1. Need for novel treatment options in hepatocellular carcinoma (HCC)
HCC is still one of the leading causes of death caused by cancer worldwide [Citation1]. Despite different curative treatment modalities available, advanced-stage HCC has a poor prognosis and high tumor recurrence rate. Since early stages of HCC are asymptomatic, many patients represent with non-resectable advanced-stage tumors. In every respect, early diagnosis of HCC is the key for a survival benefit because only in early stages treatment options such as resection, liver transplantation, or nonsurgical approaches are available and lead to improved outcomes [Citation2].
The pan-tyrosine kinase inhibitor sorafenib is the only standard drug therapy available for patients with advanced HCC, with modest effectiveness at extending the overall survival of patients for 2–3 months. The mechanism by which sorafenib acts on advanced HCC is not well understood, and no biomarkers have been identified to predict response to sorafenib treatment in patients with HCC [Citation3]. Moreover, resistance to sorafenib and severe side effects further limit its clinical efficacy [Citation3]. One factor which could play a role in the resistance to sorafenib is sirtuin 1 (SIRT1). Sirtuins are a family of nicotinamide adenine dinucleotide (NAD)-dependent enzymes that modulate distinct metabolic, energetic, and stress response pathways. In a recent study, higher SIRT1 protein levels in HCC tissue were associated with worse outcome, and SIRT1 overexpression supported resistance to sorafenib [Citation4]. SIRT1 activity depends on intracellular NAD levels. Nicotinamide phosphoribosyltransferase (NAMPT) is the key enzyme in mammalian NAD salvage from nicotinamide and therefore regulates the activity of NAD-dependent enzymes, such as sirtuins or poly-ADP-ribosyltransferases (PARPs) (reviewed in [Citation5], ).
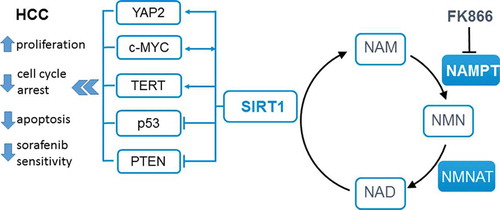
Figure 1. Potential mechanism of NAMPT-NAD-SIRT1 action in HCC.
Nicotinamide phosphoribosyltransferase (NAMPT) catalyses the salvage of nicotinamide (NAM), a reaction product of Sirtuin (SIRT) 1, to nicotinamide mononucleotide (NMN). FK866 is a specific pharmacologic inhibitor of NAMPT enzymatic activity, which leads to depletion of intracellular nicotinamide adenine dinucleotide (NAD) levels. Conversion of NMN to NAD is carried out by nicotinamide mononucleotide adenylyltransferase (NMNAT) enzymes. NAD is a substrate for SIRT1. By deacetylating specific lysine residues, SIRT1 regulates the transcriptional activity or protein stability of several crucial factors in hepatocarcinoma (HCC) tumorigenesis. YAP: Yes-associated protein; c-MYC: V-Myc Avian Myelocytomatosis Viral Oncogene Homolog; TERT: telomerase catalytic subunit; p53: tumor suppressor protein 53; PTEN: phosphatase and tensin homolog; Data from Wu et al. Tumor Biol. (2015) 36:4063–4074.
2. Inhibition of NAD biosynthesis in HCC
Cancer cells have a higher demand for NAD because of their rapid proliferation which is associated with the need for increased energy metabolism, ATP generation, nucleotide biosynthesis, DNA repair, and increased activity of NAD-consuming enzymes [Citation6]. NAMPT has been extensively studied as a target of anticancer therapy. NAMPT is overexpressed in several types of tumor tissue and its expression often correlates with cancer therapy resistance and poor outcome in cancer patients [Citation6]. NAMPT inhibitors as single agents have not been applied successfully in clinical studies so far [Citation6]. Possible successful treatment combinations with NAMPT inhibitors include DNA-damaging agents, inhibitors targeting enzymes involved in cellular stress responses or DNA repair, and rescue with nicotinic acid in nicotinic acid phosphoribosyltransferase-negative tumors to increase tolerance of non-tumor tissue to NAMPT inhibition [Citation6]. Another promising possibility is the simultaneous inhibition of NAMPT and other enzymes providing precursors for NAD biosynthesis, e.g. cluster of differentiation 73 [Citation7].
We found that NAMPT expression is lower in hepatocarcinoma cell lines compared to primary hepatocytes. NAMPT enzymatic activity, however, is higher in HCC with the result of comparable NAD levels in HCC cell lines and primary hepatocytes [Citation8]. Nevertheless, blocking NAMPT enzymatic activity by the specific inhibitor FK866 induced depletion of NAD and ATP and delayed cell death in human hepatocarcinoma cell lines. FK866 treatment induced adenosine monophosphate-activated kinase (AMPK) activation while the activity of mammalian target of rapamycin (mTOR) complex 1 and downstream targets was blocked [Citation9]. We and others showed that the catalytic subunit of AMPK is significantly downregulated while mTOR complex 1 activation is higher in HCC compared to primary hepatocytes or normal liver tissue [Citation9,Citation10]. Thus, increasing AMPK activity by inducing energy stress through NAMPT inhibition or by Metformin, an activator of AMPK, could help to antagonize the Warburg effect and could become a novel option for the treatment of HCC [Citation11].
The polyphenol resveratrol was found to inhibit carcinogenesis in different types of cancer with a pleiotropic mode of action and has been considered in HCC prevention and treatment [Citation12]. We found that resveratrol treatment of hepatocarcinoma cell lines led to cell cycle arrest and apoptosis with concomitant reduced NAMPT activity and NAD levels, which resulted in reduced SIRT1 activity [Citation8].
3. Extracellular NAMPT in HCC
In addition to its key role in intracellular NAD biosynthesis, NAMPT (also known as visfatin or pre-B cell colony-enhancing factor in this context) is also found in human circulation. Extracellular NAMPT was found to act both as enzyme, converting nicotinamide to nicotinamide mononucleotide (NMN), and as cytokine (reviewed in [Citation5]). Increased NAMPT serum concentrations have been associated with a variety of cancers and extracellular NAMPT was found to influence the microenvironment of cancer cells in a way to promote tumor progression and metastasis [Citation13]. In patients with HCC, serum NAMPT levels were significantly correlated with stage progression and tumor enlargement. Extracellular NAMPT preferentially stimulated the proliferation of human HCC cell lines compared with normal hepatocytes via activation of extracellular signal-regulated kinase and V-Akt Murine Thymoma Viral Oncogene Homolog 1 pathways and glycogen synthase kinase-3β [Citation14]. Similar results for extracellular NAMPT action have been found in breast cancer [Citation15].
4. Boosting NAD levels in HCC
One of the cellular events preceding HCC development is oncogene-induced DNA damage. Expression of unconventional prefoldin RBP5 interactor in hepatocytes was shown to reduce the expression of enzymes of de novo NAD biosynthesis. The resulting NAD depletion induced DNA damage by inhibiting the NAD-dependent DNA repair enzyme PARP-1, subsequent genotoxic stress, apoptosis, and compensatory proliferation leading to the development of liver tumors in mice [Citation16]. HCC development was blocked by the restoration of hepatocellular NAD pools by supplementing nicotinamide ribose, a precursor of NAD biosynthesis [Citation16]. HCC is also driven by hepatocyte death during progression of non-alcoholic fatty liver disease (NAFLD). Studies on NASH patients and animal models revealed that there is a negative correlation of NAMPT expression and NAFLD progression. We could demonstrate that during early stage NAFLD, NAD salvage via NAMPT is upregulated in mice [Citation17], whereas in later disease stages, NAMPT and NAD levels were reported to be downregulated (reviewed in [Citation5]). Low NAD levels may then reduce the activity of PARP-1, thereby impairing genomic integrity as was described by Tummala et al. [Citation16].
5. Conclusion
HCC is an extremely heterogeneous cancer, varying widely in etiology (genotoxic stress, metabolic disorders) and progression in individual patients [Citation2]. Both NAD depletion (e.g. via NAMPT inhibition) and NAD replenishment could be beneficial in the prevention and treatment of HCC, especially in case of sorafenib resistance. Boosting NAD levels could be a preventive treatment, especially since recent studies on long-term supplementation of NAD precursors or intermediates did not show obvious toxicity or deleterious effects and increased NAD levels in mouse liver [Citation18,Citation19]. Early therapeutic intervention prior to genomic instability may protect risk patients from tumor initiation and genotoxic-induced tumorigenesis.
Future studies focusing on inhibition of NAD biosynthesis in hepatocarcinoma animal models could help to elucidate questions regarding the timing of NAD inhibition (early vs. advanced stage tumors), finding biomarkers to predict response to NAMPT inhibitor treatment and test drug combinations that enhance the efficiency of NAD depletion. Complete inhibition of NAMPT is crucial to prevent tumor recurrence, as incomplete NAMPT inhibition, which impedes NAD+ metabolism but does not kill a tumor cell, can alter its phenotype to be more aggressive and metastatic [Citation20].
Unraveling the role of NAMPT activity in HCC development and the function of NAMPT’s non-enzymatic activity in inflammation, angiogenesis and metastasis will pave the way for future novel NAMPT-based oncotherapy options for HCC.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
- Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53.
- Chen J, Gao J. Advances in the study of molecularly targeted agents to treat hepatocellular carcinoma. Drug Discov Ther. 2014;8(4):154–164.
- Chen H-C, Jeng Y-M, Yuan R-H, et al. SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol. 2012;19(6):2011–2019.
- Garten A, Schuster S, Penke M, et al. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11(9):535–546.
- Roulston A, Shore GC. New strategies to maximize therapeutic opportunities for NAMPT inhibitors in oncology. Mol Cell Oncol. 2016;3(1):e1052180.
- Sociali G, Raffaghello L, Magnone M, et al. Antitumor effect of combined NAMPT and CD73 inhibition in an ovarian cancer model. Oncotarget. 2016;7(4):2968–2984.
- Schuster S, Penke M, Gorski T, et al. Resveratrol differentially regulates NAMPT and SIRT1 in hepatocarcinoma cells and primary human hepatocytes. PLoS One. 2014;9(3):e91045.
- Schuster S, Penke M, Gorski T, et al. FK866-induced NAMPT inhibition activates AMPK and downregulates mTOR signalling in hepatocarcinoma cells. Biochem Biophys Res Commun. 2015;458(2):334–340.
- Lee C-W, Wong LL-Y, Tse EY-T, et al. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res. 2012;72(17):4394–4404.
- Bhat A, Sebastiani G, Bhat M. Systematic review: preventive and therapeutic applications of metformin in liver disease. World J Hepatol. 2015;7(12):1652–1659.
- Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36(1):43–53.
- Grolla AA, Travelli C, Genazzani AA, et al. Extracellular nicotinamide phosphoribosyltrasferase (eNAMPT), a new cancer metabokine. Br J Pharmacol. 2016;173(14):2182–2194.
- Ninomiya S, Shimizu M, Imai K, et al. Possible role of visfatin in hepatoma progression and the effects of branched-chain amino acids on visfatin-induced proliferation in human hepatoma cells. Cancer Prev Res (Phila). 2011;4(12):2092–2100.
- Park H-J, Kim S-R, Kim SS, et al. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget. 2014;5(13):5087–5099.
- Tummala KS, Gomes AL, Yilmaz M, et al. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell. 2014;26(6):826–839.
- Penke M, Larsen PS, Schuster S, et al. Hepatic NAD salvage pathway is enhanced in mice on a high-fat diet. Mol Cell Endocrinol. 2015;412:65–72.
- Mills KF, Yoshida S, Stein LR, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806.
- Trammell SAJ, Schmidt MS, Weidemann BJ, et al. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948.
- Santidrian AF, LeBoeuf SE, Wold ED, et al. Nicotinamide phosphoribosyltransferase can affect metastatic activity and cell adhesive functions by regulating integrins in breast cancer. DNA Repair (Amst). 2014;23:79–87.
