1. Introduction
Atherosclerosis is the most common underlying cause of coronary heart disease and cerebrovascular disease and responsible for 17.5 million deaths annually [Citation1]. An early localized build-up of cholesterol-rich particles in the subendothelial intimal layer of large elastic and muscular arteries, at ostia, bifurcations, and bends initiates atherosclerosis and an early inflammatory response. Monocytes enter the intima to clear accumulated cholesterol and differentiate into macrophages and monocyte-derived dendritic cells; both contribute to foam cell formation. Macrophages frequently take on an inflammatory phenotype whilst dendritic cells contribute to regulating atherosclerotic T and B cell responses. In humans, with time, frequently decades these lesions develop into large atherosclerotic plaques, either stable or vulnerable plaques. Stable plaques that frequently encroach on the vessel’s lumen restricting blood flow are characterized by a thick fibrous cap consisting of collagen and vascular smooth muscle cells covering a necrotic core, a variety of cell types including small numbers of immune cells, most frequently lipid-laden macrophage-derived foam cells. Vulnerable lesions that are highly susceptible to rupture causing heart attacks and strokes possess thin fibrous caps with reduced collagen and vascular smooth muscle cell numbers covering a large necrotic core and a large population of pro-inflammatory immune cells [Citation2].
Cholesterol-lowering ‘statin’ therapy has greatly reduced the incidence of myocardial infarctions (MIs) and strokes. More recently targeting inflammation, specifically neutralizing interleukin-1β (IL-1β) with antibodies has been shown to have additional beneficial effects with further reductions in MIs, strokes, and cardiovascular death [Citation3]; IL-1 β is produced by inflammasomes, mostly NLRP3 inflammasomes within atherosclerotic plaques. Despite such therapies, MIs and strokes remain the major causes of death and morbidity [Citation4] and additional anti-inflammatory therapies targeting either cytokines or immune cells should further improve outcomes.
There is now a large body of evidence demonstrating the involvement of monocytes and lymphocytes in the pathogenesis of atherosclerosis; inflammatory lymphocytes accelerate development and progression of atherosclerosis as well as contribute to lesion inflammation and vulnerable plaque development. We and others have shown that both B and T cells are involved [Citation5–Citation8]. B2 B cells promote the development/progression of atherosclerosis in hyperlipidemic mice by secreting inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and pathogenic antibodies [Citation9,Citation10]. In contrast, B1a cells protect against atherosclerosis by reducing lesion apoptosis and necrosis via their low-affinity natural IgM antibodies that clear oxidized LDLs in atherosclerotic lesions [Citation11]. Depletion of B cells using anti-CD20 antibodies attenuated atherosclerosis [Citation5,Citation6], effects that could be mimicked by preventing B cells secreting pathogenic antibodies [Citation10]. Plasma cells, terminally differentiated follicular B2 cells after interacting with BAFF-responsive follicular T helper cells secrete IgG antibodies [Citation12]. Transfer of IgGs isolated from atherosclerotic hyperlipidemic mice into chimeric mice with a deficiency in plasma cell differentiation promotes vulnerable plaque development by greatly increasing cell death and necrotic core size [Citation10].
Such studies raise the possibility that targeting the cytokine BAFF (B-cell activating factor), which is required for B cell proliferation, differentiation and survival may be an appropriate therapeutic strategy to attenuate the development of vulnerable atherosclerotic plaque development and thereby further reducing the incidence of MIs and strokes. Indeed, the risk of death or recurrent heart attacks is directly related to the plasma level of BAFF determined at admission in patients with MI [Citation13], supporting the need for targeting BAFF to prevent MIs and strokes.
2. BAFF, BAFF receptors, and atherosclerosis
BAFF is a type II membrane-bound protein produced by many cell types including B cells, macrophages, and dendritic cells which can be released from membranes via proteolytic cleavage generating soluble BAFF (sBAFF). sBAFF exists as trimers (3-mers) or multimers (60-mers). Cytokines including type 1 interferons (IFNs), IFN-γ as well as Toll-like stimulation increase BAFF expression. Membrane BAFF stimulates BAFF receptors whilst sBAFF binds and activates three receptors (BAFF receptors, TACI, and BMA) [Citation14]. BAFF receptors (BAFFRs) are mostly expressed on peripheral B cells and activated/memory T cells whilst TACI is largely expressed by transitional type 2 precursor B cells, activated B cells and marginal zone B cells and BCMA by germinal center B cells () [Citation12]. Studies directly evaluating the role of BAFF in atherosclerosis have been complicated by abnormally high increases in body weight in BAFF knockout mice [Citation15]. As a consequence, effects of BAFF receptor deficiency/blockade on atherosclerosis have been studied. Genetic deletion of BAFFRs reduces the development of atherosclerosis and is associated with large reductions in B2 but not atheroprotective IgM-producing B1a cells [Citation7,Citation11]. Plaque T cells are also reduced as are IgG deposits; plaque cytokines TNF-α, IL-1β, and IFN-γ are also reduced, effects consistent with BAFF influencing peripheral B and T cells, particularly follicular CD4 + T cell proatherogenic effects increasing proinflammatory cytokines and pathogenic pro-atherosclerotic IgG levels [Citation10]. Essentially similar effects were observed using BAFFR inhibitory antibodies [Citation16]. In addition, anti-BAFFR antibody treatment attenuated the progression of established atherosclerosis indicating that targeting the BAFF–BAFFR axis may be an effective therapeutic strategy to prevent the progression of atherosclerotic lesions toward vulnerable plaques in humans. More recently BAFF has also been targeted by anti-BAFF antibodies [Citation17], and whilst B2 cells were deleted similar to studies targeting BAFFR, atherosclerosis was increased and associated with enhanced inflammation; this latter pro-atherogenic effect appeared due to BAFF affecting monocyte/macrophages responses via TACI [Citation17], indicating a need for more refined BAFF therapeutic approaches for targeting atherosclerosis.
Figure 1. Different B-cell activating factor (BAFF) subsets differentially bind their receptors. BAFF, produced by many cells, exists either as membrane form or soluble form (soluble BAFF; sBAFF) after proteolytic cleavage. After oligomerization, sBAFF is either a 3-mer or 60-mer. BAFF can interact with BAFF receptor (BAFFR), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) or B-cell maturation antigen (BCMA); however, 3-mer BAFF binds preferentially to BAFFR whilst TACI and 60-mer BAFF binds predominately to TACI and BCMA. Membrane-bound BAFF seems to have preferential binding to BCMA compared to BAFFR and TACI.
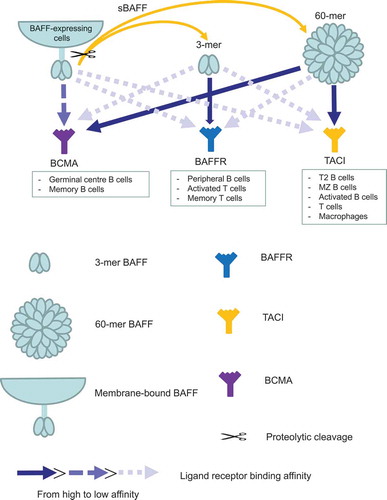
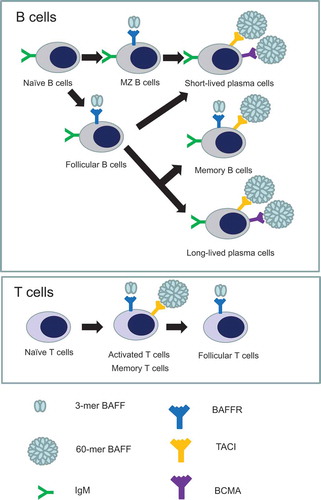
Figure 2. B and T cells considered to be required for atherosclerosis express B-cell activating factor receptors (BAFFRs) differently during their development and activation. On B cell development, 3-mer BAFF and BAFFR interaction is critically required for naïve B cells to develop into marginal zone B cells and follicular B cells. After differentiation into immunoglobulin-secreting plasma cells, both short-lived and long-lived plasma cells require 60-mer BAFF that interacts with transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and B-cell maturation antigen (BCMA) for their maintenance and survival. Memory B cells express both BAFFR and TACI that bind to 3-mer and 60-mer, respectively. Upon exposure to antigens, T cells are activated and develop into memory T cells. Whilst both activated and memory T cells express BAFFR that interacts with 3-mer BAFF and TACI that interacts with 60-mer BAFF, follicular T cells seem to have BAFFR expression.
Only BAFFR but not TACI responds to soluble 3-mer BAFF whilst soluble 60-merBAFF activates TACI () [Citation18]. The above effects on BAFF inhibition and atherosclerosis can be explained by the BAFF inhibitory antibody neutralizing both soluble 3-mer and 60-mer BAFF and possibly membrane-BAFF, as effects are similar to genetic deletion of BAFF. It should be possible to produce inhibitory monoclonal antibodies that selectively target only one or two BAFF forms and gain greater selectivity for the different forms of BAFF to treat atherosclerosis. The BAFF inhibitory antibody belimumab which is approved for the treatment of systemic lupus erythematosus possesses some selectivity; it is a weak inhibitor of 60-mer BAFF compared to potently inhibiting 3-mer BAFF and differs with respect to specificity from other inhibitory antibodies such as tabalumab () [Citation18]. Targeting atherosclerosis with inhibitory antibodies with appropriate selectivity should result in effects seen by targeting BAFFR with inhibiting antibodies. To date targeting BAFFR to inhibit atherosclerosis has only been achieved in mice and needs to be confirmed in human atherosclerosis as does the efficacy of targeting the different forms of BAFF.
3. BAFF and accelerated atherosclerosis associated with autoimmune and arthritic disorders
BAFF has been proposed as a therapeutic target for autoimmune disorders such as rheumatoid arthritis, lupus, and psoriasis. These disorders are associated with significant increases in adverse cardiovascular events compared to the general population due to atherosclerosis; affected patients are at much greater risk of cardiovascular death mostly due to ischemic heart disease [Citation19].
Development/progression of atherosclerosis is accelerated in these diseases and whilst underlying mechanisms are still unclear, systemic inflammation appears to be particularly important. BAFF is highly pro-inflammatory and elevated in patients with psoriasis and lupus and also implicated in rheumatoid arthritis [Citation20–Citation22]. Despite comparable plasma LDL levels, lupus patients with high plasma BAFF levels showed an evidence of subclinical atherosclerosis established at carotid and femoral arteries compared to those with low plasma BAFF levels. This association was observed following B cell depletion therapy (rituximab), suggesting a risk of global B cell depletion strategy in patients with autoimmune diseases [Citation23]. Taken together with increased risk of death and recurrent attacks in MI patients with increased BAFF levels during MI [Citation13] and atherogenic role of BAFF [Citation17], BAFF-selective therapeutic strategies are more beneficial than global B cell depletion therapy. Whilst BAFF modulates follicular B cells and follicular T helper cells in selection of autoreactive B cells and production of autoantibodies [Citation12], BAFF can also bind to BAFFR, TACA, and BMCA to activate NF-κB signaling pathway that is responsible for production of pro-inflammatory cytokines such as IL1-β, TNF-α, IL-2, and IL-6 [Citation24]. Targeting BAFF in such conditions may not only reduce the severity of such disorders but may also attenuate the development/progression of atherosclerosis.
Success of targeting BAFF, however, appears variable and dependent on the characteristics of the different therapies. The BAFF inhibitory antibody belimumab is approved for treating Lupus but a related inhibitory antibody tabalumab appears ineffective [Citation21]. Briobacept, a protein that also neutralizes BAFF and is composed of IgG and part of the BAFFR (i.e. decoy receptor), appears also ineffective; other agents, e.g. blisibimod, are still under evaluation [Citation21]. Therapeutic efficacy in these instances is very likely dependent on which forms of BAFF are being targeted. Also, current trials on efficacy in, for example, lupus are relatively short term (12 months treatment) and whilst sufficient to assess beneficial effects on lupus, possibly longer 5-year trials will be required to assess effects on accelerated atherosclerosis. Similar studies will also need to be performed for agents targeting different BAFF forms in psoriasis and rheumatoid arthritis. B cells also promote inflammation in obesity and type 2 diabetes and it is possible that BAFF is also an important contributor influencing accelerated atherosclerosis in type 2 diabetes [Citation25]. In the future, it will be important to determine which BAFF forms are best targeted to attenuate accelerated atherosclerosis associated with these inflammatory conditions and whether they also reduce the severity of associated inflammatory disorders.
4. Expert opinion
As a major finding, it is clear that BAFF is indispensable for B cell maturation and has an important role in enhancing systemic inflammation and immune responses, specifically B and T cell responses (). Studies in hyperlipidemic atherosclerotic mice indicate a significant role for BAFF in the development of atherosclerotic lesions, in particular, vulnerable lesions that are prone to rupture causing heart attacks and strokes. BAFF can be considered as a potential therapeutic target to prevent atherosclerosis either by targeting the BAFFR or BAFF itself. Experimental studies indicate that targeting BAFFR is one strategy to inhibit the pro-atherogenic effects of BAFF. Recently a new humanized defucosylated engineered antibody against BAFFR has been developed [Citation26], which is undergoing early clinical trials in patients with autoimmune disorders such as rheumatoid arthritis and also has potential to prevent atherosclerosis in humans, based on preclinical studies [Citation16]. Targeting BAFFR offers a two-pronged approach to attenuate atherosclerosis, attenuating both T cell activation and impairing B cell survival. Directly targeting BAFF is more complex than targeting BAFFR because of the different forms of BAFF, membrane, soluble 3-mer, and 60-mer BAFF which may exert different overall effects depending on receptors with which they interact.
Future research needs to address the major weakness and develop specific therapies, for example, monoclonal antibodies which specifically target only the sBAFF 3-mer or sBAFF 60-mer or membrane-BAFF. This is critical for atherosclerosis prevention where neutralization/inhibition of sBAFF 3-mer prevents BAFF receptor activation and neutralization/inhibition of sBAFF 60-mer prevents TACI actions which results in increased atherosclerosis [Citation17], limiting the usefulness of current BAFF therapies for treating autoimmune diseases associated with accelerated atherosclerosis.
To date, selective inhibition of these different forms has not been achieved although the clinically used anti-BAFF antibody belimumab is more selective in preventing the actions of BAFF 3-mer than BAFF 60-mer. The importance of such selectivity needs to be evaluated with respect to effects on atherosclerosis development/prevention and more specific agents developed if necessary. Agents with no specificity are clearly not suitable for atherosclerosis therapy given that BAFF deletion in hyperlipidemic atherosclerotic mice augments rather than prevents atherosclerosis [Citation16]. Understanding BAFF structure and how inhibitory antibodies interact with the different BAFFs may help design more specific therapies that target specific BAFFs. BAFF contains a loop region designated the ‘flap’ region [Citation27] which is important for signaling and facilitates the formation of BAFF 60-mer [Citation28]. The ‘flap’ of BAFF 60-mer prevents binding of the BAFF inhibitory antibody belimumab making it more specific in inhibiting BAFF 3-mer [Citation28]. BAFF knockout mice showed an increase in body weight; however, it is subcutaneous, not epididymal nor visceral, fat increase that is attributed to increased body weight and BAFF seems to modulate systemic inflammation, suggesting that targeting BAFF–BAFFR interaction has a beneficial effect in reducing systemic inflammation, particularly in cardiovascular diseases [Citation15]. Collectively, targeting BAFF 3-mer instead of targeting either BAFF 60-mer or both is expected to effectively eliminate, or at least decelerate, lesion inflammation mediated via BAFF–BAFFR interaction in patients with atherosclerosis.
Taken together, evidence indicates that BAFF is an appropriate therapeutic target to prevent MIs and strokes associated with rupture of vulnerable atherosclerotic plaques in hyperlipidemic patients and those with autoimmune disorders such as rheumatoid arthritis. In a few years’ time, we anticipate that more specific BAFF therapies should become available, designed specifically to target the different BAFF structures – sBAFF 3-mer, sBAFF 60-mer, and membrane-BAFF – and their abilities to interact with three receptors BAFFR, TACI, and BCMA.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Acknowledgments
The authors apologize for not citing several relevant publications in the literature due to space limitations.
Additional information
Funding
References
- World Health Orgaization. Cardiovascular diseases (CVDs) [internet]. cited 2017 May 17. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Stefanadis C, Antoniou CK, Tsiachris D, et al. Coronary atherosclerotic vulnerable plaque: current perspectives. J Am Heart Assoc. 2017;6:3.
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131.
- Australian Institute of Health and Welfare. Deaths in Australia. cited 2019 Jul 17. https://www.aihw.gov.au/reports/life-expectancy-death/deaths-in-australia/contents/age-at-death
- Kyaw T, Tay C, Khan A, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185(7):4410–4419.
- Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207(8):1579–1587.
- Kyaw T, Tay C, Hosseini H, et al. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS One. 2012;7(1):e29371.
- Sage AP, Murphy D, Maffia P, et al. MHC Class II-restricted antigen presentation by plasmacytoid dendritic cells drives proatherogenic T cell immunity. Circulation. 2014;130(16):1363–1373.
- Tay C, Liu YH, Hosseini H, et al. B-cell-specific depletion of tumour necrosis factor alpha inhibits atherosclerosis development and plaque vulnerability to rupture by reducing cell death and inflammation. Cardiovasc Res. 2016;111(4):385–397.
- Tay C, Liu Y-H, Kanellakis P, et al. Follicular B cells promote atherosclerosis via T cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler Thromb Vasc Biol. 2018;38(5):e71–e84.
- Kyaw T, Tay C, Krishnamurthi S, et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109(8):830–840.
- Chen M, Lin X, Liu Y, et al. The function of BAFF on T helper cells in autoimmunity. Cytokine Growth Factor Rev. 2014;25(3):301–305.
- Zouggari Y, Ait-Oufella H, Bonnin P, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19(10):1273–1280.
- Thompson N, Isenberg DA, Jury EC, et al. Exploring BAFF: its expression, receptors and contribution to the immunopathogenesis of Sjogren’s syndrome. Rheumatology (Oxford). 2016;55(9):1548–1555.
- Kim DH, Do MS. BAFF knockout improves systemic inflammation via regulating adipose tissue distribution in high-fat diet-induced obesity. Exp Mol Med. 2015;47:e129.
- Kyaw T, Cui P, Tay C, et al. BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(-/-) mice. PLoS One. 2013;8(4):e60430.
- Tsiantoulas D, Sage AP, Goderle L, et al. B cell-activating factor neutralization aggravates atherosclerosis. Circulation. 2018;138(20):2263–2273.
- Nicoletti AM, Kenny CH, Khalil AM, et al. Unexpected potency differences between B-Cell-Activating Factor (BAFF) antagonist antibodies against various forms of BAFF: trimer, 60-Mer, and membrane-bound. J Pharmacol Exp Ther. 2016;359(1):37–44.
- Hong J, Maron DJ, Shirai T, et al. Accelerated atherosclerosis in patients with chronic inflammatory rheumatologic conditions. Int J Clin Rheumatol. 2015;10(5):365–381.
- Samoud-El Kissi S, Galai Y, Sghiri R, et al. BAFF is elevated in serum of patients with psoriasis: association with disease activity. Br J Dermatol. 2008;159(3):765–768.
- Nakayamada S, Tanaka Y. BAFF- and APRIL-targeted therapy in systemic autoimmune diseases. Inflamm Regen. 2016;36:6.
- Zhou B, Zhang H, Su X, et al. Therapeutic effects of a novel BAFF blocker on arthritis. Signal Transduct Target Ther. 2019;4:19.
- Theodorou E, Nezos A, Antypa E, et al. B-cell activating factor and related genetic variants in lupus related atherosclerosis. J Autoimmun. 2018;92:87–92.
- Rihacek M, Bienertova-Vasku J, Valik D, et al. B-cell activating factor as a cancer biomarker and its implications in cancer-related cachexia. Biomed Res Int. 2015;2015:792187.
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110(13):5133–5138.
- McWilliams EM, Lucas CR, Chen T, et al. Anti-BAFF-R antibody VAY-736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. 2019;3(3):447–460.
- Schuepbach-Mallepell S, Das D, Willen L, et al. Stoichiometry of heteromeric BAFF and APRIL cytokines dictates their receptor binding and signaling properties. J Biol Chem. 2015;290(26):16330–16342.
- Vigolo M, Chambers MG, Willen L, et al. A loop region of BAFF controls B cell survival and regulates recognition by different inhibitors. Nat Commun. 2018;9(1):1199.
