ABSTRACT
Background
Real-world data are useful to guide the management of psoriasis. Here, we present data on the effectiveness and survival of guselkumab in moderate-to-severe chronic plaque psoriasis for up to 148 weeks.
Research design and methods
Cross-sectional study of 122 patients receiving guselkumab (100 mg at weeks 0 and 4, and then every 8 weeks thereafter) for>12 weeks, from November 2018 to April 2022.
Main outcome measures
Clinical features and drug survival were analyzed up to 148 weeks.
Results
Obese patients (32.8%) and those receiving prior biologics (64.8%) were included. Guselkumab treatment was associated with a rapid decrease in PASI, from 16.2 to 3.2 at week 12, and long-term improvements in all subgroups (97.6%, 82.9%, and 63.4% of patients, respectively, achieved PASI 75, 90, and 100 after 148 weeks). More non-obese than obese patients achieved PASI 100 at week 148 (86.4% vs 38.9%), as did bio-naïve vs bio-experienced patients (86.7% vs 50.0%). Previous biologic therapy was a negative prognostic factor for achieving PASI 100 over the long-term by multivariate analysis (p = 0.005). Overall, 96% of patients were on treatment after 2 years.
Conclusions
Real-world data confirm the long-term effectiveness of guselkumab in patients with psoriasis.
KEYWORDS:
1. Introduction
Psoriasis is a chronic disease that affects 2–3% of the population globally [Citation1]. The relatively high prevalence of psoriasis, disease-associated comorbidities, and the high burden on quality of life and economic resources, highlight the need for effective and safe therapies [Citation2,Citation3]. Increased knowledge of the pathogenesis of psoriasis led to the development of new targeted therapies [Citation4]. Among these, biologic agents have dramatically changed the management of psoriasis and provide good results in terms of their safety and efficacy [Citation5].
In addition to other pathways, the interleukin (IL)23–T helper (Th)17 axis is a key player in the pathogenesis of psoriasis. IL-23 targeting agents are the most recent biologics approved to treat moderate-to-severe psoriasis in both the U.S.A and Europe, and include ustekinumab, guselkumab, tildrakizumab, and risankizumab [Citation6]. Guselkumab is a fully human monoclonal antibody that selectively binds the p19 subunit of IL-23, leading to the specific inhibition of intracellular signaling needed for terminal differentiation and survival of Th17 cells [Citation7].
Two phase 3 studies, VOYAGE 1 and VOYAGE 2, demonstrated that guselkumab was associated with significant efficacy compared with placebo and was superior to adalimumab in terms of the Psoriasis Area Severity Index (PASI) 90 at weeks 16, 24, and 48 [Citation8,Citation9]. In addition, the NAVIGATE trial showed that patients with an inadequate response to ustekinumab who switched to guselkumab at week 16 of treatment, experienced greater benefits in improving the severity of psoriasis than patients who remained on ustekinumab for 1 year [Citation10]. Extension studies of the VOYAGE trials found that continuous guselkumab therapy maintained clinical responses over 4–5 years [Citation11–13]. These studies also showed that guselkumab had a predictable safety profile and was associated with improvements in health-related quality of life over the longer term [Citation13,Citation14].
Despite these encouraging findings, real-world studies are essential to understand the overall efficacy and utility of new biologic therapies in daily clinical practice. Real-world data on the efficacy of guselkumab are becoming available, although they are generally limited to relatively short follow-up times of around one year [Citation15–17], with the exception of a few recently published analyses [Citation18–20]. Since psoriasis is a chronic disease and treatment is often lifelong, demonstration of the long-term effectiveness in a real-life setting is needed to provide clinicians with greater confidence in prescribing the drug. In this analysis, we present real-world outcomes on the effectiveness of guselkumab in patients with moderate-to-severe chronic plaque psoriasis who were treated with guselkumab for 148 weeks. We also present data on drug survival during long-term treatment with guselkumab.
2. Materials and methods
2.1. Study design
The overall aim of the study was to evaluate the long-term clinical efficacy of guselkumab in patients with moderate-to-severe chronic plaque psoriasis, eligible for treatment with a biologic. Adult patients (≥18 years old) who were receiving monotherapy treatment at the Dermatology Unit of the Polyclinic Tor Vergata Foundation, Rome, Italy, were included. Data were collected from November 2018 to April 2022. Patients were excluded from the study if they were receiving any co-medication with systemic or topical therapies (topical corticosteroids, vitamin D derivatives). Participants were included in this retrospective, cross-sectional, ‘snapshot’ study on the basis of medical records, which were reviewed by the clinicians who authored this manuscript. Administration of guselkumab to patients with moderate-to-severe plaque psoriasis followed the Summary of Product Characteristics (briefly, an induction with a subcutaneous administration of 100 mg at weeks 0 and 4 and a maintenance administration every 8 weeks) including patients who did not respond to or showed contraindications or side effects to at least one conventional systemic therapy [Citation21] and with a: i) PASI>10 and Body Surface Area (BSA) >10 at baseline; or ii) PASI<10 and BSA<10 at baseline and involvement of specific sensitive areas (i.e. face, scalp, hands, nails, soles of the feet or genital areas) [Citation15], according to Italian law.
Psoriasis was diagnosed following clinical criteria. At enrollment, data on patient demographics, clinical history, PASI score, and comorbidities were recorded. Before enrollment, all patients gave written informed consent to participate in the study. The study was conducted in accordance with the 1975 Declaration of Helsinki ethical standards. According to Italian law, formal ethical committee approval is not required for this type of study [Citation22].
The efficacy of guselkumab treatment was evaluated by measuring PASI at week 0, week 4, and every 8 weeks thereafter. Clinical efficacy was evaluated using PASI 75, PASI 90, and PASI 100 (75%, 90%, 100% reduction in PASI score, respectively). The proportion of patients achieving PASI 75, PASI 90, and PASI 100 responses with guselkumab was examined in relation to Body Mass Index (BMI) class (obese versus non-obese) and biologic status of the patient (bio-naïve versus bio-experienced.
As the patients included in this study started treatment at different times, the data showed here are to be considered as a cross-sectional ‘snapshot’ of our experience up to April 2022.
2.2. Drug survival
Drug survival was defined as the period a patient stayed on treatment, from start until discontinuation for any reason (i.e. loss of treatment efficacy, safety, change to another treatment, loss at follow-up, patient decision).
2.3. Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables, and number and percentage for categorical variables. Univariate logistic regression analysis was carried out considering sex, age, age of disease onset, disease duration, weight, BMI, PASI score at baseline, number of comorbidities, number of previous biologics, last class of biologic drug used, and presence of disease in difficult-to-treat sites. Multivariate logistic regression analysis was carried out using the same variables tested in univariate analysis.
Drug survival analysis was performed applying the Kaplan-Meier estimator and compared using the long-rank test.
When a value for an intermediate visit was missed, it was imputed using the last observation carried forward (LOCF) method. Statistical significance was set for p < 0.05. All analyses were carried out using SPSS (IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp).
3. Results
3.1. Study population
In all, 151 patients with moderate-to-severe plaque psoriasis were included in this study. Data were analyzed on 122 patients who were treated with at least two doses of guselkumab for>12 weeks (). The mean age of the study population was 51.5 (SD 14.6) years; 52.6% of patients were male. The mean disease duration was 24 (SD 15.9) years; nineteen patients had a disease onset at ≤12 years of age, 17 patients were between 13 and 17 years of age, while the remainder of the patient population had disease onset as adults (≥18 years). Mean BMI was 28.6 (SD 6.1) kg/m2, and 32.8% of patients were obese; 31.1% of patients had hypertension. In addition, 64.8% of patients had received prior biologic therapy (38 patients had received one prior biologic, 24 patients had received two, 10 patients had received three, and 7 patients had received≥4 prior biologics), and 35 patients received guselkumab after failing therapy with ustekinumab. At the time of analysis, 122 (100%), 107 (87.7%), 93 (76.2%), 77 (63.1%), 69 (56.5%), 51 (41.8%) and 37 (30.3%) patients had completed 12 weeks, 28 weeks, 52 weeks, 76 weeks, 100 weeks, 124 weeks, and 148 weeks of guselkumab treatment, respectively.
Table 1. Demographic and clinical characteristics of the cohort (122 patients).
Eleven patients presented with infections: one patient was HIV+ and was on antiretroviral therapy; four patients had prior HBV infection and were anti-HBc Ag positive, HBs Ag negative, and HBV DNA negative, and guselkumab was started without antiviral therapy; one patient had prior HCV infection that was eradicated; five patients were TB GOLD+ (one patient with previous TB disease and four TB GOLD+ patients without disease: the latter received prophylactic therapy with isoniazid contextually with the initiation of guselkumab).
3.2. PASI response
Overall, treatment with guselkumab induced a rapid decrease in mean PASI score, from 16.2 (SD 12.9) at baseline to 7.0 (SD 6.9) at week 4, 3.2 (SD 4.0) at week 12, 1.4 (SD 1.7) at week 28, 1.2 (SD 0.9) at week 52, and 1.3 (SD 0.9) at week 100 (). PASI score remained low throughout the observation period, with a mean of 1.2 (SD 2.0) at week 148. At week 12, 65.6%, 44.2%, and 31.7% of patients achieved PASI 75, PASI 90, and PASI 100, respectively. At week 52, a higher percentage of patients achieving these endpoints was observed, and PASI 75 was reached by 88.5%, PASI 90 by 83.3%, and PASI 100 by 70.8% of patients. After 148 weeks of guselkumab treatment, PASI 75, PASI 90, and PASI 100 was reached by 97.6%, 82.9%, and 63.4% of patients, respectively (analyzed as LOCF) ().
Figure 1. Effect of guselkumab on Psoriasis Area and Severity Index (PASI) score and achievement of PASI 75, 90, and 100 responses over 148 weeks in patients with moderate-to-severe psoriasis. Missing values for intermediate visits were imputed with the last observation carried forward (LOCF) method. a: mean PASI scores in the overall population. B: improvement in PASI 75, 90, and 100 responses in the cohort of all patients. C: mean PASI scores in obese (BMI≥30) and non-obese (BMI<30) patients. Improvement in PASI 75 (D), 90 (E), and 100 (F) responses in obese and non-obese patients.
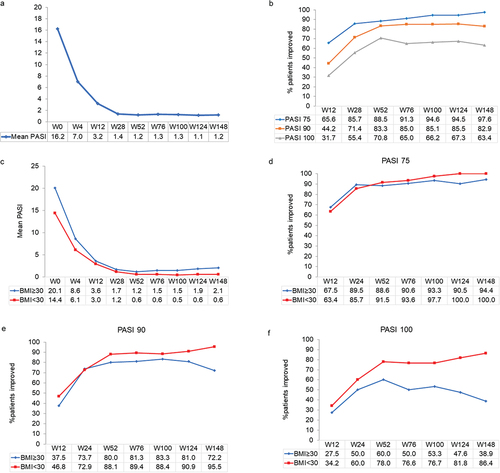
The decrease in mean PASI score was similar in the 40 obese patients (BMI≥30 kg/m2) and non-obese patients (n = 82), but tended to be slightly less in those with a BMI≥30 kg/m2 (). At week 148, the mean PASI score was 2.1 (SD 4.1) in those with a BMI≥30 kg/m2 compared with 0.6 (SD 1.7) in patients with a lower BMI. While differences in PASI 75 were similar in obese and non-obese patients (), clear differences were seen in PASI 90 and PASI 100, especially at longer follow-up times (, and f). At week 148, PASI 90 and PASI 100 were achieved in 95.5% and 86.4% of patients with a BMI<30 kg/m2 vs. 72.2% and 38.9% in obese patients, respectively.
PASI 75 and PASI 90 were slightly improved in patients who were bio-naïve compared with patients previously treated with a biologic (). Over the short term, bio-naïve patients easily reached an excellent efficacy compared to bio-experienced (97.5% of patients reaching PASI 75 vs 79.5%, 85.0% reaching PASI 90 vs 64.4% and 80% reaching PASI 100 vs 42.5% already at week 28, respectively). These differences were most apparent at week 148 for PASI 100, which was achieved by 86.7% and 50% of patients who were bio-naïve and bio-experienced, respectively. PASI responses observed for patients receiving<3 vs ≥ 3 lines of previous biologic therapy were similar for PASI 75 and PASI 90 (), but were markedly different for PASI 100 at week 148, and favored patients with<3 lines than those with≥3 lines of previous biologic therapy (64.7% vs 22.5%, respectively) ().
Figure 2. Effect of guselkumab on PASI 75, 90, and 100 responses over 148 weeks in bio-naïve and bio-experienced patients with moderate-to-severe psoriasis (panels a, b and c), in bio-experienced patients with moderate-to-severe psoriasis who had received<3 or ≥ 3 previous lines of biologic therapy (PBT) (panels d, e and f), and in patients with moderate-to-severe psoriasis who switched from ustekinumab over 100 weeks (panel g). Missing values for intermediate visits were imputed with the last observation carried forward (LOCF) method.
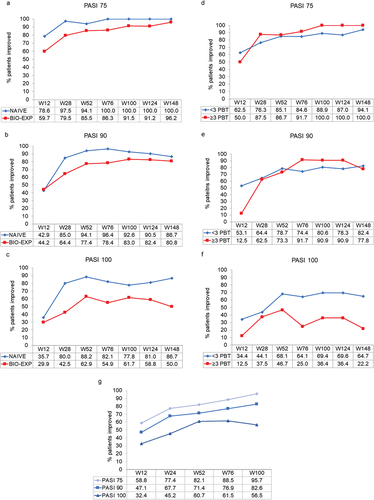
We also assessed PASI 75, 90, and 100 responses after guselkumab in 35 patients who received the drug after failing therapy with ustekinumab. Of these, 19 patients had switched for non-complete remission (PASI≥5) and 16 for loss of efficacy. In this group, the mean age was 55.4 (SD 12.9) years, mean disease duration was 27.5 (SD 15.2) years, mean BMI was 27.9 (SD 5.2) kg/m2, and baseline PASI was 12.6 (SD 11.3). At week 100, PASI 75, 90, and 100 were seen in 95.7%, 82.6%, and 56.5% of these patients, respectively ().
3.3. Univariate and multivariate analysis
A univariate logistic regression analysis was carried out considering sex, age, age of disease onset, disease duration, weight, BMI, PASI score at baseline, number of comorbidities, number of previous biologics, last class of biologic drug used, and presence of disease in difficult-to-treat sites. The number of comorbidities at week 4 (OR 0.63, p = 0.018) resulted a negative prognostic factor in achieving PASI 75, while being bio-naïve was significantly associated with a better probability of achieving PASI 75 response at week 12 than being bio-experienced (OR 2.44, P = 0.45) ().
Table 2. Univariate logistic regression analysis of variables associated with PASI 75 response.
A large number of variables were associated with achieving PASI 90 response (). Of note, anti-IL-12/IL-23 last therapy before guselkumab was significantly associated with a reduced ability of achieving PASI 90 at both 76 (OR 0.19, p = 0.010) and 124 (OR 0.18, p = 0.033) weeks. Achieving PASI 100 was also associated with a large number of variables (). Notably, the number of previous biologic therapies was a significantly negative prognostic factor for achieving PASI 100 at 28 (p = 0.019), 52 (p = 0.008), 76 (p = 0.004), 100 (p = 0.013), and 124 (p = 0.005) weeks.
Table 3. Univariate logistic regression analysis of variables associated with PASI 90 response.
Table 4. Univariate logistic regression analysis of variables associated with PASI 100 response.
In multivariate logistic regression analysis of predictors of PASI 75, PASI 90 and PASI 100 response after treatment with guselkumab, the number of comorbidities remained significant for the achievement of PASI 75 at weeks 4 and 12. Male sex was significantly associated with a better probability of achieving PASI 90 at week 28 (p = 0.037) and for PASI 100 at week 100 (p = 0.008). In addition, anti-IL-12/IL-23 as the last therapy before guselkumab was significantly associated with a reduced ability of achieving PASI 75 at week 28 (OR 0.13, p = 0.017) and PASI 90 at week 76 (OR 0.16, p = 0.014). Moreover, anti-IL-17 as the last therapy before guselkumab significantly associated with a reduced achievement of PASI 75 at week 28 (OR 0.15, p = 0.022) and PASI 90 at week 28 (OR 0.30, p = 0.022). Lastly, the number of previous biologic therapies was significantly associated with a reduced achievement of PASI 100 at week 76 (OR 0.47, p = 0.006) ().
Table 5. Multivariate logistic regression analysis (stepwise analysis) of predictors of PASI 75, PASI 90, and PASI 100 response after treatment with guselkumab.
3.4. Drug survival
Drug survival rates are shown by Kaplan-Meier analysis (). In the entire cohort, the probability that a patient would still be on treatment after 2 years was 96% (). Four patients discontinued guselkumab during the first year of treatment, 3 patients between the first and second year, and 2 patients within the third year (see drop-outs section).
Figure 3. Drug survival rates for guselkumab monotherapy in the entire cohort (a) and grouped according to sex (b), BMI<30 or ≥30 kg/m2 (c), or prior treatment with a biologic (d).
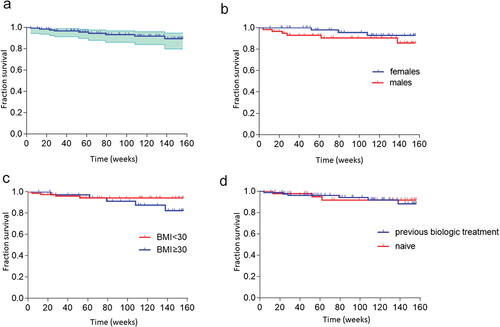
Stratification according to sex showed no significant difference in drug survival between males and females (). Differences in drug survival were not significant when comparing obese and non-obese patients (). Interestingly, no major differences were seen in drug survival rates between bio-naïve and bio-experienced patients ().
3.5. Dropouts
Two patients were lost to follow-up during COVID-19 lockdown (being in another region where they continued to be followed). Three male patients discontinued guselkumab for loss of secondary effectiveness at weeks 52, 79, and 108. Lastly, one female patient discontinued guselkumab at week 28 due to a diagnosis of lymphoma not correlated to the study drug. No safety concerns were reported during the study.
4. Case studies of guselkumab use in two patient categories
4.1. Patients naïve to biologic therapies
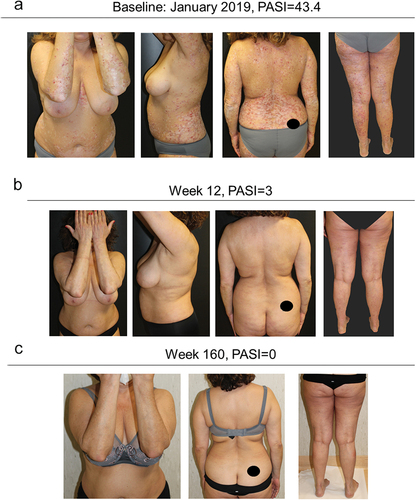
A 56-year-old woman, smoker and with dyslipidemia, affected by chronic plaque psoriasis from the age of 47, presented in January 2019 with severe psoriasis and arthralgia in the small joints of the hands. Evaluation of PASI and VAS score were 43.4 and 80, respectively. The patient had previously been treated with cyclosporine from 2014 until December 2018, with loss of response.
In January 2019, therapy with guselkumab was started. An excellent response was obtained in the first 12 weeks of treatment, both in skin and joints. The patient is currently on 4 years of continuous treatment with guselkumab, and she is still in complete remission from both skin and articular disease (both PASI and VAS equal to 0) ().
4.2. Patients with multi-drug resistance, comorbidities, and BMI≥30
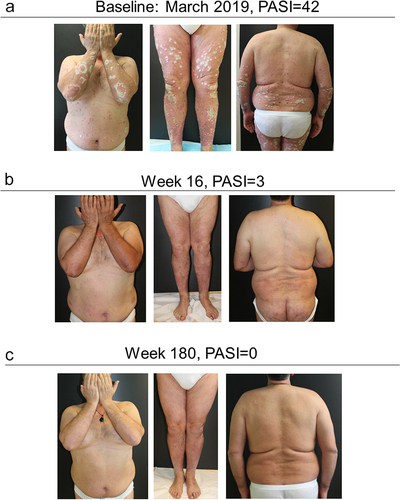
A 55-year-old male, smoker, obese (BMI = 35.3), with diverticulitis and arterial hypertension was referred to our center in 2007. The patient reported a long history of psoriatic disease from the age of 24, chronic plaque psoriasis from the age of 45, and he was cyclically in therapy from 2001 to 2007 with acitretin and PUVA therapy. In 2007, the patient started cyclosporine, discontinued due to adverse events. In the same year, infliximab was administered, and then discontinued in 2010 due to loss of efficacy. The combination of infliximab with methotrexate showed no benefit.
From 2010 to 2012 the patient was administered several cycles of corticosteroid therapy. In 2012, etanercept was started until discontinuation in 2014 due to loss of efficacy.
In 2015 secukinumab showed an optimal response over the first 24 weeks of treatment, allowing complete disease remission. After 52 weeks, a progressive disease reappearance led to therapy discontinuation after 160 weeks, with a PASI score of 42.
In March 2019 therapy with guselkumab was started. Patient response was excellent from the first 16 weeks of treatment, and subsequent complete disease remission, which is ongoing after almost four years of continuous treatment ().
5. Discussion
Guselkumab was the first IL-23 inhibitor approved by the EMA and FDA for moderate-to-severe plaque psoriasis, and its efficacy and safety have been documented in both randomized clinical trials and real-life studies. However, real-life data on the long-term use of guselkumab in patients with psoriasis are limited [Citation8–10,Citation12,Citation14,Citation18,Citation19,Citation23]. The present study aimed to assess long-term data in patients treated with guselkumab for up to 148 weeks in a real-life setting, which included patients failing a previous biologic therapy. In this cohort of 122 patients, guselkumab treatment showed a rapid, lasting decrease in the mean PASI score that remained low throughout 148 weeks. Responses tended to be better in non-obese patients and in those who were bio-naïve, and significantly favored patients with less than three lines of prior biologic therapy; this evidence was confirmed in multivariate analysis. Good responses were also observed in the subgroup of patients who had failed previous therapy with ustekinumab. The rate of drop-outs due to loss of effectiveness was also low.
In a long-term extension of the pivotal trials, 82.8% in VOYAGE 1 and 77.2% in VOYAGE 2 achieved PASI 90 at week 156, while 50.8% and 48.4%, respectively, achieved PASI 100 [Citation24]. In our analysis, after 148 weeks of guselkumab, PASI 90 and PASI 100 were reached by 82.9% and 63.4% of patients, which is broadly similar to the results reported from the pivotal trials. In the real-life trial by Megna et al., 77.4% and 58.1% of patients, respectively, achieved PASI 90 and PASI 100 at 144 weeks [Citation18]. A number of real-life studies with shorter follow-up times have reported similar rates of achieving PASI 90 and PASI 100, with rapid reductions in mean PASI score as confirmed herein [Citation16,Citation17,Citation19,Citation23,Citation25]. An analysis of data from an extension of VOYAGE 2 has documented that response is maintained for up to 5 years [Citation13].
While good responses were observed in both obese and non-obese patients, they tended to be better in the latter subgroup. Bardazzi et al. reported that reduction in PASI with guselkumab does not seem to be significantly influenced by the presence of ≥ 3 comorbidities, BMI>30 kg/m2, or a previous failure to ≥ 2 biologic therapies [Citation19]. In our analysis, the differences between patients with BMI≥30 kg/m2 vs BMI<30 kg/m2 were particularly noticeable when considering PASI 100, with non-obese patients easily achieving completely clear skin (at 76 and 124 weeks BMI<30 kg/m2 was associated with an OR of 3.13 and 3.70 for PASI 100 respectively). In addition, the number of previous biologic therapies was significantly associated with a worse prognosis in achieving PASI 90 and PASI 100 at different time points. Gargiulo et al., reported similar effectiveness in obese and non-obese patients with psoriasis in terms of PASI 75, 90, and 100 at week 104 [Citation20]. Although the values for non-obese patients were slightly lower than what we observed at week 100, the patients had already been subjected to prior treatment with biologic therapy (mainly ustekinumab, and in 40% of cases to more than one biologic), and this could have influenced the final outcome. In line with our results, Lytvyn et al. also reported that drug survival was higher for bio-naïve patients and lower for patients who had previously failed at least 2 biologics [Citation26].
In the 35 patients who failed ustekinumab in our study, PASI 90 and 100 responses were seen in 82.6% and 56.5% of patients at week 100, similar to the entire cohort, even if at some time points a prior therapy with an anti-IL12/23 inhibitor resulted to be a negative prognostic factor in achieving PASI response. In the 52-week real-life analysis in 13 patients by Ruggiero et al., no significant differences were found between patients who had previously received an anti-IL-12/23 agent compared with an anti-IL-17 (either secukinumab, ixekizumab, or both) [Citation25]. Gargiulo et al., in a multicenter study including patients previously exposed to biologics, showed that 74% and 60% patients reached PASI 90 and 100 at 104 weeks, respectively [Citation20], similar to our results.
In addition to the good long-term effectiveness, this analysis also reports a high rate of drug survival of guselkumab, with more than 90% of patients still on treatment after 2 years. Discontinuation rates did not appear to be influenced by gender differences, previous treatment with biologics, or comorbidities such obesity. These data confirm guselkumab as a therapy associated with the highest survival, as reported previously in short-term studies (91.7% at around 3 months) [Citation27], in longer studies with larger cohorts (90.2% at 2 years) [Citation28], and in a multi-centric observational study including centers in Europe, U.S.A, and Canada (80.0% at 2 years) [Citation29]. Nevertheless, the study by Torres et al. highlighted a higher discontinuation rate among subgroups such as female and obese patients [Citation29], while the present analysis does not seem to support this difference, with only a trend of higher discontinuation identified in these specific populations. These results are likely due to the favorable safety of the drug, as shown in the VOYAGE-1 and −2 trials [Citation30].
This study has some limitations, mainly due to its retrospective design and to the inclusion of patients treated at one center. Nevertheless, the detailed characterization of the patients and the long-term treatment outcomes reported, in line with the main findings of efficacy in randomized clinical trials and other long-term real-life analyses, provide reliable support for the evaluation of treatment of moderate-to-severe psoriatic patients in clinical practice.
6. Conclusion
The effectiveness of guselkumab is confirmed in our real-life experience, with a rapid improvement of mean PASI score and good long-term results in different subgroups of patients. Treatment showed excellent results in achieving PASI 100 in non-obese patients and in those naïve to biologic therapy. Overall, drug survival was good and was not significantly affected by differences in gender, BMI, or previous biologic therapies.
Declaration of interest
M Galluzzo and M Talamonti declare to have acted as speakers and/or consultants for AbbVie, Almirall, Eli-Lilly, Janssen-Cilag, LeoPharma, Novartis and Sanofi, outside the submitted work. L Bianchi declares to have acted as a speaker and consultant for AbbVie, Novartis, Janssen-Cilag, Pfizer, UCB, and LeoPharma, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed consultancy work for Abbvie, Leo, Eli Lilly, Bristol Meyers Squibb and Amgen, and that they have worked as an investigator for Janssen on clinical trials with guselkumab. A reviewer on this manuscript has disclosed research, speaking and/or consulting support from Eli Lilly and Company, GlaxoSmithKline/Stiefel, AbbVie, Janssen, Alovtech, vTv Therapeutics, Bristol-Myers Squibb, Samsung, Pfizer, Boehringer Ingelheim, Amgen, Dermavant, Arcutis, Novartis, Novan, UCB, Helsinn, Sun Pharma, Almirall, Galderma, Leo Pharma, Mylan, Celgene, Ortho Dermatology, Menlo, Merck & Co, Qurient, Forte, Arena, Biocon, Accordant, Argenx, Sanofi, Regeneron, the National Biological Corporation, Caremark, Teladoc, Bristol Meyers Squibb, Ono, Micreos, Eurofins, Informa, UpToDate and the National Psoriasis Foundation. They are a part owner of Causa Research and hold stock in Sensal Health. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Ethics statement
The authors state that all patients gave written informed consent to participate in the study. The study was conducted in accordance with the 1975 Declaration of Helsinki ethical standards. According to Italian law, formal ethical committee approval is not required for this type of study. Consent to publish was obtained from the individuals featured in .
Author contributions
M Galluzzo and M Talamonti conceived the study. All authors collected and analyzed the data, drafted the manuscript and agree to be accountable for all aspects of the work.
Acknowledgments
Editorial assistance was provided by Dr Patrick Moore, an independent medical writer, on behalf of Health Publishing & Services Srl.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Gudjonsson JE, Elder JT. Psoriasis: epidemiology. Clin Dermatol. 2007 Nov;25(6):535–546.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014 Aug 17;20(8):13030/qt48r4w8h2.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015 Jun;72(6):961–7 e5.
- Elyoussfi S, Thomas BJ, Ciurtin C. Tailored treatment options for patients with psoriatic arthritis and psoriasis: review of established and new biologic and small molecule therapies. Rheumatol Int. 2016 May;36(5):603–612.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020 Oct 11;21(20):7488.
- Teng MW, Bowman EP, McElwee JJ, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015 Jul;21(7):719–729.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017 Mar;76(3):405–417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017 Mar;76(3):418–431.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018 Jan;178(1):114–123.
- Griffiths CEM, Papp KA, Song M, et al. Continuous treatment with guselkumab maintains clinical responses through 4 years in patients with moderate-to-severe psoriasis: results from VOYAGE 1. J DermatolTreat. 2022 Jul 13;33(2):848–856.
- Reich K, Armstrong AW, Foley P, et al. Maintenance of response through up to 4 years of continuous guselkumab treatment of psoriasis in the VOYAGE 2 phase 3 study. Am J Clin Dermatol. 2020 Dec;21(6):881–890.
- Reich K, Gordon KB, Strober BE, et al. Five-year maintenance of clinical response and health-related quality of life improvements in patients with moderate-to-severe psoriasis treated with guselkumab: results from VOYAGE 1 and VOYAGE 2. Br J Dermatol. 2021 Dec;185(6):1146–1159.
- Blauvelt A, Tsai TF, Langley RG, et al. Consistent safety profile with up to 5 years of continuous treatment with guselkumab: pooled analyses from the phase 3 VOYAGE 1 and VOYAGE 2 trials of patients with moderate-to-severe psoriasis. J Am Acad Dermatol. 2022 Nov 17;86(4):827–834.
- Galluzzo M, Talamonti M, Bernardini N, et al. Real-world outcomes in patients with moderate-to-severe plaque psoriasis treated with guselkumab for up to 1 year. Expert Opin Biol Ther. 2022 Jun 22;22(12):1585–1592.
- Galluzzo M, Tofani L, Lombardo P, et al. Use of guselkumab for the treatment of moderate-to-severe plaque psoriasis: a 1 year real-life study. J Clin Med. 2020 Jul 9;9(7):2170.
- Megna M, Potestio L, Ruggiero A, et al. Guselkumab is efficacious and safe in psoriasis patients who failed anti-IL17: a 52-week real-life study. J DermatolTreat. 2022 Feb 7;33(5):2560–2564.
- Megna M, Potestio L, Fabbrocini G, et al. Long-term efficacy and safety of guselkumab for moderate to severe psoriasis: a 3-year real-life retrospective study. Psoriasis (Auckl). 2022;12:205–212.
- Bardazzi F, Viviani F, Merli Y, et al. Guselkumab for the treatment of psoriasis: a 60-week real-life multicenter retrospective experience. Expert Opin Biol Ther. 2022 Apr 13;22(12):1561–1566.
- Gargiulo L, Ibba L, Malagoli P, et al. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: a 104-week multicenter retrospective study - IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol. 2023 Jan 25. DOI:10.1111/jdv.18913.
- European Medicines Agency. Guselkumab, summary of product characteristics. [3 Mar 2023]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/tremfya
- Ministero della Salute. Attività dei Comitati Etici istituiti ai sensi del decreto ministeriale 18 marzo 1998. 2002. [cited 2023 3 Mar]. Available from: https://www.aifa.gov.it/documents/20142/0/Cir_Min_2_Settembre_2002_CE.pdf
- Megna M, Fabbrocini G, Cinelli E, et al. Guselkumab in moderate to severe psoriasis in routine clinical care: an Italian 44-week real-life experience. J DermatolTreat. 2022 Mar;33(2):1074–1078.
- Reich K, Griffiths CEM, Gordon KB, et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol. 2020 Apr;82(4):936–945.
- Ruggiero A, Fabbrocini G, Cinelli E, et al. Efficacy and safety of guselkumab in psoriasis patients who failed ustekinumab and/or anti-interleukin-17 treatment: a real-life 52-week retrospective study. Dermatol Ther. 2021 Jan;34(1):e14673.
- Lytvyn Y, Zaaroura H, Mufti A, et al. Drug survival of guselkumab in patients with plaque psoriasis: a 2 year retrospective, multicenter study. JAAD Int. 2021 Sep;4:49–51.
- Lee EB, Reynolds KA, Pithadia DJ, et al. Drug survival of guselkumab for psoriasis in a real-world setting: a single-center retrospective chart review. J DermatolTreat. 2020 Jun;31(4):342–343.
- Yiu ZZN, Becher G, Kirby B, et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022 Oct 1;158(10):1131–1141.
- Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021 Jul;22(4):567–579.
- Reich K, Papp KA, Armstrong AW, et al. Safety of guselkumab in patients with moderate-to-severe psoriasis treated through 100 weeks: a pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol. 2019 May;180(5):1039–1049.