ABSTRACT
Introduction: Epidermal growth factor receptor (EGFR) mutations are well-described drivers of non-small cell lung cancer (NSCLC) and EGFR tyrosine kinase inhibitors (TKIs) have become key components of the NSCLC front-line treatment landscape. Tumors inevitably develop resistance to these agents, and development efforts continue to focus on identifying mechanisms of resistance and drugs to target these mechanisms.
Areas covered: With several EGFR TKIs approved for use in the first-line or in later-line settings, an understanding of the efficacy and safety of these inhibitors in various populations is warranted. Furthermore, given the frequent emergence of drug resistance in NSCLC, examination of tumor tissue throughout the disease course provides the opportunity to select treatments based on the tumor’s mutation profile. Here, we discuss: key efficacy and safety findings for approved and investigational EGFR TKIs; known mechanisms of resistance, particularly the T790M acquired EGFR mutation; and recent advances in EGFR mutational testing that may facilitate less invasive tissue testing and guide treatment selection.
Expert commentary: The expanding armamentarium of EGFR TKIs, improvements in the understanding of resistance mechanisms and technological developments in the molecular analysis of tumors may help render EGFR mutation-positive NSCLC a chronic disease in many patients by facilitating optimal sequential therapy.
1. Introduction
An estimated 230,000 patients will be diagnosed with lung cancer in the United States in 2018 [Citation10]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, and recent advances in the understanding of its biology have led to the development of novel therapeutic strategies. An example of this was the identification of activated epidermal growth factor receptor (EGFR) as a driver in NSCLC [Citation11], which led to the development of targeted EGFR inhibitors and expanded the therapeutic options for this disease [Citation12]. The frequency of EGFR mutations varies depending on tumor histology and patient population, ranging from 7% to 14% in Caucasian populations and 27% to 34% in Asian populations with adenocarcinoma [Citation13–Citation15]. EGFR mutations are more prevalent in females than males and never smokers than ever smokers [Citation14,Citation15].
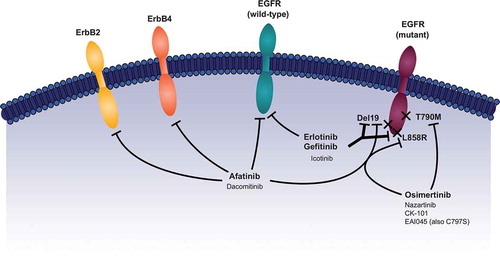
Currently, five EGFR tyrosine kinase inhibitors (TKIs) are approved by the US Food and Drug Administration and the European Medicines Agency for the first-line treatment of patients with EGFR mutation-positive NSCLC: the first-generation reversible EGFR TKIs, erlotinib and gefitinib; the second-generation irreversible ErbB family blockers, afatinib and dacomitinib; the third-generation irreversible EGFR TKI, osimertinib. Patients with EGFR mutation-positive NSCLC are highly sensitive to EGFR TKIs with response rates and progression-free survival (PFS) typically ranging from 56% to 83% and 8.4‒18.9 months, respectively [Citation12]. However, regardless of which agent is chosen as first-line treatment, acquired resistance is inevitable. Third-generation EGFR TKIs were initially developed in an effort to combat resistance to first- and second-generation EGFR TKIs. Indeed, osimertinib is approved for the treatment of metastatic NSCLC harboring the T790M EGFR resistance mutation, a common mechanism of resistance to first- and second-generation EGFR TKIs [Citation16–Citation18], following progression on EGFR-TKI therapy. Resistance mechanisms to third-generation TKIs are currently not as well characterized as those for first- and second-generation TKIs. Given the expanding armamentarium of highly effective TKIs for the treatment of EGFR mutation-positive NSCLC, it is important that the characteristics of each agent are understood so that treatment can be tailored according to individual patient characteristics. Moreover, the assessment of the molecular evolution of tumors at the point of acquired resistance is becoming increasingly important with respect to optimizing the sequence of therapy. Here we review efficacy and safety data for approved and investigational EGFR TKIs for both the front-line treatment of NSCLC and for tumors developing resistance to EGFR-TKI therapy (). In an effort to improve treatment selection and efficacy, newer and more convenient methods for detecting EGFR mutations, including from circulating tumor cells (CTCs) and cell-free tumor DNA (cftDNA), are evolving and are highlighted here.
Table 1. Mechanisms of resistance to EGFR TKIs [Citation4].
2. FDA approved front-line EGFR tkis
2.1. Gefitinib
Gefitinib (IRESSA®, AstraZeneca) reversibly inhibits the kinase activity of EGFR and has greater binding affinity for deletion 19 (del19) and L858R mutant EGFR than for wild-type EGFR protein (). It is indicated for the first-line treatment of metastatic NSCLC harboring del19 or L858R EGFR mutations, as detected by a United States Food and Drug Administration (FDA)-approved test. In the IPASS study, gefitinib led to longer PFS than carboplatin/paclitaxel as first-line therapy in patients with advanced lung adenocarcinoma (hazard ratio [HR] for progression or death, 0.74 [95% confidence interval (CI): 0.65–0.85];
Figure 1. Mechanism of action of EGFR TKIs. Reprinted with permission from Journal of the Advanced Practitioner in Oncology.
Note: EGFR = epidermal growth factor receptor, TKI = tyrosine kinase inhibitors, del19 = deletion 19.
While gefitinib has proven activity against common activating EGFR mutations (del19, L858R) few prospective data are available that have assessed its activity against uncommon mutations such as exon 18 mutations, exon 20 insertions, and mutations in the extracellular domain of EGFR, which collectively account for >10% of all cases of EGFR mutation-positive NSCLC [Citation25]. Available data from clinical trials [Citation26], or real-world studies [Citation27,Citation28], indicate that gefitinib is less active against uncommon mutations such as G719X, L861Q and S768I than common mutations. Of note, certain uncommon mutations including exon 20 insertions and de novo T790M are intrinsically resistant to gefitinib [Citation27,Citation28].
2.2. Erlotinib
Erlotinib (TARCEVA®, Genentech) also reversibly inhibits the kinase activity of EGFR and has greater binding affinity for del19 – and L858R-mutant EGFR than for wild-type EGFR protein (). Erlotinib is indicated for the treatment of patients with metastatic NSCLC harboring del19 or L858R EGFR mutations (as detected by an FDA-approved test) who are receiving first-line, maintenance, or second- or later-line treatment after disease progression following ≥1 prior chemotherapy regimen. Results from the BR.21 (previously treated patients) and SATURN (maintenance therapy) trials demonstrated the efficacy of erlotinib in unselected (i.e. wild-type and mutant EGFR) NSCLC patient populations [Citation29,Citation30], although erlotinib has recently become indicated only for patients with del19 or L858R EGFR mutation-positive NSCLC. Approval in the first-line setting was based on results from the EURTAC trial, which compared erlotinib and standard chemotherapy (cisplatin/docetaxel and cisplatin/gemcitabine) in Caucasian EGFR mutation-positive NSCLC patients. Erlotinib demonstrated significantly longer PFS versus chemotherapy (9.7 vs 5.2 months, respectively; HR, 0.37 [95% CI: 0.25–0.54]; p < 0.0001) [Citation31]. In subgroup analyses, PFS was also significantly longer with erlotinib compared with chemotherapy in patients with del19 (11.0 vs 4.6 months, respectively; p < 0.0001), but not L858R (8.4 vs 6.0 months; p = 0.0539) mutations [Citation31]. Two randomized Phase III trials, ENSURE and OPTIMAL, demonstrated that erlotinib significantly improved PFS versus gemcitabine/cisplatin (median 11.0 vs 5.5 months; HR, 0.34 [95% CI: 0.22–0.51]; p < 0.0001) and gemcitabine/carboplatin (median 13.1 vs 4.6 months; HR, 0.16 [95% CI: 0.10–0.26]; p < 0.0001) in Asian, predominantly Chinese, patients with EGFR mutation-positive NSCLC [Citation32,Citation33]. Like gefitinib, erlotinib appears to have limited or no clinical activity against most uncommon EGFR mutations, including exon 20 insertions and de novo T790M [Citation27,Citation28].
The combination of erlotinib and bevacizumab recently gained approval in Europe for the first-line treatment of patients with unresectable advanced, metastatic, or recurrent non-squamous, EGFR mutation-positive NSCLC. Approval was based on results from the Phase II JO25567 trial comparing erlotinib plus bevacizumab with erlotinib alone in Japanese patients. The combination of erlotinib and bevacizumab led to significantly longer PFS compared with erlotinib alone (median 16.0 vs 9.7 months, respectively; HR, 0.54 [95% CI: 0.36–0.79]; p = 0.0015) [Citation34]. The PFS benefit seen with the combination was also significant in patients with del19 EGFR mutations (median 18.0 vs 10.3 months, respectively; p = 0.0011), and showed a non-significant trend in patients with L858R mutations (median 13.9 vs 7.9 months; p = 0.1653) [Citation34]. However, there was no difference in OS with the combination compared with erlotinib alone (median 48.4 vs 48.5 months, respectively; HR, 0.91 [95% CI: 0.56–1.46]; p = 0.6838) [Citation35]. In the second-line setting, a meta-analysis of five studies comparing combination treatment with erlotinib plus bevacizumab versus bevacizumab or erlotinib alone, pooled estimates did not show significant improvement in OS with combination therapy (HR, 0.96 [95% CI: 0.83–1.11]; p = 0.573) [Citation36]. The erlotinib plus bevacizumab combination resulted in greater PFS (HR, 0.63 [95% CI: 0.53–0.75]; p = 0.000) and ORR (risk ratio = 1.91 [95% CI: 1.19–3.06]; p = 0.007) compared with either bevacizumab or erlotinib alone [Citation36].
2.3. Afatinib
Afatinib (GILOTRIF®, Boehringer Ingelheim) is an irreversible ErbB family blocker that targets EGFR/ErbB1, HER2/ErbB2, and HER4/ErbB4, which results in the inhibition of HER3/ErbB3 phosphorylation; afatinib can inhibit both wild-type and mutant EGFR (), with higher affinity for mutant EGFR [Citation37–Citation39]. Afatinib is indicated for the first-line treatment of metastatic NSCLC harboring non-resistant EGFR mutations, as detected by an FDA-approved test. In the global LUX-Lung 3 study, afatinib demonstrated significantly longer PFS (11.1 months) compared with cisplatin/pemetrexed (6.9 months) in NSCLC patients with activating EGFR mutations (HR, 0.58 [95% CI: 0.43–0.78]; p = 0.001) [Citation9]. This PFS benefit was more pronounced with afatinib in patients with del19 and L858R EGFR mutations (median 13.6 vs 6.9 months for afatinib and cisplatin/pemetrexed, respectively; HR, 0.47 [95% CI: 0.34–0.65]; p = 0.001) [Citation9]. While OS benefit was not observed in the overall population, in a pre-specified subgroup analysis by EGFR mutation type, afatinib significantly improved OS compared with chemotherapy in patients with del19 mutation (median 33.3 vs 21.1 months; HR, 0.54 [95% CI: 0.36–0.79]; p = 0.0015) [Citation40]. In the Phase III LUX-Lung 6 trial, afatinib significantly improved PFS versus cisplatin/gemcitabine in Asian, predominantly Chinese, patients with EGFR mutation-positive NSCLC (median 11.0 vs 5.6 months; HR, 0.28 [95% CI: 0.20–0.39]; p < 0.0001) [Citation41]. Notably, this study independently replicated the significant OS benefit observed in LUX-Lung 3 in del19-positive patients (median 31.4 vs 18.4 months; HR, 0.64 [95% CI: 0.44–0.94]; p = 0.023) [Citation40].
In contrast to many Phase III trials of EGFR TKIs, LUX-Lung 3 and 6 permitted the recruitment of patients whose tumors harbored uncommon EGFR mutations. In a post hoc analysis of these trials, along with the Phase II LUX-Lung 2 trial, 75 patients were identified with uncommon activating mutations [Citation42]. In patients with L861Q, G719X or S768I, the response rate to afatinib was 56.3, 77.8 and 100.0%, respectively. Based on these findings, the indication for afatinib was recently expanded to include these mutations. In contrast, afatinib appeared to have poor activity against exon 20 insertions and de novo T790M [Citation42]. Patients with stable brain metastases were also permitted in LUX-Lung 3 and 6. In a prespecified subgroup analysis, similar overall clinical benefit with afatinib versus chemotherapy was observed in patients with, or without, brain metastases [Citation43]. Competing risk analysis of patients with brain metastases treated with afatinib indicated that the risk of CNS progression was lower than that of non-CNS progression (31% and 52%, respectively [Citation12]). Moreover, the frequency of de novo CNS progression in LUX-Lung 3 and 6 was only 6%, compared with a non-CNS progression frequency of 78% [Citation12]. These findings suggest that afatinib may protect against metastatic spread to the brain.
The LUX-Lung 7 head-to-head trial compared afatinib with gefitinib as first-line treatment in EGFR mutation-positive NSCLC patients. Afatinib treatment led to longer PFS (median 11.0 vs 10.9 months for gefitinib; HR, 0.73 [95% CI: 0.57–0.95]; p = 0.017) and time-to-treatment failure (median 13.7 vs 11.5 months; HR, 0.73 [95% CI: 0.58–0.92]; p = 0.0073) compared with gefitinib [Citation1]. However, median OS was not significantly different (27.9 vs 24.5 months; HR, 0.86 [95% CI: 0.66–1.12]; p = 0.258), regardless of mutational subtype (del19 or L858R) in subgroup analyses [Citation44].
Afatinib is also approved for the treatment of metastatic squamous cell lung cancer, regardless of EGFR mutation status, based on results from the LUX-Lung 8 trial of afatinib versus erlotinib in patients who had progressed on ≥4 cycles of platinum-based chemotherapy. Patients in the afatinib group had significantly longer PFS than those in the erlotinib group (2.4 vs 1.9 months; HR, 0.82 [95% CI: 0.68–1.00]; p = 0.0427). OS was also significantly longer with afatinib (7.9 months) compared with erlotinib (6.8 months; HR, 0.81 [95% CI: 0.69–0.95]; p = 0.0077) and DCR was significantly higher (51% vs 40%, respectively; p = 0.002), although ORR was not significantly different between the two groups (6% vs 3%, p = 0.0551) [Citation45].
2.4. Dacomitinib
Dacomitinib (Pfizer) irreversibly inhibits tyrosine autophosphorylation of EGFR, HER2/ErbB2, and HER4/ErbB4 () [Citation46]. In a single-arm, Phase II study, dacomitinib showed promising activity with a four-month PFS rate of 95.5% in patients with EGFR mutation-positive disease [Citation47]. These data were recently substantiated by the Phase III ARCHER 1050 trial [Citation2]. In this study, dacomitinib significantly improved PFS versus gefitinib in patients with EGFR mutation-positive (del19 or L858R) NSCLC (median 14.7 vs 9.2 months; HR, 0.59 [95% CI: 0.47–0.74]; p < 0.0001). In contrast to the LUX-Lung 3, 6 and 7 and FLAURA trials patients with stable brain metastases were excluded. Dacomitinib improved duration of response versus gefitinib (median 14.8 vs 8.3 months, respectively), but there was no significant difference in response rate between the two groups (75 vs 72%; p = 0.4234). ARCHER 1050 had a hierarchical statistical testing order of PFS followed by ORR then OS. No formal testing of OS was conducted because ORR was not statistically significant [Citation48]. Nevertheless, exploratory OS analysis from ARCHER 1050 was recently reported [Citation49]. After a median follow-up of 31.3 months, dacomitinib significantly prolonged OS versus gefitinib (median 34.1 vs 26.8 months; HR, 0.76 [95% CI: 0.58–0.99]; p = 0.044). Similar OS benefit was observed across patient subgroups including age (<65 vs ≥65 years), race (Asian vs non-Asian) and EGFR mutation type (del19 vs L858R).
2.5. Osimertinib
Osimertinib (TAGRISSO™, AstraZeneca) covalently binds and inhibits mutant forms of EGFR, including T790M, del19, and L858R, with minimal activity against wild-type EGFR () [Citation50,Citation51]. In the Phase III FLAURA trial, undertaken in patients with EGFR mutation-positive (del19 or L858R) NSCLC, including patients with stable brain metastases, osimertinib conferred superior investigator-assessed PFS versus erlotinib or gefitinib (median 18.9 vs 10.2 months; HR, 0.46 [95% CI: 0.37–0.57]; p < 0.001) [Citation3]. Consistent PFS benefit was observed in patients with (HR, 0.47 [95% CI: 0.30–0.74]), or without (HR, 0.46 [95% CI: 0.36–0.59]), brain metastases. The response rate with osimertinib was similar to that observed with first-generation EGFR TKIs; however, the duration of response was prolonged with osimertinib (median 17.2 vs 8.5 months, respectively). At data cutoff, OS data were immature. Based on the results of FLAURA, osimertinib has recently been approved by the US FDA for the first-line treatment of patients with EGFR mutation-positive NSCLC Second-generation EGFR TKIs were not included in the comparator arm of FLAURA. Phase III trials are currently assessing osimertinib in an adjuvant setting following surgical resection (ADAURA; NCT02511106) or chemoradiation (LAURA; NCT03521154) in patients with non-metastatic EGFR mutation-positive NSCLC.
Recent preplanned subgroup analyses of FLAURA indicated that osimertinib has CNS activity. In patients with measurable and/or non-measurable CNS lesions, CNS PFS was longer with osimertinib than gefitinib/erlotinib (median not reached vs 13.9 months; HR, 0.48 [95% CI: 0.26–0.86]; p = 0.014 [Citation52]). CNS ORR was 66% and 43%, respectively. The frequency of CNS progression (20% vs 39%) and the development of de novo CNS lesions (12% vs 30%), was lower with osimertinib than gefitinib/erlotinib, indicating a protective effect against metastatic spread to the brain [Citation52], These findings are consistent with preclinical observations that osimertinib effectively crosses the blood-brain barrier [Citation50,Citation53].
3. Treatment of EGFR TKI–resistant NSCLC
Resistance to front-line EGFR-TKI therapy is a major challenge that limits durable responses to these agents. Selected patients with minimal or isolated progression may be able to continue with front-line EGFR TKIs in conjunction with local therapies, such as radiation, to address the site of disease progression [Citation54–Citation56]. However, it is important to consider possible systemic treatment options in the second-line setting and beyond, as well as potential combination strategies with the aim of delaying resistance in a first-line setting or providing novel treatment options following acquired resistance. Accordingly, many experimental regimens are currently being assessed in clinical trials (ongoing Phase 1b-3 trials are summarized in ). Central to the development of novel treatments is an understanding of the key molecular resistance mechanisms to EGFR TKIs, which appear to be somewhat different between first-/second-generation TKIs and third-generation TKIs.
Table 2. Key ongoing trials of combination therapy with EGFR TKIs in NSCLC.
3.1. Resistance to first – and second-generation EGFR TKIs
Currently, multiple mechanisms of resistance to first- and second-generation EGFR TKIs have been identified and include secondary EGFR mutations and upregulation of various compensatory kinases or signaling pathways () [Citation4,Citation57,Citation58]. The first resistance mechanism discovered was the T790M mutation, which results in increased affinity for adenosine triphosphate (ATP) over TKIs at their binding site to EGFR [Citation57]. At least half of first- and second-generation EGFR TKI–resistant lung cancers arise through the T790M secondary EGFR mutation [Citation58,Citation59] and, as a result, there is a large focus on developing EGFR TKIs that target this particular acquired mutation.
Osimertinib is highly effective in this setting and is approved by the FDA for the treatment of metastatic NSCLC harboring T790M EGFR mutations (detected by an FDA-approved test) after progression on EGFR-TKI therapy. In a Phase I trial of osimertinib in NSCLC patients who progressed on EGFR-TKI therapy (AURA), the confirmed ORR was 51% and DCR was 84%; median PFS was 8.2 months [Citation60]. Additionally, patients with detectable T790M EGFR mutation by central testing had a higher ORR (61% vs 21% for those without) and longer PFS (9.6 vs 2.8 months, respectively) than patients with no detectable T790M [Citation60]. The single-arm, Phase II AURA2 study confirmed the clinical benefit of osimertinib in patients with T790M mutation-positive NSCLC after progression on EGFR-TKI therapy, with an ORR of 70% [Citation61]. The Phase III AURA3 study, which compared osimertinib with platinum-based chemotherapy plus pemetrexed in patients with T790M mutation-positive NSCLC after progression on EGFR-TKI therapy, reported significantly longer PFS (median 10.1 vs 4.4 months; HR, 0.30 [95% CI: 0.23–0.41]; p < 0.001) and significantly higher ORR (71% vs 31%; p < 0.001) with osimertinib versus chemotherapy, respectively [Citation62].
T790M-independent mechanisms of resistance to first- and second-generation TKIs are heterogeneous and are generally unresponsive to EGFR blockade. Thus, treatment options for T790M-negative tumors remains an area of unmet medical need [Citation63]. One T790M-independent resistance mechanism, the constitutive activation of MET, activates the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway, signaling cell proliferation and survival, which EGFR inhibitors cannot overcome [Citation57]. HER2 mutations allow HER2 receptor activation independent of heterodimerization with EGFR and phosphorylate EGFR even when TKIs are present [Citation57]. Occasionally, resistance to TKIs is mediated by histologic changes in the tumor. In a study performed at the Massachusetts General Hospital, tumor biopsies were undertaken in 37 patients with EGFR-mutated NSCLC at diagnosis and at the time of EGFR-TKI resistance. Of these patients, 5 (14%) experienced transformation to small-cell lung cancer at the time of clinical TKI resistance. In all 37 patients, the original EGFR mutation was maintained at the time of TKI resistance [Citation59].
3.2. Resistance to osimertinib
With the approval of first-line osimertinib in patients with EGFR mutation-positive NSCLC, it is important that resistance mechanisms are defined, with a view to identifying targeted treatment options following acquired resistance. There is an ongoing effort to identify resistance mechanisms. Small cohort studies have identified several putative resistance mechanisms, including the tertiary EGFR mutation, C797S, or molecular aberrations in other intracellular signaling pathways, such as amplification of MET, HER2 or KRAS [Citation64–Citation67]. A recent subanalysis of the FLAURA study indicated that resistance mechanisms to first-line osimertinib are highly heterogeneous. In 91 evaluable patients treated with first-line osimertinib, the most common resistance mechanism was MET amplification (15% of patients). C797S was present in only 7% of patients. Other less common resistance mechanisms were HER2 amplification and PIK3CA and RAS mutations [Citation5]. No putative resistance mechanisms were identified in ~60% of patients. As expected, no osimertinib-treated patients showed evidence of T790M-mediated acquired resistance [Citation5]. Some of the identified mechanisms of resistance offer rationale for potential targeted treatments. For example, C797S-positive tumors could be sensitive to EAI045, an allosteric inhibitor of mutant EGFR in early stages of development () [Citation68]. EAI045, when combined with cetuximab, has shown antitumor activity in preclinical models of EGFR-mutated lung cancer, including C797S [Citation68], suggesting that agents targeting this newly discovered resistance mutation may be viable treatment options. Furthermore, C797S-positive tumors may be sensitive to first-generation EGFR TKIs [Citation69,Citation70] if T790M is not present. However, recent data indicate that tertiary EGFR mutations, including C797S, occur in tumors that remain T790M-positive at the point of resistance [Citation55]. Moreover C797S and T790M generally occur in cis i.e. on the same allele [Citation71], suggesting that first- and second-generation EGFR TKIs would have limited activity after osimertinib failure. The involvement of other signaling pathways in patients with acquired resistance to osimertinib suggests that novel combination strategies may be effective in some of these patients. Ongoing Phase I studies, for example, are assessing the combination of osimertinib with MEK, MET, VEGFR2 or BCL-2 family inhibitors (NCT02143466, NCT02789345, NCT02520778). At this time, the most likely treatment following failure of osimertinib is a clinical trial or chemotherapy.
4. Other EGFR TKIs currently in development
4.1. Icotinib
Icotinib (Beta Pharma), a reversible inhibitor of wild-type and mutant forms of EGFR (), is in development for the treatment of patients with advanced, EGFR mutation-positive NSCLC, both in the first-line and later-line settings [Citation72]. In a Phase III trial comparing icotinib with gefitinib in Chinese patients with previously treated, advanced NSCLC (ICOGEN), icotinib was non-inferior to gefitinib (median PFS, 4.6 vs 3.4 months, respectively; p = 0.13) [Citation73]. The efficacy of icotinib was further demonstrated in patients with advanced or metastatic NSCLC who failed ≥1 platinum-based chemotherapy regimen (median PFS, 5.0 months; ORR, 25.8%; DCR, 67.7%; median OS, >17.6 months) [Citation74]. In a pharmacokinetic study, ORR with icotinib was 25% in Chinese patients with advanced NSCLC [Citation75]. Results from the recent CONVINCE trial in EGFR mutation-positive NSCLC patients demonstrated significantly improved PFS (median 11.2 vs 7.9 months for icotinib and cisplatin/pemetrexed, respectively; HR 0.61 [95% CI: 0.43–0.87]; p = 0.006) although there was no difference in OS [Citation76]. Icotinib is approved in China as first-line therapy in patients with advanced NSCLC and activating EGFR mutations [Citation77]. Ongoing Phase III trials are evaluating adjuvant icotinib versus placebo or vinorelbine in EGFR mutation-positive NSCLC patients (NCT02125240, NCT02448797).
4.2. Nazartinib
Nazartinib (Novartis) is an irreversible, mutant-selective EGFR TKI in development for the treatment of T790M mutation-positive NSCLC () [Citation78]. A first-in-human study of nazartinib recently demonstrated an ORR of 55% and a DCR of 86% in NSCLC patients with T790M mutation-positive tumors; this trial is ongoing [Citation79]. Nazartinib is also being assessed in combination with gefitinib in previously untreated patients with EGFR mutation-positive NSCLC, and in combination with nivolumab in patients with T790M-positive tumors following progression on an EGFR-TKI ().
4.3. CK-101
CK-101 (CheckPoint Therapeutics) is a novel third-generation EGFR TKI. An ongoing phase I/II trial is assessing CK-101 for the treatment of patients with EGFR mutation-positive NSCLC in both EGFR TKI treatment naïve and previously-treated (T70M positive settings). In 37 patients treated to date there was 100% disease control. The PR rate in previously treated patients was 75%. The PR rate in patients with brain metastases was 50% [Citation80].
5. Safety profile of EGFR TKIs
Across studies, treatment with EGFR TKIs has been associated with specific adverse events (AEs) related to inhibition of this key pathway. Not surprisingly, approved TKIs and those in development share similar safety profiles in the treatment of NSCLC; a summary of EGFR TKI–related AEs is provided in . The most commonly reported AEs with EGFR TKIs are mainly gastrointestinal and dermatologic in nature and include diarrhea, rash, mucosal inflammation (e.g. stomatitis, mucositis), and paronychia [Citation81]. Rates of diarrhea and stomatitis appear to be greater with afatinib and dacomitinib compared to erlotinib or gefitinib, possibly reflecting their broader inhibitory profiles. Gefitinib appears to also have a slightly lower rate of dermatologic AEs compared to erlotinib, afatinib and dacomitinib. While rates of these events can be high with EGFR TKIs compared to cytotoxic chemotherapy, the vast majority of events are of low severity and can be managed with appropriate supportive care [Citation81]. Of note, tolerability-guided dose adjustment appears to reduce the incidence of AEs associated with afatinib, dacomitinib and gefitinib without compromising PFS [Citation82–Citation84]. For example, a recent retrospective analysis of 228 patients with EGFR mutation-positive NSCLC who received first-line afatinib in a ‘real world’ clinical setting demonstrated that while dose reductions were common (67% of patients) and effectively reduced frequency and intensity of adverse drug reactions, they did not adversely affect time on treatment or time to progression [Citation85]. Furthermore, an observational study in Japanese patients (n = 22) suggested that erlotinib can be dose-adjusted for body surface area and poor performance status without a significant effect on survival [Citation86].
Table 3. Rates of common EGFR TKI–associated AEs from key randomized NSCLC trials.
As osimertinib was engineered to specifically inhibit mutant forms of EGFR, and spare wild-type EGFR, it had a better tolerability profile than gefitinib in the FLAURA trial; overall, the frequency of Grade ≥ 3 AEs was 34% and 45%, respectively [Citation46]. The most frequent AEs were rash/acne (all grades: 58% vs 78%; Grade ≥ 3: 1% vs 7%), diarrhea (all grades: 58% vs 57%; Grade ≥ 3: 2% vs 2%), and dry skin (all grades: 36% vs 36%; Grade ≥ 3: <1% vs 1%).
Of note, effective management of AEs with dose-reduction schemes and supportive care allow most patients to remain on TKI therapy for as long as they experience clinical benefit. In clinical trials, discontinuations due to treatment-related AEs were generally low (around 10% or less) [Citation2,Citation3,Citation9,Citation20,Citation22,Citation31–Citation33,Citation41]
6. Advances in EGFR mutation testing
With the increased number of agents in development targeting specific resistance mutations in NSCLC, as well as our increased understanding of the mechanisms that drive EGFR TKI resistance, it is becoming increasingly important to re-biopsy tumors at subsequent stages of treatment. In recent years, the development of techniques to isolate CTCs and cftDNA from liquid biopsies has provided less invasive methods for detecting EGFR mutations in NSCLC patients [Citation87]. Less invasive tumor sampling allows for repeat biopsies, which can guide treatment selection and monitor therapeutic efficacy. Also, liquid biopsy techniques facilitate mutation testing when tissue biopsy or repeat biopsies are not possible or refused.
Liquid biopsies can potentially be obtained from blood, saliva, or urine [Citation88], and cftDNA or DNA isolated from CTCs can undergo EGFR mutational testing by a variety of methods. Studies have demonstrated correlation between EGFR mutations detected in plasma and urine tumor DNA and mutations detected in tissue samples, suggesting cftDNA is a potentially valid screening tool for EGFR mutation detection [Citation89–Citation91]. Furthermore, in a prespecified analysis of the EURTAC trial, L858R EGFR mutations detected in cftDNA were associated with significantly shorter OS compared with del19 EGFR mutations [Citation92]. The FDA approved testing of peripheral blood for del19/L858R mutations in June 2016 and the T790M mutation in September 2016.
Many liquid biopsy platforms are now available, and the advantages/disadvantages of specific assays have been reviewed in detail elsewhere [Citation93]. At present, few assays have been reliably and prospectively validated, so questions remain about their clinical applicability. In general, while current assays have high specificity, sensitivity is more limited [Citation93]. Consequently, mutations detected by liquid biopsy are actionable but negative results should be followed by tumor biopsy testing in order to confirm the negative result [Citation93,Citation94]. Also, liquid biopsy does not provide information on possible coexisting resistance mechanisms. For example, while plasma assays can detect T790M, they will not detect cases of concomitant small-cell transformation [Citation95]. Despite these limitations, liquid biopsy can play an important complementary role to standard tissue biopsy and may, in cases, assist when tissue biopsy is unavailable at initial screening [Citation96].
7. Expert opinion
EGFR TKIs are an important component of the NSCLC treatment landscape. Resistance to EGFR TKIs is inevitable, and effective treatment of resistant tumors depends on the ability to easily obtain repeat tumor samples. Non-invasive liquid biopsy methods are more convenient for repeated sampling, and we need to incorporate cftDNA testing into the NSCLC diagnostic pathway to improve our ability to identify the patients who will benefit from EGFR-TKI therapy.
The promising efficacy of the combination of erlotinib and bevacizumab in NSCLC underscores the need to explore newer combinations of EGFR TKIs with other targeted agents. EGFR TKIs are being evaluated in NSCLC in combination with programmed death 1 (PD1) and PD-L1 inhibitors, including nivolumab, pembrolizumab, and durvalumab. Subgroup analyses and a subsequent meta-analysis have demonstrated that the HRs for survival outcomes in EGFR mutation-positive NSCLC patients favored docetaxel rather than immune checkpoint inhibitors (nivolumab, atezolizumab, or pembrolizumab) [Citation97–Citation99], suggesting that patients with EGFR mutation-positive tumors may not respond well to PD1 and PD-L1 inhibitors when compared to wild-type. Indeed, a recent phase II study of 11 patients with EGFR mutation-positive NSCLC treated with pembrolizumab indicated that monotherapy with checkpoint inhibitors is not an appropriate treatment choice in this setting [Citation100]. Combination of checkpoint inhibitors with EGFR TKIs in EGFR mutation-positive patients may overcome this observation, though this remains to be seen. Of note, preliminary data suggest an increased risk of interstitial lung disease after sequential or combination treatment with a PD1/PD-L1 inhibitor (e.g. nivolumab, durvalumab) and osimertinib and thus further investigation is warranted [Citation101–Citation104].
The approval of osimertinib and continuing clinical development of mutation-specific EGFR TKIs aim to target the emergence of EGFR resistance mutations, such as T790M, a common mechanism of resistance to EGFR-TKI therapy. Furthermore, with increased use of T790M mutation-specific EGFR TKIs, additional resistance mechanisms will need to be identified and targeted. There is a need to incorporate comprehensive tumor genomic testing in the clinic to identify emerging resistance to EGFR TKIs, which includes not only T790M mutations but also other mechanisms such as MET and HER2 amplification [Citation4], which can be targeted with currently available agents. Also, it is becoming clear that acquired resistance to the mutation-selective EGFR TKIs (e.g. osimertinib) via C797S mutation represents a clinical challenge, and EAI045 is in development to target this particular resistance mechanism. Osimertinib has recently demonstrated striking clinical activity and favorable tolerability in a first-line setting, and is thus a first-line treatment of choice in patients with EGFR mutation-positive NSCLC. However, targeted therapeutic options following osimertinib are unclear at present because resistance mechanisms are not clearly defined at this time. There is some discussion whether to reserve osimertinib for second-line use, since the majority of patients treated with first- or second-generation TKIs develop T790M-positive tumors. Post-hoc analysis of LUX-Lung 7 demonstrated that around 90% of 43 patients treated sequentially with afatinib or gefitinib followed by third-generation EGFR TKIs survived for at least 3 years [Citation44]. Furthermore, in a recent observational study of 204 patients who received sequential afatinib and osimertinib, overall median time on treatment was 27.6 months overall, 30.3 months in patients with tumors harboring an EGFR del19 mutation, and 46.7 months in Asian patients [Citation8]. A recent study that utilized a highly sensitive droplet-digital PCR liquid biopsy technique indicated that the frequency of T790M following afatinib may be as high as 73%, with some tumors having a low allelic frequency of T790M below the detection threshold of other platforms [Citation6]. Importantly, response to osimertinib was independent of the allelic frequency of T790M in this study. Therefore, sequential afatinib followed by osimertinib may be an option for some patients with EGFR mutation-positive NSCLC and could be facilitated by wider availability of sensitive liquid biopsy assays. Recent exploratory analysis of ARCHER 1050 demonstrated that dacomitinib confers an OS benefit in a first-line setting, the first TKI to demonstrate such a benefit against another TKI [Citation49]. Only 22 patients received a sequential third-generation TKI after dacomitinib, but OS (median of 36.7 months) in these patients was encouraging [Citation49], thus suggesting that sequential dacomitinib and osimertinib could also be an attractive strategy. However, there are few data regarding the CNS activity of dacomitinib and real-world data assessing the activity of dacomitinib are limited at present.
While there is some evidence to support reserving osimertinib for second-line use, these findings have to be balanced with the impressive improvement in PFS in patients receiving front-line osimertinib compared to erlotinib and gefitinib in the FLAURA trial. In addition, data from the FLAURA trial indicates that osimertinib has a better toxicity profile and CNS penetration than first-generation EGFR TKIs [Citation3,Citation52]. Also, up to a third of the patients receiving front-line EGFR-TKI therapy in Phase III trials do not go on to receive any second-line therapy [Citation105]. Furthermore, if reserved for second-line use, a proportion of patients, i.e. those who develop T790M-independent mechanisms of acquired resistance, will not be eligible to receive osimertinib and will therefore not benefit from its favorable efficacy and tolerability. Thus, there is a risk of reserving osimertinib for second-line use. Ultimately, further studies will help determine whether there is any benefit in sequencing osimertinib after second-generation EGFR TKIs rather than using it in a first-line setting.
In the last few years there has been significant progress in several fronts against EGFR TK mutation-positive NSCLC including the ability to non-invasively detect EGFR TK mutations and the development of third-generation EGFR TKIs. However, we need to better understand the resistance mechanisms to EGFR-TKIs, and develop effective strategies to target them with the eventual goal of making EGFR mutation-positive NSCLC a chronic disease.
8. Five-year view
The development of EGFR TKIs represented a quantum leap in terms of treatment options for patients with EGFR mutation-positive NSCLC. A little over a decade ago, these patients received front-line platinum doublet chemotherapy. Even contemporary doublets such as pemetrexed-cisplatin would only offer median OS of 12 months or less [Citation106]. EGFR TKIs have eliminated chemotherapy in the front-line setting for these patients, and each new generation of drug improves on the last. Given the ever expanding armamentarium of EGFR TKIs, including osimertinib, improvements in the understanding of resistance mechanisms to different agents, and technological developments in the molecular analysis of tumors, it is likely that median OS rates of at least three years will become commonplace, as effective sequential regimens are employed according to individual patient and tumor characteristics. As such, EGFR mutation-positive NSCLC could become a chronic manageable disease in many patients.
Key issues still need to be addressed, not least of which is that no targeted treatment options have been defined following osimertinib failure. It is likely that such treatment options will be identified within the next five years such as fourth-generation EGFR TKIs to target tertiary EGFR mutations or novel combination regimens in patients with EGFR independent resistance mechanisms.
Key issues
EGFR TKIs are undoubtedly the first-line treatment of choice for patients with EGFR mutation-positive NSCLC.
Five agents are currently available to clinicians: the first-generation reversible EGFR TKIs, erlotinib and gefitinib; the second-generation irreversible ErbB family blockers, afatinib and dacomitinib, and the irreversible wild-type sparing EGFR TKI, osimertinib.
Head-to-head clinical trial data have demonstrated that second- and third-generation TKIs are superior to first-generation EGFR TKIs in terms of PFS improvement. Dacomitinib has demonstrated OS advantage over gefitinib. Osimertinib OS data are immature.
Regardless of which agent is chosen as first-line therapy, most patients will become resistant to therapy so the availability of treatment options beyond progression is an important consideration.
At least half of patients treated with first- and second-generation EGFR TKIs acquire the T790M resistance mutation which can be effectively treated with second-line osimertinib. Resistance mechanisms to osimertinib are heterogeneous.
Targeted treatment options after failure of osimertinib, and for patients with T790M-independent acquired resistance to first- and second-generation EGFR TKIs, are an unmet medical need.
Further development of liquid biopsy techniques may help to facilitate the screening and monitoring of tumor evolution in the clinic thus optimizing sequential treatment.
Declaration of interest
J Subramanian has served on the advisory boards for AstraZeneca, Bristol-Myers Squibb, Alexion, Pfizer, and Boehringer Ingelheim. They have also served on speaker bureaus for Lilly, AstraZeneca, and Boehringer Ingelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Lauren Fink, of MedErgy and Lynn Pritchard, of GeoMed, an Ashfield Company, part of UDG Healthcare plc, for their medical writing assistance.
Additional information
Funding
References
- Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589.
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125.
- Stewart EL, Tan SZ, Liu G, et al. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res. 2015;4(1):67–81.
- Ramalingam SS, Cheng Y, Zhou C et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29(Suppl 9):ix173-ix178.
- Hochmair MJ, Buder A, Schwab S, et al. Liquid-biopsy-based identification of EGFR T790M mutation-mediated resistance to afatinib treatment in patients with advanced EGFR mutation-positive NSCLC, and subsequent response to osimertinib. Target Oncol. 2019;14(1):75–83.
- Hirsh V. Turning EGFR mutation-positive non-small-cell lung cancer into a chronic disease: optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther Adv Med Oncol. 2018;10:1758834017753338.
- Hochmair MJ, Morabito A, Hao D, et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 2018;27:2861–2874.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- Rosell R, Moran T, Carcereny E, et al. Non-small-cell lung cancer harbouring mutations in the EGFR kinase domain. Clin Transl Oncol. 2010;12(2):75–80.
- Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018;11:1117–1132.
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371–2376.
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967.
- Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346.
- Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12(21):6494–6501.
- Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12(19):5764–5769.
- Wu SG, Liu YN, Tsai MF, et al. The mechanism of acquired resistance to irreversible EGFR tyrosine kinase inhibitor-afatinib in lung adenocarcinoma patients. Oncotarget. 2016;7(11):12404–12413.
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–2874.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957.
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer. 2014;110(1):55–62.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388.
- Nakamura A, Inoue A, Morita S, et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). Ann Oncol. 2018;29(suppl_8):viii493-viii547.
- Tu HY, Ke EE, Yang JJ, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96–102.
- Watanabe S, Minegishi Y, Yoshizawa H, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol. 2014;9(2):189–194.
- Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–3821.
- Chiu CH, Yang CT, Shih JY, et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol. 2015;10(5):793–799.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11(6):521–529.
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246.
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742.
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244.
- European Medicines Agency. Avastin® (bevacizumab) summary of product characteristics; 2009 [cited 2019 Apr 29]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf (2009).
- Zhang S, Mao XD, Wang HT, et al. Efficacy and safety of bevacizumab plus erlotinib versus bevacizumab or erlotinib alone in the treatment of non-small-cell lung cancer: a systematic review and meta-analysis. BMJ Open. 2016;6(6):e011714.
- Ioannou N, Seddon AM, Dalgleish A, et al. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF-IR inhibitor, NVP-AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer. 2013;13:41.
- Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–4711.
- Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342–350.
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222.
- Yang JC-H, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838.
- Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11(3):380–390.
- Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270–277.
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907.
- Gonzales AJ, Hook KE, Althaus IW, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2008;7(7):1880–1889.
- Jänne PA, Ou SH, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15(13):1433–1441.
- Food And Drug Administration (FDA). Highlights of Prescribing Information. VIZIMPRO® (dacomitinib); 2018 [cited 2019 Apr 29]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211288s000lbl.pdf
- Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250.
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061.
- Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014;57(20):8249–8267.
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(33):3290–3297.
- Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140.
- Lim SW, Park S, Kim Y, et al. Continuation of gefitinib beyond progression in patients with EGFR mutation-positive non-small-cell lung cancer: A phase II single-arm trial. Lung Cancer. 2018;124:293–297.
- Le X, Puri S, Negrao MV, et al. Landscape of EGFR -dependent and -independent resistance mechanisms to osimertinib and continuation therapy post-progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24:6195–6203.
- Faehling M, Eckert R, Kamp T, et al. EGFR-tyrosine kinase inhibitor treatment beyond progression in long-term Caucasian responders to erlotinib in advanced non-small cell lung cancer: a case-control study of overall survival. Lung Cancer. 2013;80(3):306–312.
- Lin Y, Wang X, Jin H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am J Cancer Res. 2014;4(5):411–435.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26.
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699.
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17(12):1643–1652.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640.
- Corallo S, D’Argento E, Strippoli A, et al. Treatment options for EGFR T790M-negative EGFR tyrosine kinase inhibitor-resistant non-small cell lung cancer. Target Oncol. 2017;12:153–161.
- Ou Q, Wu X, Bao H, et al. Investigating novel resistance mechanisms to third generation EGFR TKI osimertinib in non-small cell lung cancer patients using next generation sequencing. J Clin Oncol. 2017;35(15_suppl):2572.
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:841–849.
- Song HN, Jung KS, Yoo KH, et al. Acquired C797S mutation upon treatment with a T790M-specific third-generation EGFR inhibitor (HM61713) in non-small cell lung cancer. J Thorac Oncol. 2016;11(4):e45–e47.
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560–562.
- Jia Y, Yun CH, Park E, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature. 2016;534(7605):129–132.
- Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015;21(17):3913–3923.
- Niederst MJ, Hu H, Mulvey HE, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res. 2015;21(17):3924–3933.
- Hidaka N, Iwama E, Kubo N, et al. Most T790M mutations are present on the same EGFR allele as activating mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;108:75–82.
- Tan F, Shi Y, Wang Y, et al. Icotinib, a selective EGF receptor tyrosine kinase inhibitor, for the treatment of non-small-cell lung cancer. Future Oncol. 2015;11(3):385–397.
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953–961.
- Hu X, Zhang L, Shi Y, et al. The efficacy and safety of icotinib in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a single-arm, multi-center, prospective study. PLoS One. 2015;10(11):e0142500.
- Liu D, Zhang L, Wu Y, et al. Clinical pharmacokinetics, safety, and preliminary efficacy evaluation of icotinib in patients with advanced non-small cell lung cancer. Lung Cancer. 2015;89(3):262–267.
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450.
- Shi Y, Sun Y, Ding C, et al. China experts consensus on icotinib for non-small cell lung cancer treatment (2015 version). J Thorac Dis. 2015;7(10):e468–e472.
- Lelais G, Epple R, Marsilje TH, et al. Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imid azol-2-yl)-2-methylisonicotinamide (EGF816), a novel, potent, and WT sparing covalent inhibitor of oncogenic (L858R, ex19del) and resistant (T790M) EGFR mutants for the treatment of EGFR mutant non-small-cell lung cancers. J Med Chem. 2016;59(14):6671–6689.
- Tan DSW, Seto T, Leighl NB, et al. First-in-human phase I study of EGF816, a third generation, mutant-selective EGFR tyrosine kinase inhibitor, in advanced non-small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol. 2015;33(15_suppl):8013.
- Johnson M, Karlix J, Burris H, et al. OA02.05 CK-101 (RX518), a third generation mutant-selective inhibitor of EGFR in NSCLC: results of an ongoing phase I/II trial. J Thorac Oncol. 2018;13(10):S323.
- Melosky B, Hirsh V. Management of common toxicities in metastatic NSCLC related to anti-lung cancer therapies with EGFR-TKIs. Front Oncol. 2014;4:238.
- Satoh H, Inoue A, Kobayashi K, et al. Low-dose gefitinib treatment for patients with advanced non-small cell lung cancer harboring sensitive epidermal growth factor receptor mutations. J Thorac Oncol. 2011;6(8):1413–1417.
- Yang JC, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol. 2016;27(11):2103–2110.
- Mok T, Nakagawa K, Rosell R, et al. MA26.11 effects of dose modifications on the safety and efficacy of dacomitinib for EGFR mutation-positive NSCLC. J Thorac Oncol. 2018;13:S454.
- Halmos B, Tan E-H, Lee MK, et al. Real-world dose adjustment study of first-line afatinib in pts with EGFR mutation-positive (EGFRm+) advanced NSCLC. J Clin Oncol. 2018;36(suppl_15):e21060.
- Inagaki M, Shinohara Y, Kaburagi T, et al. Efficacy of first-line erlotinib in non-small cell lung cancer patients undergoing dose reduction and those with a low body surface area: A population-based observational study by the Ibaraki Thoracic Integrative (POSITIVE) Research Group. Mol Clin Oncol. 2016;4(3):425–428.
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377.
- Bianchi F. Molecular profile of liquid biopsies: next generation biomarkers to improve lung cancer treatment. Ecancermedicalscience. 2015;9:598.
- Lam DC, Tam TC, Lau KM, et al. Plasma EGFR mutation detection associated with survival outcomes in advanced-stage lung cancer. Clin Lung Cancer. 2015;16(6):507–513.
- Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep. 2014;4:6269.
- Reckamp KL, Melnikova VO, Karlovich C, et al. A highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasma. J Thorac Oncol. 2016;11(10):1690–1700.
- Karachaliou N. Mayo-de las CC, Queralt C et al. Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol. 2015;1(2):149–157.
- Sacher AG, Komatsubara KM, Oxnard GR. Application of plasma genotyping technologies in non-small cell lung cancer: a practical review. J Thorac Oncol. 2017;12(9):1344–1356.
- Normanno N, Denis MG, Thress KS, et al. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget. 2017;8(7):12501–12516.
- Minari R, Bordi P, Del Re M, et al. Primary resistance to osimertinib due to SCLC transformation: issue of T790M determination on liquid re-biopsy. Lung Cancer. 2018;115:21–27.
- Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(28):3375–3382.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265.
- Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–1145.
- Ahn MJ, Yang J, Yu H, et al. 136O: osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol. 2016;11(4 Suppl):S115.
- Fujiwara Y, Goto Y, Kanda S, et al. Efficacy and safety of osimertinib in a Japanese compassionate use program. Jpn J Clin Oncol. 2017;47(7):625–629.
- Kotake M, Murakami H, Kenmotsu H, et al. High incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapy. Ann Oncol. 2017;28(3):669–670.
- Mamesaya N, Kenmotsu H, Katsumata M, et al. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Invest New Drugs. 2017;35(1):105–107.
- Sequist L, Wu Y, Schuler M, et al. Subsequent therapies post-afatinib among patients (pts) with EGFR mutation-positive (EGFRm+) NSCLC in LUX-Lung (LL) 3, 6 and 7. Ann Oncol. 2017;28(suppl_5):v460–v496.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551.
