1. Background
Thrombus formation is the result of the disruption of the complex homeostatic system involving platelets, coagulation factors, inflammatory elements, cytokines, and the endothelium [Citation1]. Platelets play a central role in the initiation and propagation of thrombus formation [Citation1]. After platelet adhesion – which is mediated by the interaction between the glycoprotein (GP) Ib-IX-V and von Willebrand factor, and the GPVI receptor which binds directly to collagen – multiple intracellular pathways are responsible for platelet activation [Citation1]. The released adenosine diphosphate (ADP) acts on P2Y1 and, most importantly, on P2Y12 receptors on the platelet surface [Citation1]. While P2Y1 receptor activation leads to an initial mobilization of intracellular calcium ions from internal stores resulting in platelet shape change, the P2Y12 receptor is a G protein-coupled receptor that plays a central role in the amplification of platelet activation resulting in platelet aggregation through activation of GP IIb/IIIa receptors on the platelet surface that binds fibrinogen [Citation1]. Given the central role of the P2Y12 signaling pathway in platelet activation and amplification processes leading to platelet aggregation and thrombus formation, there has been relentless interest over the past 35 years in developing compounds that selectively inhibit this pathway () [Citation2].
Figure 1. PubMed research updated as of November 7th 2023 on the number and temporal trends of the published articles involving the currently approved P2Y12 inhibitors.
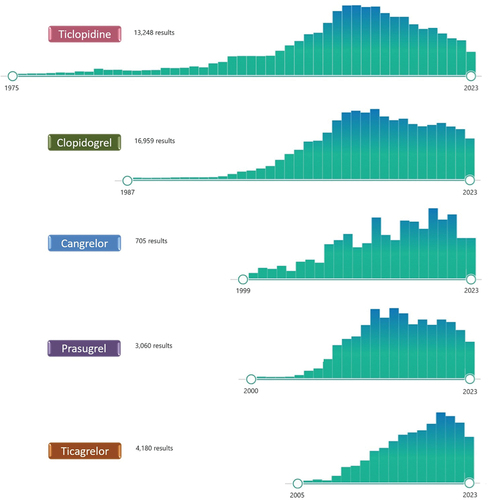
Several P2Y12 inhibitors have been developed for clinical use in patients with cardiovascular disease. The first generation thienopyridine ticlopidine, introduced in the late 1990s, was associated with effective antiplatelet effects but also with a number of side effects, including mild (diarrhea, nausea, and vomiting and skin rash) side effects in 30–50% of recipients and severe (neutropenia, bone marrow aplasia, thrombotic thrombocytopenic purpura and death) side effects in 1–2% of recipients [Citation3]. Ticlopidine was rapidly replaced in the early 2000s by the second generation thienopyridine clopidogrel, characterized by a more favorable side-effect profile, and no reported fatal complications [Citation3]. Clopidogrel has been consistently shown to reduce thrombotic events at the cost of increased bleeding when used on top of aspirin in patients with atherosclerotic cardiovascular disease including those with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI) [Citation4,Citation5]. However, ischemic recurrences continue to occur in patients with cardiovascular disease treated with dual antiplatelet therapy (DAPT) with aspirin and clopidogrel, in part attributed to variability in the antiplatelet effects achieved by clopidogrel leading to inadequate platelet inhibition in up to 30% of patients [Citation6,Citation7].
Indeed, clopidogrel is a pro-drug that requires a 2-step biotransformation oxidative process by the hepatic cytochrome P450 (CYP) system to be activated, in which the CYP2C19 enzyme is both involved, making genetic variants affecting its function of particular interest [Citation7]. In fact, carrier status for loss-of-function (LoF) alleles of the CYP2C19 gene are as frequent as 20–40% in the general population and are associated with reduced generation of clopidogrel’s active metabolite and high platelet reactivity (HPR), a marker of thrombotic complications [Citation6,Citation7]. To this extent, platelet function or genetic testing has been proposed to identify ‘clopidogrel non responders’ and allow for the use of alternative antiplatelet agents in these patients, but its systematic implementation in clinical practice remains debated [Citation6,Citation7]. These observations have prompted the development of P2Y12 inhibitors with less variability in effects and more potent platelet inhibition.
The third generation thienopyridine prasugrel and the cyclopentyltriazolopyrimidine (CPTP) ticagrelor are new generation P2Y12 inhibitors characterized by faster and more potent antiplatelet effects but also by more predictable pharmacodynamic (PD) profiles resulting in reduced thrombotic events at the cost of increased bleeding, compared with clopidogrel [Citation8–10].
However, prasugrel and ticagrelor are oral agents and inevitably associated with a time delay in exerting their platelet inhibitory effects underscoring the need for P2Y12 inhibitors that can be administered parentally. The need for a rapid and potent platelet inhibition is particularly critical in ACS or during PCI to reduce periprocedural thrombotic complications. Cangrelor is an intravenous direct reversible P2Y12 receptor antagonist characterized by a quick onset and a rapid offset of action after infusion discontinuation. Compared to oral P2Y12 inhibitors, cangrelor achieves fast and consistent platelet inhibition, and it is effective in reducing periprocedural thrombotic complication of PCI, such as periprocedural myocardial infarction or acute stent thrombosis [Citation11]. The use of cangrelor in the modern setting of ACS and PCI is also supported by the increasing understanding of the prognostic impact of periprocedural MI, the downgrade in the recommendation on pre-treatment with oral P2Y12 inhibitors in ACS resulting in an increase of P2Y12 inhibitor naïve patients at the time of PCI and the acknowledgment that oral P2Y12 inhibitor absorption is delayed in ACS patients, especially those treated with opioids. Furthermore, the use of cangrelor is an attractive option as bridging strategy in patients deemed at high thrombotic risk requiring non-deferrable surgery in whom discontinuation of oral P2Y12 inhibition is necessary. Although cangrelor allows for a fast and potent P2Y12 inhibition, it requires to be administered intravenously (bolus followed by continuous infusion), preventing from smoothly using this strategy in the prehospital setting. To overcome this limitation, the subcutaneous administration of the P2Y12 inhibitor selatogrel is currently under investigation with the aim of facilitating self-administration in the prehospital setting, after onset of symptoms, reducing early thrombotic complications among out-of-hospital ACS patients (NCT04957719) [Citation12].
P2Y12 inhibitors have been associated with both class- and drug-specific pleiotropic effects including the modulation of inflammatory pathways, endothelial function, pre- and post-conditioning, the extent of which, however, remain to be fully elucidated [Citation2,Citation13,Citation14]. More recently, the increasing understanding that bleeding complications carry important prognostic implications together with the fact that local thrombotic complications are rare (~1%) with new stent platforms and that the thrombotic risk remains highest in the early phase (1–3 months) after ACS or PCI while tends to decrease thereafter, have sparked the interest toward de-escalation antiplatelet strategies, including the use of P2Y12 inhibitor monotherapy [Citation2,Citation15]. To this extent, it is important to note that the vast majority of the clinical evidence on P2Y12 inhibitors over years has been generated on the background of aspirin therapy, which has represented the backbone of antiplatelet therapy in cardiovascular disease for over 40 years. Therefore, the use of P2Y12 inhibitor monotherapy has been the focus for a number of more recent dedicated investigations showing promising results [Citation2]. Specifically, P2Y12 inhibitor monotherapy may be used (1) after a short course of DAPT; (2) in leu of aspirin for primary or secondary prevention; (3) early after ACS/PCI in patients with concomitant indication for oral anticoagulation (OAC); and (4) in adjunct to low dose of OAC (the so called dual-pathway inhibition, DPI) [Citation2,Citation16].
2. Expert opinion
There are two main issues surrounding the use of P2Y12 inhibitor monotherapy in clinical practice. First, the term ‘P2Y12 inhibitor’ encompasses different compounds with very different PD profiles resulting in different clinical outcomes [Citation7–9]. In particularly, a major concern when using clopidogrel monotherapy lies in the fact that about 30% of patients is expected to be non-responder to clopidogrel while non-responders are rare with aspirin, ticagrelor or prasugrel. Although a guided selection of P2Y12 inhibiting therapy has shown promising results, further evidence is warranted to shed light on the use of platelet function or genetic testing could be advantageous compared to an unguided use of clopidogrel [Citation6,Citation7]. It is important to note that a patient-centered rather than a randomized trial-centered approach needs to be implemented when interpreting the results of RCTs in this setting, considering that, although rare, thrombotic events are predictable, associated with poor prognosis, and can be significantly reduced by the use of a tailored approach based on the implementation of these tools [Citation17]. Second, a large part of the RCTs testing P2Y12 monotherapy vs. DAPT or aspirin monotherapy have been performed in East Asian patients, who are characterized by a peculiar bleeding and thrombotic risk profile, preventing from generalizing the results of these trials to other populations [Citation18]. Therefore, dedicated RCTs focusing on a specific P2Y12 inhibitor and on different populations as well as focusing in a specific clinical setting (acute vs. chronic) are warranted before this strategy could be safely implemented.
Finally, a promising field of research is that of combining a P2Y12 inhibitor with low dose of OAC [Citation16]. Indeed, DPI has been proposed as a synergistic approach not only in terms of antithrombotic effects but also in light of its effects on coagulation and inflammatory processes involved in the pathogenesis of atherosclerosis [Citation16]. Although DPI has traditionally consisted in aspirin plus low dose of OAC, early PD and clinical evidence support the encouraging safety and efficacy profiles of a P2Y12 inhibitor-based DPI, particularly with ticagrelor [Citation19]. However, further clinical evidence is needed to support this approach.
3. Conclusion
P2Y12 inhibitors have written a piece of history in the treatment of patients with cardiovascular disease over the last 30 years and their future evolution will lie on their personalized use taking into consideration the different pharmacological properties of the available agents and on the understanding of their pleiotropic and synergistic effects with other compounds, potentially affecting their safety and efficacy profiles.
Declaration of interest
M Galli declares that he has received consulting fees or honoraria from Terumo, outside the present work. DJ Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, Novo Nordisk, PhaseBio, PLx Pharma, Pfizer, Sanofi and Ventura, outside the present work. DJ Angiolillo also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and Scott R. MacKenzie Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082
- Angiolillo DJ, Galli M, Collet JP, et al. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17(17):e1371–e1396. doi: 10.4244/EIJ-D-21-00904
- Quinn MJ, Fitzgerald DJ. Ticlopidine and clopidogrel. Circ. 1999;100(15):1667–1672.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–2420. doi: 10.1001/jama.288.19.2411
- Galli M, Franchi F, Rollini F, et al. Role of platelet function and genetic testing in patients undergoing percutaneous coronary intervention. Trends Cardiovasc Med. 2023;33(3):133–138. doi: 10.1016/j.tcm.2021.12.007
- Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(16):1521–1537. doi: 10.1016/j.jcin.2019.03.034
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57. doi: 10.1056/NEJMoa0904327
- Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet (London, England). 2020;395(10233):1374–1381. doi: 10.1016/S0140-6736(20)30325-1
- Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–1313. doi: 10.1056/NEJMoa1300815
- Milluzzo RP, Franchina GA, Capodanno D, et al. Selatogrel, a novel P2Y(12) inhibitor: a review of the pharmacology and clinical development. Expert Opin Investig Drugs. 2020;29(6):537–546. doi: 10.1080/13543784.2020.1764533
- D’Amario D, Galli M, Restivo A, et al. Ticagrelor enhances the cardioprotective effects of ischemic preconditioning in stable patients undergoing percutaneous coronary intervention: the TAPER-S randomized study. Eur Heart J Cardiovasc Pharmacother. 2023. doi: 10.1093/ehjcvp/pvad092
- Siegel PM, Sander L, Fricke A, et al. P(2)Y(12) receptor blockers are anti-inflammatory drugs inhibiting both circulating monocytes and macrophages including THP-1 cells. Sci Rep. 2021;11(1):17459. doi: 10.1038/s41598-021-95710-3
- Galli M, Capodanno D, Andreotti F, et al. Safety and efficacy of P2Y(12) inhibitor monotherapy in patients undergoing percutaneous coronary interventions. Expert Opin Drug Saf. 2021;20(1):9–21. doi: 10.1080/14740338.2021.1850691
- Galli M, Franchi F, Rollini F, et al. Dual pathway inhibition in patients with atherosclerotic disease: pharmacodynamic considerations and clinical implications. Expert Rev Clin Pharmacol. 2023;16(1):27–38. doi: 10.1080/17512433.2023.2154651
- Galli M, Ortega-Paz L, Franchi F, et al. Precision medicine in interventional cardiology: implications for antiplatelet therapy in patients undergoing percutaneous coronary intervention. Pharmacogenomics. 2022;23(13):723–737. doi: 10.2217/pgs-2022-0057
- Galli M, Laborante R, Occhipinti G, et al. Impact of ethnicity on antiplatelet treatment regimens for bleeding reduction in acute coronary syndromes: a systematic review and pre-specified subgroup meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2023. doi: 10.1093/ehjcvp/pvad085
- Galli M, Franchi F, Rollini F, et al. Platelet P2Y12 inhibiting therapy in adjunct to vascular dose of rivaroxaban or aspirin: a pharmacodynamic study of dual pathway inhibition vs. dual antiplatelet therapy. Eur Heart J Cardiovasc Pharmacother. 2022;8(7):728–737. doi: 10.1093/ehjcvp/pvac022
