0 mg/kg) intravenously (IV) injected weekly for 12 weeks, into DMD boys aged 5–15 years. Treatment was well tolerated and induced dystrophin expression in seven out of 19 participants [Citation36] (https://clinicaltrials.gov/ct2/show/NCT00844597). The next placebo-controlled double-blind clinical study was conducted on 12 DMD boys who were 7–13 years old. The study tested the ability of higher doses (30 and 50 mg/kg) not only to induce the production of dystrophin in fibers but also to improve the distance walked on the 6MWT (https://clinicaltrials.gov/ct2/show/NCT01396239). After 25 weeks, placebo-treated patients were assigned to PMO treatment cohorts, and all participants continued participation in an open-label clinical trial for an additional 4 years (https://clinicaltrials.gov/ct2/show/NCT01540409). Matched natural history (NH) patients amenable to exon 51 skipping were used for comparison of functional effects. The percentage of dystrophin-positive fibers was increased [Citation37,Citation38] and improvement in the distance walked on the 6MWT was observed after 144 weeks of eteplirsen treatment compared to NH [Citation39]. Based on the ability of eteplirsen to induce functional dystrophin production in treated participants, FDA granted accelerated approval to eteplirsen for exon skipping therapy of DMD patients in September 2016. However, since the clinical effect of eteplirsen on walking distance was demonstrated in a small group of patients without a placebo group, FDA concluded that the functional effect of eteplirsen cannot be confirmed yet, and required to conduct additional clinical trials to verify the clinical benefit of eteplirsen [Citation40].
Additional clinical trials of eteplirsen have been focusing on endpoints that could confirm the clinical benefits of eteplirsen treatment on DMD boys aged 7–16 years. In the first trial, 30 DMD patients not amenable to exon 51 skipping were enrolled as an untreated control group (https://clinicaltrials.gov/ct2/show/NCT02255552). However, half of the control group discontinued the trial mostly to be involved in other clinical trials. Moreover, since mutations of DMD patients from the untreated control group did not match to mutations of the exon 51 skippable group, these groups are not clinically equal, e.g., the untreated group contained also exon 44 skippable patients known to have a slower disease progression [Citation41]. These factors impede group comparison and receiving a statistically significant result. Therefore, external NH patients and patients from the phase 2 clinical study of eteplirsen were chosen as a comparator for analysis. The level of dystrophin proteins measured by western blot (WB) increased by 0.516% after 96 weeks of eteplirsen treatment. Analysis of the 6MWT data demonstrated that patients from eteplirsen-treated cohorts walked 67.3 m less from the baseline, while NH patients walked 133.8 m less suggesting a beneficial functional effect of eteplirsen [Citation42].
Previous trials investigated the effect of eteplirsen on DMD boys in a middle to late ambulatory stage aged from 7 years. Two clinical trials have been initiated to further investigate eteplirsen in younger DMD boys that could reveal the efficiency and safety of exon skipping therapy started in earlier stages of DMD. Results of the eteplirsen effect on DMD boys in the early ambulatory stage aged from 4 to 6 years reported that 4 out of 26 patients of the eteplirsen group had serious adverse events (not attributable to eteplirsen treatment) and no death has been reported (https://clinicaltrials.gov/ct2/show/NCT02420379). Dystrophin protein levels measured by WB in muscle biopsies have been quantified in 14 patients after 48 weeks of therapy and in 11 patients after 96 weeks. The WB analysis showed an average increase of 0.102% and 0.312%, respectively.
In another dose-escalating study (2, 4, 10, 20, and 30 mg/kg) of eteplirsen young participants were divided into two cohorts: cohort 1 are boys aged 24–48 months and cohort 2 are boys aged 6–24 months (https://clinicaltrials.gov/ct2/show/NCT03218995). Adverse events were reported in all patients, while a severe adverse event of bronchiolitis was observed in 1 patient, which was thought not to be drug related. As eteplirsen therapy was well tolerated by the young patients, participants who successfully completed the trial have been enrolled in the next safety clinical trial. The study is currently ongoing and focuses on the effect of long-term therapy of eteplirsen administered from a very young age (https://clinicaltrials.gov/ct2/show/NCT03985878).
DMD boys are currently being recruited in the clinical trial of high doses of eteplirsen. In this trial, participants will be administered 100 or 200 mg/kg weekly IV doses, and the incidence of adverse events and functional effects of therapy will be investigated (https://clinicaltrials.gov/ct2/show/NCT03992430).
2.2.2 Golodirsen, viltolarsen, and casimersen
In addition to eteplirsen, Sarepta Therapeutics and NS Pharma have also conducted clinical studies of other PMO-modified AONs developed to target other exons.
Sarepta Therapeutics developed AONs targeting exons 53 and 45 named golodirsen and casimersen, respectively. The first clinical trial of golodirsen has been initiated in 2014, where the primary endpoint was the safety of IV infusions of four escalating doses (4, 10, 20, and 30 mg/kg). As the compound was well tolerated, the study of golodirsen efficiency has been continued. In the second part of the clinical trial, primary endpoints were the expression of dystrophin and the 6MWT (https://clinicaltrials.gov/ct2/show/NCT02310906). An increase of 0.924% of dystrophin from baseline was observed after 48 weeks of golodirsen treatment, and participants walked 99 m less in 144 weeks than at baseline versus to 181.4 m in NH controls [Citation43,Citation44]. Golodirsen received accelerated approval from the FDA in 2019 based on dystrophin restoration [Citation4]. Two other clinical trials are currently ongoing to confirm the safety and functional effects of golodirsen in a placebo-controlled (https://clinicaltrials.gov/ct2/show/NCT02500381) and an NH-controlled setting.
The first clinical trial of casimersen was initiated in 2015 where safety was studied as a primary endpoint (https://clinicaltrials.gov/ct2/show/NCT02530905). Four escalating doses (4, 10, 20, and 30 mg/kg) were well tolerated. In the follow-up placebo-controlled clinical trial (https://clinicaltrials.gov/ct2/show/NCT02500381), the mean change of dystrophin of 0.81% was reported 48 weeks after casimersen therapy initiation [Citation45]. Based on the safety efficacy data, in 2021 the FDA granted accelerated approval to casimersen and required Sarepta Therapeutics to provide verification of the clinical benefits. A placebo-controlled trial to measure the functional effect of casimersen on the 6MWT is ongoing. Regarding safety, while kidney toxicity has been observed in an animal study, it has not been observed in human trials. Nevertheless, the FDA requires monitoring of kidney function [Citation6].
Viltolarsen has been developed by NS Pharma for the treatment of DMD patients who are amenable to exon 53 skipping. It targets the same mRNA sequence as golodirsen but is four nucleotides shorter. The first dose-finding clinical trial has been initiated in 2013 where three escalating doses tested on 10 DMD patients were well tolerated [Citation46] (https://clinicaltrials.gov/ct2/show/NCT02081625). In a phase 2 dose-finding clinical study, safety and dystrophin production were tested for 16 participants who received 40 and 80 mg/kg doses of IV infusion after 24 weeks (https://clinicaltrials.gov/ct2/show/NCT02740972). No serious adverse events were observed and mean increases from baseline of 5.7% and 5.9% of dystrophin have been induced in 40 and 80 mg/kg dose cohorts, respectively [Citation47]. Viltolarsen received accelerated approval based on its safety data and dystrophin-restoring potential in Japan and the USA [Citation5,Citation48]. The FDA requires NS Pharma to confirm the clinical benefits of viltolarsen and several clinical trials are currently ongoing to assess this (https://clinicaltrials.gov/ct2/show/NCT04060199; https://clinicaltrials.gov/ct2/show/NCT04687020, https://clinicaltrials.gov/ct2/show/NCT04768062, https://clinicaltrials.gov/ct2/show/NCT04956289). The effect of long-lasting (2 years) viltolarsen administration in 16 patients treated in an open-label study following a dose-finding trial demonstrated that long-term treatment is well tolerated and induces stabilization of muscle function when patients are compared to a natural history cohort (https://clinicaltrials.gov/ct2/show/NCT03167255) [Citation49].
2.2.3 Exon 44 skipping
To expand the applicability of exon skipping therapy, NS Pharma has initiated an exploratory clinical trial of PMO NS-089/NCNP-02 for exon 44 skipping in Japan in 2019. In the study, the PMO infusion of 1.62, 10, 40, and 80 mg/kg in 6 DMD boys for 24 weeks was well tolerated (https://clinicaltrials.gov/ct2/show/NCT04129294). Dystrophin levels increased by 10.27% of the normal level in the 40 mg/kg dose cohort and by 15.79% of the normal in the dose 80 mg/kg cohort as was announced in press release [Citation50]. Based on the results, NS Pharma initiated an extension study (https://clinicaltrials.gov/ct2/show/NCT05135663). In addition, NS Pharma has more PMOs compounds for exons 50, 51, 45, and 55 skipping in preclinical studies [Citation51].
3. Modifications for improvement of PMO efficiency
PMOs have been approved based on their potential to induce dystrophin restoration and their good tolerability. However, the level of restored dystrophin is low and the clinical benefits of PMOs therapy have not yet been confirmed. The main reason for the low efficiency is likely the poor uptake of the PMO by muscles and the absence of PMO uptake in the heart at doses used in clinical applications. Current efforts focus on improving the delivery of PMO by muscles, as this will increase exon skipping and dystrophin restoration levels and thus is anticipated to result in larger therapeutic effects.
3.1. Cell-penetrating peptides
One way to improve the overall PMO delivery to tissues is to conjugate them to positively charged cell-penetrating peptides (CCPs). CCPs are chains of 5–30 amino acids that can be internalized by cells without interaction with receptors through energy-independent direct penetration of the plasma membrane or energy-dependent endocytosis. CPPs are often rich in positively charged amino acids (arginine and lysine) and lead to an increased delivery in all tissues. Various CPPs have been investigated, including short CPPs, and PMO internalization peptides (Pips), which comprise arginine-rich domains flanking a hydrophobic core. Systemically delivered peptide conjugated PMOs (PPMOs) were shown to have enhanced uptake both by skeletal and heart muscle in vivo and induce exon skipping and dystrophin restoration at a lower dose than unconjugated PMOs [Citation52,Citation53].
In parallel with confirmatory studies for eteplirsen, Sarepta Therapeutics also developed a PPMO targeting exon 51 called SRP-5051 (vesleteplirsen), containing a CCP coupled to eteplirsen. The first phase 1 clinical trial () of SRP-5051 tested the safety and tolerability of five single doses (https://clinicaltrials.gov/ct2/show/NCT03375255). In the further two-part clinical trial, four escalating doses (4, 10, 20, and 30 mg/kg) were IV infused every 4 weeks for 12 weeks and tested. Results were compared to weekly dosing of eteplirsen from the PROMOVI clinical trial (https://clinicaltrials.gov/ct2/show/NCT04004065). Measurement of restored dystrophin by WB revealed a mean increase of 3.06% and 6.55% in 20 and 30 mg/kg cohorts, respectively, while the level of restored dystrophin was 8 times higher in the 30 mg/kg cohort than in the eteplirsen cohort. It was hypothesized that long-term therapy of SRP-5051 can result in greater exon skipping and dystrophin protein levels, as the patient who received five infusions of 30 mg/kg doses had the highest exon skipping and dystrophin protein levels compared to patients who received 3 infusions of the same dose. A serious drug-related adverse event (hypomagnesemia) was observed in two patients [Citation54,Citation55]. The second part of this clinical trial is currently ongoing aiming to further evaluate the effectiveness and safety of the 30 mg/kg dose. Notably, this trial has been put on temporary hold by the FDA after another event of severe hypomagnesemia, despite prophylactic supplementation [Citation56].
PepGen announced the initiation of a clinical trial to test their PPMO called PGN-EDO51 based on the Enhanced Delivery Oligonucleotide (EDO) platform technology (i.e. the pip peptides) developed in collaboration with Wood and Gait research groups [Citation57]. PGN-EDO51 is a PMO coupled to a novel pip CCP with enhanced properties enable to diminish toxicity while maintaining increased delivery. The company presented preclinical data demonstrating 4 times higher exon skipping capacity after a single dose IV injection in wild type (WT) mice compared to a PPMO containing more arginine residues. In addition, the preclinical study demonstrated effective delivery and exon skipping in the cardiac muscle of non-human primates [Citation58]. Notably, in these animals, hypomagnesemia was reported. Based on the results of the preclinical study, PepGen has initiated a phase 1 clinical trial of ascending doses (1, 5, 10 , and 15 mg/kg) of PGN-EDO51 targeting exon 51 in healthy volunteer participants [Citation59]. In a press release, PepGen announced that the highest dose induced mean exon skipping levels of 2%. However, also here two cases of hypomagnesemia were observed [Citation60]. Phase 2 clinical trial in DMD patients is planned to be in 2023.
Further investigation into ways to reduce the toxicity of arginine-rich peptides resulted in the development of a new family of cyclic arginine-rich CCPs, Endosomal Escape Vehicles (EEV). These were found to internalize into cells through energy-dependent endocytosis and escape from the endosomes [Citation61]. Single IV administration of ENTR-601-44, a PMO targeting exon 44 coupled to an EEV, exon skipping in skeletal and cardiac muscle in hDMD mice carrying the full-size human gene and non-human primates [Citation62]. Entrada Therapeutics is preparing a clinical study with this compound.
3.2. Antibodies
Conjugation of antibodies to PMOs is another way to improve oligonucleotide delivery to muscle. While peptides increase PMO delivery in general, antibodies enable the delivery of oligonucleotides specifically to muscles. The approach is based on using antibodies targeting receptors, which are highly expressed on the muscle cell membrane that allow internalization of the oligonucleotides directly to muscle cells. Currently, Dyne Therapeutics and Avidity Biosciences are developing PMOs conjugated to antibody targeting transferrin receptor 1 (Trf 1), a candidate for the direct delivery of PMO to muscles. Trf1 is located throughout the surface of the cell membrane of skeletal, smooth, and cardiac muscles but is also expressed in the brain.
Dyne Therapeutics developed the FORCE platform, which is based on an antigen-binding fragment (Fab) coupled to a PMO through a valine-citrulline linker. For a preclinical study of the FORCE platform, a single IV dose of FORCE-M23D comprising Fab linked to a PMO targeting exon 23 was tested in mdx mice. Both exon skipping and restored dystrophin levels were enhanced after IV administration of a single dose of FORCE-M23D compared to the M23D PMO alone, in a dose-dependent manner. Exon skipping and expression of restored dystrophin were observed in skeletal and cardiac muscle for up to 8–12 weeks after IV injection, suggesting that Fab-based PMOs can be dosed in DMD patients once a month or less frequently. The functional effect of FORCE-M23D was demonstrated with lowered serum CK levels and a small increase in distance traveled in the open field [Citation63]. The first clinical trial of DYNE-251 based on the FORCE platform developed by Dyne Therapeutics to target exon 51 is currently ongoing to test the safety and tolerability of Fab-based PMO (https://clinicaltrials.gov/ct2/show/NCT05524883). AON compounds for exons 53, 45, and 44 and other muscular genetic diseases using the FORCE platform are also under Dyne Therapeutics development.
While Dyne Therapeutics develops a platform using a fragment of an antibody, Avidity Biosciences designed Antibody Oligonucleotide Conjugates (AOC) platform based on the full monoclonal antibody (mAb) specific for TfR1. Their AOC 1044 targeting exon 44 is currently under IND-enabling studies in order to receive approval for initiating clinical trials. The same technology is used by Avidity Biosciences to develop ASOs targeting exons 51 and 45 skipping and for other genetic muscle diseases such as myotonic dystrophy [Citation64].
4. Alternative approaches
4.1. AAV9 U7 snRNA gene therapy
Since approved AONs have to be administered repeatedly (weekly intravenous infusions for currently approved AONs) in order to maintain steady exon skipping and expression of restored dystrophin, a technology decreasing the frequency of drug administration and keeping sufficient exon skipping and dystrophin restoration would be preferred. Previously, it was confirmed that adeno-associated viral vectors (AAV) can deliver micro-dystrophin transgenes to skeletal muscles resulting in durable expression of micro-dystrophin in animal models and DMD patients [Citation65–67]. Thus, AAV was proposed to deliver the uridine-rich seven small nuclear RNA (U7 snRNA) gene. Normally, the U7 snRNA is a part of a small nuclear ribonucleoprotein (snRNP) complex where U7 snRNA targets histone pre-mRNA and binds proteins that induce pre-mRNA processes [Citation68,Citation69].
To allow using U7 snRNA as a tool for exon skipping, the gene was modified by replacing the histone pre-mRNA binding sequence with an antisense oligonucleotide sequence [Citation70,Citation71]. Incorporation of antisense oligonucleotide sequences into the binding site enables U7 snRNA to target exons in the DMD gene and modulate their splicing. Thus, delivering the gene of U7 snRNA using AAV will enable continuous production of antisense oligonucleotides as long as the gene is present. The therapeutic potential of AAV-U7 snRNA technology has been shown in mdx mice, the golden retriever muscular dystrophy dog model, and non-human primates (NHP) [Citation72–74]. Notably, high exon skipping levels were observed in heart muscles due to the efficiency of AAV delivery to the heart [Citation75].
In 2020, Nationwide Children’s Hospital initiated a clinical trial () of scAAV9.U7.ACCA for exon 2 skipping (https://clinicaltrials.gov/ct2/show/NCT04240314). The approach was developed for patients with an exon 2 duplication in which skipping of a single exon 2 can lead to restoration of full-length functional dystrophin, while skipping of both exons can result in recognition of the internal ribosome entry site located in exon 5 by the translation machinery and translation of functional shortened dystrophin [Citation76]. Notably, scAAV9.U7.ACCA can also be administered by DMD patients with a mutation in exons 1–5 as also here skipping of exon 2 will facilitate translation starting at exon 5 [Citation76].
Dystrophin production was tested in a muscle biopsy 4 months after a single dose of peripheral IV injections of scAAV9.U7.ACCA in 3 DMD boys. The level of full-length dystrophin was increased by 70% of the normal level in the youngest DMD boy (7 months old), while in 8.9 and 13.7 years old patients full dystrophin was increased by 6% and 1–2%, respectively [Citation77]. In addition, treatment was well tolerated except for transient nausea and vomiting and no serious events have been observed 3 months after injection [Citation78]. Thus, this result indicates that early DMD therapy could be more beneficial and more tolerable for patients. In addition, Astellas Pharma was developing gene therapy programs with an AAV-based exon skipping approach for exon 2 (AT702), 51 (AT751), and 53 (AT753) but development was terminated by Astellas [Citation79].
5. Conclusion
DMD is a slowly progressing genetic disorder characterized by lack of functional dystrophin. Exon skipping therapeutics, aiming to restore dystrophin in DMD muscles, have been approved by FDA but their clinical benefits are currently under investigation in clinical trials with placebo-controlled and NH-controlled settings. Moreover, development of additional oligonucleotides chemical modifications (i.e. backbone modifications, conjugated peptides, conjugated antibodies, and alternative approach based on U7 snRNA tool) are currently ongoing in preclinical and clinical investigations.
6. Expert opinion
Proof-of-concept that the exon skipping approach can restore dystrophin production in DMD patients has been provided. However, there is room for improvement and many different approaches have been used to optimize exon skipping compounds, since the first approval of eteplirsen in 2016. Currently, four exon skipping drugs based on the PMO chemistry are approved by FDA for DMD patients, and other AONs modifications are being tested in preclinical and clinical studies. Improved AON modifications demonstrate more efficient exon skipping and dystrophin restoration in vitro and in vivo. While peptide conjugates enhance oligonucleotide uptake by muscles and the antibody approach imparts more targeted AONs delivery to muscle cells, the AAV U7 snRNA-based approach enables continuous production of dystrophin reducing dose frequency.
Despite the advantages of each of these new techniques, there is limited expertise with these modifications in humans, and safety and efficiency need to be carefully studied. Even though hypomagnesemia observed in DMD patients receiving SRP-5051 or healthy volunteers receiving PGN-EDO51 was successfully corrected with magnesium supplementation, the root cause of the hypomagnesemia needs to be carefully studied. Hypomagnesemia could be a signal of proximal tubuli toxicity as reabsorption of magnesium takes relatively more energy compared to other ions, and magnesium is likely to be the first to be excreted in case of renal toxicity [Citation80].
Regarding efficacy, while suvodirsen was successfully tested in preclinical studies, in a clinical trial it was not able to induce dystrophin restoration. This underlines the need to develop multiple alternative AONs in parallel, as safety and efficacy are not guaranteed.
Despite muscle degeneration and motor delay beginning in early childhood, DMD is diagnosed at the mean age of 4–5 years [Citation2,Citation81]. Thus, patients receive treatment when motor functions have already declined, and part of the muscle tissue is irreversibly replaced with fibrotic or adipose tissue. Early diagnosis is necessary to start early treatment, in order to preserve muscle quality and muscle function as much as possible and have the largest possible impact on slowing down disease progression. Newborn screening (NBS) was proposed to overcome the diagnostic odyssey and enable early DMD treatment [Citation82]. NBS of DMD is based on testing dried blood spots for creatine kinase (CK) activity, which is increased in DMD already in newborns. Recently, a pilot in NBS has been successfully completed where more than 36.000 newborn babies were tested and four babies with DMD or BMD were detected. In 2022, DMD was nominated for inclusion in the Recommended Uniform Screening Panel (RISP), a list of disorders that benefit from NBS [Citation83]. However, as so far trials have been done in older patients, there is no data on optimal dosing and safety in very young DMD patients. The current phase 2 clinical trial of eteplirsen is studying the effect of early treatment and dystrophin restoration in young patients of 2–5 years old, while a clinical trial of scAAV9.U7.ACCA has already shown very successful restoration of dystrophin in a patient of 7 months old. However, long-term clinical benefits of dystrophin restoration still need to be investigated, and more studies in young patients are necessary to see whether dosing is similar in these patients and whether for the scAAV9.U7.ACCA the dystrophin restoring effect is not diluted when the patient and his muscle grow.
To increase the chance of being able to pick up a therapeutic effect in a clinical trial one needs either a very effective drug with a large response in the primary endpoint, a large cohort of patients, or a very long trial. DMD progresses slowly and currently tested therapeutic interventions aim to slowdown disease progression, ruling out the first criterion. Due to the rarity of the disease, especially for exon skipping, where subsets of eligible patients get smaller and smaller with different target exons, large trials are not an option either. To reduce the cohort sizes, DMD patients not amenable to specific exon skipping (open-label untreated control) or external NH patients have been used as control groups. However, the experience of the untreated control trial of eteplirsen, where the vast majority of untreated patients did not complete the study due to being enrolled in other trials, underlines that this approach is probably not viable for DMD given that there are so many opportunities for patients to be enrolled in clinical trials. Using NH reference data is a more likely alternative, but this requires a detailed understanding of disease trajectories for the outcome measure used as a primary endpoint to allow proper matching of trial patients with NH patients. The collaborative trajectory analysis project (cTAP) is working on providing this data [Citation84,Citation85]. However, for now regulators insist on randomized placebo-control trials being necessary for the investigation of drug safety and efficacy, meaning the only option available is to have longer trials. This raises ethical questions because the placebo group in a placebo-controlled DMD receives placebo for a long period (currently 96 weeks) and it is known that patients will irreversibly lose muscle function during this period [Citation86,Citation87].
We want to stress that because so far there is no confirmatory data that the dystrophin levels observed for the approved PMOs slowdown disease progression, the relation between low level of dystrophin restoration and functional effects is unknown. Furthermore, the confirmatory studies are mostly conducted in ambulant patients, and it is not known whether the same amounts will slowdown disease progression in arms and respiratory function in non-ambulant patients. Therefore, more clinical trials are necessary to receive comprehensive data about disease progression and motor function after AON treatment in different stages of the disease.
7. Concluding remarks
Significant progress in the exon skipping approach for DMD has been made in the last 20 years, which resulted in the approval of four AONs for DMD therapy, despite many limitations. Various AON modifications are under ongoing investigation and hold promise for the improvement of exon skipping compounds due to enhanced muscle uptake, targeted delivery, and reduction of dose frequency. In addition, hopefully future adaptations such as implementation of NBS and allowing the use of NH controls in clinical trials can benefit in exon skipping field. Looking back, the exon skipping approach for DMD has made a lot of progress over the past 25 years, going from proof-of-concept in cultured cells to approved drugs. Hopefully, in the next 25 years, the exon skipping approach will be even further developed as a clinically proven therapy for DMD.
Article highlights
Duchenne muscular dystrophy (DMD) is a severe heritable muscle disorder caused by mutations in the DMD gene.
FDA granted accelerated approval to four exon skipping compounds for DMD therapy.
Functional effect of the approved exon skipping therapies has to be established.
Application of natural history (NH) controls was suggested to assist in an evaluation of the functional effect.
Additional chemical modifications of AONs showing enhanced oligonucleotides properties are being investigated in preclinical and clinical studies.
Declaration of interest
A Aartsma-Rus is an advisory board member for Sarepta Therapeutics, Silence Therapeutics, Eisai, and Hybridize Therapeutics. She is also an ad hoc consultant for PTC Therapeutics, Alpha Anomeric, BioMarin, Entrada, Takeda, Splicesense, Galapagos, and Astra Zeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Duan D, Goemans N, Takeda S, et al. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021 Feb 18;7(1):13.
- Dj B, Bushby K, CM B, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018 Mar;17(3):251–267.
- Syed YY. Eteplirsen: first global approval. Drugs. 2016 Nov;76(17):1699–1704.
- Heo YA. Golodirsen: first approval. Drugs. 2020 Feb;80(3):329–333.
- Dhillon S. Viltolarsen: first approval. Drugs. 2020 July 01;80(10):1027–1031.
- Shirley M. Casimersen: first approval. Drugs. 2021 May;81(7):875–879.
- Aartsma-Rus A, van Ommen GJ. Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications. Rna. 2007 Oct;13(10):1609–1624.
- A-FE S, Aartsma-Rus A. Developments in reading frame restoring therapy approaches for Duchenne muscular dystrophy. Expert Opin Biol Ther. 2021 Mar 04;21(3):343–359.
- Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003 Apr;270(8):1628–1644.
- Phosphorothioates EF. Essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014;24(6):374–387.
- Järver P, O’Donovan L, Gait MJ. A chemical view of oligonucleotides for exon skipping and related drug applications. Nucleic Acid Ther. 2014 Feb;24(1):37–47.
- Amantana A, Iversen PL. Pharmacokinetics and biodistribution of phosphorodiamidate morpholino antisense oligomers. Curr Opin Pharmacol. 2005 Oct;5(5):550–555.
- Aartsma-Rus A, Kaman WE, Bremmer-Bout M, et al. Comparative analysis of antisense oligonucleotide analogs for targeted DMD exon 46 skipping in muscle cells. Gene Ther. 2004 Sep;11(18):1391–1398.
- Heemskerk H, de Winter C, van Kuik P, et al. Preclinical PK and PD studies on 2’-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol Ther. 2010 Jun;18(6):1210–1217.
- Yokota T, Hoffman E, Takeda S. Antisense oligo-mediated multiple exon skipping in a dog model of Duchenne muscular dystrophy. Methods Mol Biol. 2011;709:299–312.
- Yokota T, Lu QL, Partridge T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009 Jun;65(6):667–676.
- Shrewsbury SB, Sazani P, Muntoni F. P1.10 Comparative pharmacokinetics (PK) in primates and humans of AVI-4658, a phosphorodiamidate morpholino oligomer (PMO) for treating DMD patients. Neuromuscul Disord. 2011;21(9):644.
- van Deutekom JC, Janson AA, Ginjaar IB, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007 Dec 27;357(26):2677–2686.
- Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011 Apr 21;364(16):1513–1522.
- Voit T, Topaloglu H, Straub V, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebo-controlled phase 2 study. Lancet Neurol. 2014 Oct;13(10):987–996.
- McDonald CM, Wong B, Flanigan KM, et al. Placebo-controlled phase 2 trial of drisapersen for Duchenne muscular dystrophy. Ann Clin Transl Neurol. 2018 Aug;5(8):913–926.
- Goemans N, Mercuri E, Belousova E, et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul Disord. 2018 Jan;28(1):4–15.
- Kyndrisa: Withdrawal of the marketing authorisation application [Internet]. 2016 [cited 2022 Oct 24]. Available from: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/kyndrisa
- Declines FDA Approval for Drisapersen in DMD [Internet]. 2016 [cited 2022 Oct 24]. Available from: https://www.medscape.com/viewarticle/857406
- Wave life sciences announces discontinuation of suvodirsen development for Duchenne muscular dystrophy [internet]. 2019 [cited 2022 Oct 24]. Available from: https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-announces-discontinuation-suvodirsen
- Rigo F, Hua Y, Chun SJ, et al. Synthetic oligonucleotides recruit ILF2/3 to RNA transcripts to modulate splicing. Nat Chem Biol. 2012 Apr 15;8(6):555–561.
- Jirka SM, Tanganyika-de Winter CL, Jw B-VDM, et al. Evaluation of 2’-deoxy-2’-fluoro antisense oligonucleotides for exon skipping in Duchenne muscular dystrophy. Mol Ther Nucleic Acids. 2015 Dec 1;4(12):e265.
- Chen S, Le BT, Chakravarthy M, et al. Systematic evaluation of 2’-Fluoro modified chimeric antisense oligonucleotide-mediated exon skipping in vitro. Sci Rep. 2019 Apr 15;9(1):6078.
- Kandasamy P, McClorey G, Shimizu M, et al. Control of backbone chemistry and chirality boost oligonucleotide splice switching activity. Nucleic Acids Res. 2022 Jun 10;50(10):5443–5466.
- Wave Life Sciences Announces Initiation of Dosing in Phase 1b/2a clinical trial of WVE-N531 in Duchenne muscular dystrophy [internet]. 2021 [cited 2022 Oct 24]. Available from: https://ir.wavelifesciences.com/news-releases/news-release-details/wave-life-sciences-announces-initiation-dosing-phase-1b2a
- Morita K, Hasegawa C, Kaneko M, et al. 2’-O,4’-C-ethylene-bridged nucleic acids (ENA) with nuclease-resistance and high affinity for RNA. Nucleic Acids Res Suppl. 2001;12(1):241–242
- Lee T, Awano H, Yagi M, et al. 2’-O-methyl RNA/ethylene-bridged nucleic acid chimera antisense oligonucleotides to induce dystrophin exon 45 skipping. Genes (Basel). 2017 Feb 10;8:2.
- Ito K, Takakusa H, Kakuta M, et al. Renadirsen, a novel 2ʹOMeRNA/ENA(®) chimera antisense oligonucleotide, induces robust exon 45 skipping for dystrophin in vivo. Curr Issues Mol Biol. 2021 Sep 25;43(3):1267–1281.
- Daiichi Sankyo Announces the Results Summary of Phase 1/2 Clinical Trial in Japan for DS-5141 [Internet]. 2021 [cited 2022 Oct 24]. Available from: https://www.daiichisankyo.com/media/press_release/detail/index_4112.html
- Kinali M, Arechavala-Gomeza V, Feng L, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009 Oct;8(10):918–928.
- Cirak S, Arechavala-Gomeza V, Guglieri M, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011 Aug 13;378(9791):595–605.
- Mendell JR, Rodino-Klapac LR, Sahenk Z, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013 Nov;74(5):637–647.
- Charleston JS, Schnell FJ, Dworzak J, et al. Eteplirsen treatment for Duchenne muscular dystrophy: exon skipping and dystrophin production. Neurology. 2018 Jun 12;90(24):e2146–e2154.
- Mendell JR, Goemans N, Lowes LP, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016 Feb;79(2):257–271.
- FDA grants accelerated approval to first drug for Duchenne muscular dystrophy [Internet]. 2016 [cited 2022 Oct 24]. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-first-drug-duchenne-muscular-dystrophy
- Wang RT, Barthelemy F, Martin AS, et al. DMD genotype correlations from the Duchenne Registry: endogenous exon skipping is a factor in prolonged ambulation for individuals with a defined mutation subtype. Hum Mutat. 2018 Sep;39(9):1193–1202.
- McDonald CM, Shieh PB, Abdel-Hamid HZ, et al., Open-Label Evaluation of Eteplirsen in Patients with Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping: PROMOVI Trial. J Neuromuscul Dis. 2021. 8(6): 989–1001.
- Frank DE, Schnell FJ, Akana C, et al. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology. 2020 May 26;94(21):e2270–e2282.
- Servais L, Mercuri E, Straub V, et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with Duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther. 2022 Feb;32(1):29–39.
- Iannaccone S, Phan H, Straub V, et al. P.132 casimersen in patients with Duchenne muscular dystrophy amenable to exon 45 skipping: interim results from the phase 3 ESSENCE trial. Neuromuscul Disord. 2022 Oct 01;32:S102.
- Komaki H, Nagata T, Saito T, et al. Systemic administration of the antisense oligonucleotide NS-065/NCNP-01 for skipping of exon 53 in patients with Duchenne muscular dystrophy. Sci Transl Med. 2018;10(437):eaan0713.
- Clemens PR, Rao VK, Connolly AM, et al. Safety, tolerability, and efficacy of viltolarsen in boys with Duchenne muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol. 2020 Aug 1;77(8):982–991.
- Approves Targeted FDA Treatment for rare Duchenne muscular dystrophy mutation [internet]. 2020 [cited 2022 Oct 24]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation
- Clemens PR, Rao VK, Connolly AM, et al. Long-term functional efficacy and safety of viltolarsen in patients with Duchenne muscular dystrophy. J Neuromuscul Dis. 2022;9:493–501.
- Study shows the efficacy of antisense oligonucleotide-based exon 44 skipping drug, NS-089/NCNP-02, for patients with Duchenne muscular dystrophy (DMD) [Internet]. 2022 [cited 2022 Oct 24]. Available from: https://www.ncnp.go.jp/topics/2022/20220317e.html
- NS pharma pipeline [Internet]. [cited 2022 Oct 24]. Available from: https://www.nspharma.com/pipeline
- Moulton HM, Moulton JD. Morpholinos and their peptide conjugates: therapeutic promise and challenge for Duchenne muscular dystrophy. Biochim Biophys Acta - Biomembr. 2010;1798(12):2296–2303.
- Gan L, Wu LCL, Wood JA, et al. A cell-penetrating peptide enhances delivery and efficacy of phosphorodiamidate morpholino oligomers in mdx mice. Mol Ther Nucleic Acids. 2022;30:17–27.
- Clinical Update: Results from 30 mg/kg cohort of MOMENTUM study of SRP-5051 for Duchenne muscular dystrophy [internet]. 2021 [cited 2022 Oct 24]. Available from: https://investorrelations.sarepta.com/events/event-details/clinical-update-results-30-mgkg-cohort-momentum-study-srp-5051-duchenne
- Sheikh O, Yokota T. Pharmacology and toxicology of eteplirsen and SRP-5051 for DMD exon 51 skipping: an update. Arch Toxicol. 2022 Jan 01;96(1):1–9.
- Sarepta therapeutics announces that FDA has lifted its clinical hold on SRP-5051 for the treatment of Duchenne muscular dystrophy [Internet]. 2022 [cited 2022 Oct 24]. Available from: https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-has-lifted-its-clinical-hold
- Tsoumpra MK, Fukumoto S, Matsumoto T, et al. Peptide-conjugate antisense based splice-correction for Duchenne muscular dystrophy and other neuromuscular diseases. EBioMedicine. 2019 Jul;45:630–645.
- A novel enhanced delivery oligonucleotide (EDO) therapeutic demonstrates considerable potential in treating Duchenne muscular dystrophy [Internet]. [cited 2022 Oct 24]. Available from: https://pepgen.com/wp-content/uploads/2022/02/PepGen_A-novel-enhanced-delivery-oligonucleotide-EDO-therapeutic.pdf
- PepGen Announces First Participant Dosed in a Phase 1 Clinical Trial of PGN-EDO51 for the treatment of Duchenne muscular dystrophy [Internet]. 2022 [cited 2022 Oct 24]. Available from: https://investors.pepgen.com/news-releases/news-release-details/pepgen-announces-first-participant-dosed-phase-1-clinical-trial
- PepGen Reports Positive Data from Phase 1 Trial of PGN-EDO51 for the treatment of Duchenne muscular dystrophy [internet]. 2022 [cited 2022 Oct 24]. Available from: https://investors.pepgen.com/news-releases/news-release-details/pepgen-reports-positive-data-phase-1-trial-pgn-edo51-treatment
- Qian Z, LaRochelle JR, Jiang B, et al. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry. 2014 Jun 24;53(24):4034–4046.
- Entrada therapeutics presents new data supporting its growing pipeline of endosomal escape vehicle (EEV™) Therapeutics at TIDES USA 2022 [Internet]. 2022 [cited 2022 Oct 24]. Available from: https://ir.entradatx.com/news-releases/news-release-details/entrada-therapeutics-presents-new-data-supporting-its-growing
- Desjardins CA, Yao M, Hall J, et al. Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice. Nucleic Acids Res. 2022;50:11401–11414.
- Avidity biosciences pipeline overview [Internet]. [cited 2022 Oct 24]. Available from: https://www.aviditybiosciences.com/pipeline/pipeline-overview/
- Systemic DD. AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol Ther. 2018 Oct 3;26(10):2337–2356.
- Potter RA, Griffin DA, Heller KN, et al. Dose-Escalation Study of Systemically Delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther. 2021 Apr;32(7–8):375–389.
- Mendell JR, Sahenk Z, Lehman K, et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with Duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol. 2020 Sep 1;77(9):1122–1131.
- Müller B, The SD. U7 snRNP and the hairpin binding protein: key players in histone mRNA metabolism. Semin Cell Dev Biol. 1997 Dec;8(6):567–576.
- Schümperli D, Pillai RS. The special Sm core structure of the U7 snRNP: far-reaching significance of a small nuclear ribonucleoprotein. Cell Mol Life Sci. 2004 Oct;61(19–20):2560–2570.
- Gorman L, Suter D, Emerick V, et al. Stable alteration of pre-mRNA splicing by modified U7 small nuclear RNAs. Proc Natl Acad Sci U S A. 1998;95(9):4929–4934.
- Lesman D, Rodriguez Y, Rajakumar D, et al. U7 snRNA, a small RNA with a big impact in gene therapy. Hum Gene Ther. 2021 Nov;32(21–22):1317–1329.
- Vulin A, Barthélémy I, Goyenvalle A, et al. Muscle function recovery in golden retriever muscular dystrophy after AAV1-U7 exon skipping. Mol Ther. 2012 Nov;20(11):2120–2133.
- Goyenvalle A, Vulin A, Fougerousse F, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004 Dec 3;306(5702):1796–1799.
- Aupy P, Zarrouki F, Sandro Q, et al. Long-term efficacy of AAV9-U7snRNA-mediated exon 51 skipping in mdx52 mice. Mol Ther Methods Clin Dev. 2020 Jun 12;17:1037–1047.
- Zincarelli C, Soltys S, Rengo G, et al. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010 Jun;3(3):81–89.
- Wein N, Vulin A, Falzarano MS, et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nat Med. 2014 Sep;20(9):992–1000.
- Nationwide researchers announce restoration of full-length dystrophin in humans [internet]. 2022 [cited 2022 Oct 24]. Available from: https://cureduchenne.org/all-news/nationwide-researchers-announce-restoration-of-full-length-dystrophin-in-humans/
- Waldrop DM, Lawlor DM, Vetter TMD, et al. LATE BREAKING NEWS ORAL PRESENTATION: LBO 3 Expression of apparent full-length dystrophin in skeletal muscle in a first-in-human gene therapy trial using the scAAV9.U7-ACCA vector. Neuromuscul Disord. 2020;30:S166–S167.
- Notice regarding impairment loss for products under development [internet]. 2022 [cited 2022 Oct 24]. Available from: https://www.astellas.com/en/news/25731
- JHFd B, Hoenderop JGJ, Bindels RJM. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46.
- Lurio JG, Peay HL, Mathews KD. Recognition and management of motor delay and muscle weakness in children. Am Fam Physician. 2015 Jan 1;91(1):38–44.
- Ke Q, Zhao Z-Y, Mendell JR, et al. Progress in treatment and newborn screening for Duchenne muscular dystrophy and spinal muscular atrophy. World J Pediatr. 2019 June 01;15(3):219–225.
- PPMD submits RUSP nomination package for duchenne muscular dystrophy [Internet]. 2022 [cited 2022 Oct 24]. Available from: https://www.parentprojectmd.org/ppmd-submits-rusp-nomination-package-for-duchenne-muscular-dystrophy/
- Muntoni F, Signorovitch J, Sajeev G, et al. Real-world and natural history data for drug evaluation in Duchenne muscular dystrophy: suitability of the North Star ambulatory assessment for comparisons with external controls. Neuromuscul Disord. 2022 Apr;32(4):271–283.
- Goemans N, Wong B, Van den Hauwe M, et al. Prognostic factors for changes in the timed 4-stair climb in patients with Duchenne muscular dystrophy, and implications for measuring drug efficacy: a multi-institutional collaboration. PLoS One. 2020;15(6):e0232870.
- Merlini L, Sabatelli P. Improving clinical trial design for Duchenne muscular dystrophy. BMC Neurol. 2015 Aug;26(15):153.
- Straub V, Mercuri E, Aartsma-Rus A, et al. Report on the workshop: meaningful outcome measures for Duchenne muscular dystrophy, London, UK, 30–31 January 2017. Neuromuscular Disorders. 2018;28(8):690–701.
