1. Introduction
Obesity is an emerging global health threat affecting all people irrespective of their age. It is predicted that one billion people will become obese by 2030, including 1 in 5 women and 1 in 7 men [Citation1]. Obesity stems from the imbalance in energy homeostasis as a result of overnutrition and sedentary lifestyle, subsequently leading to life-threatening conditions, such as metabolic dysfunctions associated with Fatty Liver Disease, insulin resistance, Type 2 Diabetes, and cancer development. There are numerous nutrition programs, weight-loss meal plans and exercise paradigms prescribed by physicians for the continuous management of obesity. However, the efficacy of exercise and dietary changes in the obesity management is hampered by issues of patient compliance. The most effective way to tackle obesity is to reduce energy intake through the regulation of food consumption, but people living with obesity often encounter difficulties in controlling their appetites. Therefore, identifying a host factor that specifically controls body weight through regulation of satiety is crucial for the development of therapeutic approaches for obesity.
1.1. Pathological role of MT1-MMP in obesity
Matrix metalloproteinases (MMPs), a family of zinc-dependent proteinases, are indispensable for diverse biological processes, such as tissue repair and remodeling, by extracellular matrix remodeling and pericellular proteolysis. Among all reported MMPs knockout mouse models, only deficiency in MT1-MMP, also known as MMP14, leads to severe spontaneous phenotypes [Citation2], highlighting its diverse roles in both physiological and pathological conditions. Recent studies showed that MT1-MMP is critical for the postnatal development of white adipose tissues [Citation3]. Furthermore, mutations in MMP14 have been associated with human obesity and diabetic traits [Citation4]. Despite the genetic association between MMP14 gene polymorphisms and obesity in humans, the molecular mechanism underlying MT1-MMP-mediated regulation of body weight remains unknown. Our recent study reveals that MT1-MMP regulates the mechanism of issuing satiety signals through controlling the actions of Growth and differentiation factor 15 (GDF15), an appetite-regulatory hormone for the non-homeostatic regulation of body weight via the brain-stem-restricted receptor namely GDNF family receptor-α-like (GFRAL). In mice with diet-induced obesity, we demonstrated that the increased activation of neuronal MT1-MMP reduced the sense of satiety and increased desire to consume high-calorie food [Citation5], which is in alignment with the clinical study showing genetic variants in human MT1-MMP gene have been positively correlated with obesity traits in Japanese population [Citation4]. To further understand the pathological role of MT1-MMP in obesity, we studied the response of mouse model with hemizygous depletion of MT1-MMP to the high-fat-diet challenge. Interestingly, in standard diet, Mmp14± mice were indistinguishable from the wild-type littermate controls in terms of body weight and food intake capacity [Citation5]. However, when Mmp14± mice and their wild-type littermates were fed with high fat diet, Mmp14± mice ate significantly less than wild-type littermates did, resulting in reduced weight gain and improved metabolic parameters in Mmp14± mice. These results suggest that MT1-MMP plays an essential role in the non-homeostatic regulation of body weight through the control of food intake. Mesenchymal MT1-MMP has been found to control adipogenesis through promoting collagen degradation [Citation3,Citation4]. However, the changes in appetite observed in high-fat-diet challenged Mmp14± mice is unlikely the consequence of inadequate collagen remodeling, suggesting that mechanism apart from collagen degradation contributes to MT1-MMP-dependent regulation of body weight.
2. Non-homeostatic regulation of body weight by the MT1-MMP/GDF15/GFRAL axis
GDF15 has been implicated in multiple disorders of metabolism, such as obesity, insulin resistance, and cancer cachexia [Citation6]. Loss of GDF15 leads to increased body weight, whereas overexpression of GDF15 results in reduced food intake and lower body weight accompanied with improved metabolic parameters in mice with diet-induced obesity [Citation6]. Subcutaneous administration of recombinant GDF15 induces weight loss in both mice with diet-induced obesity and cynomolgus monkeys with spontaneous obesity [Citation6–10]. GFRAL has recently been identified as a potent receptor for GDF15 and is specifically expressed in a subset of neurons in both the area postrema and the nucleus of the solitary tract, regions of the hindbrain responsible for the regulation of energy intake [Citation7–10]. GDF15 binds to and activates GFRAL, thereby controlling a vagus-mediated neuronal circuit for satiety control [Citation7–10]. Loss of GFRAL abrogates the GDF-15-induced reduction in food intake and body weight in mice with diet-induced obesity [Citation7–10], revealing the essentiality of GFRAL for GDF15 appetite-regulatory functions. In addition, it was also shown that the anorexigenic effects of GDF15 are mediated through a direct effect on hypothalamic arcuate nucleus neurons [Citation6], suggesting that diverse receptors in addition to brainstem-restricted GFRAL may be involved in GDF15-regulated food intake. Considering the functional similarity between MT1-MMP and GDF15/GFRAL signaling, we investigated the potential interplay between MT1-MMP and GDF15/GFRAL axis in the regulation of body weight. By challenging Mmp14± mice and their wild-type littermates with subcutaneous doses of recombinant GDF15, we demonstrated that loss of MT1-MMP enhanced GDF15-induced suppression of food intake and body weight, indicating that MT1-MMP regulates body weight through suppressing GDF15 signaling [Citation5]. To further confirm the regulatory role of MT1-MMP in GDF15/GFRAL axis, we generated two transgenic mouse lines: a mouse model with compound loss of MT1-MMP and GFRAL and a mouse model of targeted deletion of MT1-MMP in GFRAL-expressing neurons (Mmp14f/f Gfralcre+). We demonstrated that genetic ablation of GFRAL abolished the anti-obesity effects conferred by MT1-MMP deficiency in mice, showing the essentiality of GFRAL for the body weight-regulatory function of MT1-MMP [Citation5]. In addition, we found that Mmp14f/f Gfralcre+ mice recapitulated the weight-losing phenotype resulted from global deletion of MT1-MMP [Citation5], confirming the importance of neuronal MT1-MMP in the regulation of energy hemostasis via GDF15/GFRAL axis.
2.1. MT1-MMP as a therapeutic target for obesity management
To uncover the mechanism by which MT1-MMP suppresses GDF15/GFRAL satiety signaling, we demonstrated that MT1-MMP is a novel endogenous regulator of GFRAL expression. Loss of MT1-MMP not only increase the protein expression of GFRAL in brainstem neurons, but also potentiates GDF15-induced GFRAL activation in neurons [Citation5]. In contrast, our unpublished data revealed that AAV-mediated overexpression of MT1-MMP in the brainstem led to the progressive depletion of GFRAL expression (). In line with the depletion of GFRAL expression, mice with MT1-MMP overexpression were resistant or much less responsive to the treatment of GDF15. GDF15 at the pharmacological dose could induce bodyweight loss along with reduction in food intake in mice infected with control vector, but its effects were dramatically reduced in mice with AAV-MT1-MMP (). These data collectively reveal that ectopic MT1-MMP suppresses the expression of GFRAL and abolishes the action of GDF15 in vivo. Mechanistically, we demonstrated that MT1-MMP directly clips GFRAL from the surface of brain neurons, thereby blocking GDF15 from binding to GFRAL and thus reduces GDF15-mediated satiety signals () [Citation5]. With the use of a neutralizing antibody against MT1-MMP, we demonstrated that inhibition of MT1-MMP activities promoted weight loss and improved metabolic functions in various mouse models of obesity without the induction of overt adverse effects [Citation5], suggesting that pharmacological inhibition of MT1-MMP could be a viable strategy for the development of effective pharmacotherapy for the treatment of obesity.
Figure 1. MT1-MMP ectopic expression in the brainstem abolishes the GDF15 action in vivo. The mice transduced with either AAV-MMP14 or AAV-control were sacrificed at around 2 weeks post-injection for analysis. (a) Immunostaining of GFRAL (green) in the brainstem of mice transduced with either AAV-MMP14 or AAV-control. The expression of ectopic MT1-MMP was visualized by the detection of mCherry signal (red). AP region limited with the white dashed lines. Scale bar 50 μm. (b) The bodyweight, (c) the change in bodyweight (d), cumulative 6-day food intake (e) and the percentage change in food intake in response to GDF15 treatment (10 nmol/kg) for 6 days in mice transduced with either AAV-MMP14 or AAV-control. (n = 4). Data are reported as average ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001.
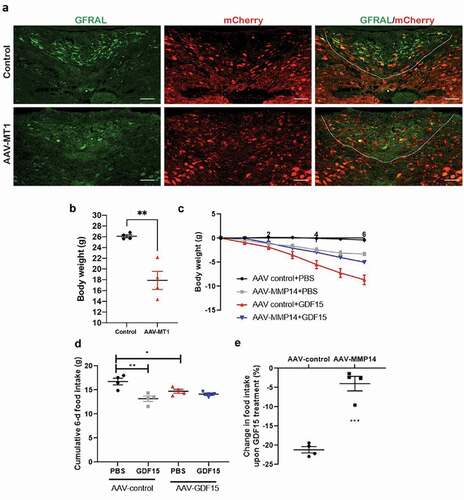
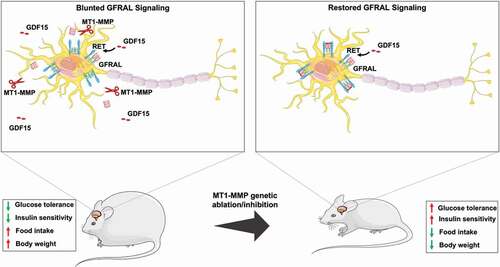
Figure 2. Diagram illustrating genetic ablation of MT1-MMP restores the GFRAL expression. Obesity induced MT1-MMP modulate protein levels of GFRAL in hindbrain neurons by proteolytic clipping it from the surface and inhibiting the GDF15 activity. Genetic ablation of MT1-MMP restores the GFRAL expression and potentiates the response to GDF15. Upregulation of GDF15-GFRAL signaling pathway prevented the diet induced obesity in mice by reducing the food intake and improving the glucose tolerance.
3. Expert opinion
It is well known that endogenous level of GDF15 is remarkably elevated during obesity and related metabolic complications. A fold change of up to 10-fold, with serum levels in the range of ~0.02–0.1 ng/ml in lean versus ~0.15–0.6 ng/ml in obese mice has been reported [Citation11]. This suggests that obesity may be a state of resistance for endogenous GDF15 at least in the physiological condition. Our findings indeed showed a potential mechanism driven by MT1-MMP underlying the obesity-associated resistance for endogenous GDF15. The expression of MT1-MMP may determine the threshold of circulating GDF15 required to induce positive effects on metabolism in the context of obesity. Our study thus identified the physiological setting in which MT1-MMP plays the role in relation to the phenomenon of GDF15 resistance, an emerging research area with important clinical implications. In addition to GDF15/GFRAL signaling axis, MT1-MMP has been shown to regulate diverse intracellular signaling pathways associated with energy metabolism, including fibroblast growth factor (FGF)/FGFRs signaling [Citation12–15]. Further investigations will be required to further delineate the molecular mechanism underlying MT1-MMP-dependent regulation of energy homeostasis.
In addition to the context of obesity, our recent study also reported that MT1-MMP regulates the mechanism by which obesity causes the development of insulin resistance [Citation16]. We demonstrated that increased activation of MT1-MMP resulted from obesity promotes the ectodomain shedding of insulin receptor in the peripheral metabolic tissues, thereby leading to insulin resistance, a major comorbid of obesity. Inhibition of MT1-MMP activities by pharmacological antagonism effectively improved insulin sensitivity and glucose tolerance in diabetic mouse models including leptin receptor-deficient db/db mice and mice with high fat diet-induced obesity. Although the differential roles of neuronal and peripheral MT1-MMP in the regulation of glucose metabolism and body weight warrant further investigations, our study nicely demonstrates that targeting MT1-MMP represents a potential therapeutic approach for the management of both obesity and diabetes, two diseases with a need for combined treatment strategies.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lobstein T, Brinsden H, Neveux M. World obesity Atlas 2022. 2022.
- Holmbeck K, Bianco P, Caterina J, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999 October 01;99(1):81–92.
- Chun TH, Hotary KB, Sabeh F, et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006 May 5;125(3):577–591.
- Chun TH, Inoue M, Morisaki H, et al. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010 October;59(10):2484–2494.
- Chow CFW, Guo X, Asthana P, et al. Body weight regulation via MT1-MMP-mediated cleavage of GFRAL. Nat Metab. 2022 February;4(2):203–212.
- Johnen H, Lin S, Kuffner T, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007 November;13(11):1333–1340.
- Mullican SE, Lin-Schmidt X, Chin CN, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017 October;23(10):1150–1157.
- Emmerson PJ, Wang F, Du Y, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017 October;23(10):1215–1219.
- Yang L, Chang CC, Sun Z, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017 October;23(10):1158–1166.
- Hsu JY, Crawley S, Chen M, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017 October 12;550(7675):255–259.
- Patel S, Alvarez-Guaita A, Melvin A, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019 March 5;29(3):707–718 e8.
- Chan KM, Wong HL, Jin G, et al. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev Cell. 2012 June 12;22(6):1176–1190.
- Tassone E, Valacca C, Mignatti P. Membrane-type 1 matrix metalloproteinase downregulates fibroblast growth factor-2 binding to the cell surface and intracellular signaling. J Cell Physiol. 2015 February;230(2):366–377.
- Sugiyama N, Varjosalo M, Meller P, et al. FGF receptor-4 (FGFR4) polymorphism acts as an activity switch of a membrane type 1 matrix metalloproteinase-FGFR4 complex. Proc Natl Acad Sci U S A. 2010 September 7;107(36):15786–15791.
- Gomez-Ambrosi J, Gallego-Escuredo JM, Catalan V, et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr. 2017 June;36(3):861–868.
- Guo X, Asthana P, Gurung S, et al. Regulation of age-associated insulin resistance by MT1-MMP-mediated cleavage of insulin receptor. Nat Commun. 2022 June 29;13(1):3749.
