1. Introduction
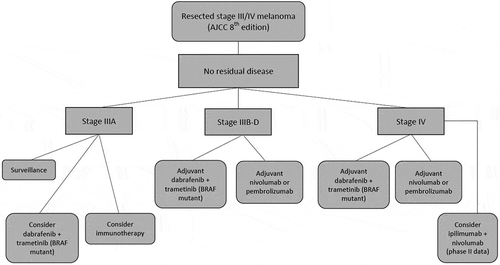
Patients with melanoma who have regional nodal or in-transit involvement at diagnosis (stage III) are at a high risk for recurrence, with the 5-year survival ranging from 93% for IIIA to 32% for IIID disease [Citation1]. Highly selected patients with resected distant metastases (stage IV) have an even higher chance of recurrence and death [Citation2]. In the last decade, immune checkpoint inhibitors and targeted therapy with BRAF plus MEK inhibitors have improved outcomes for patients with metastatic melanoma dramatically, with 5-year overall survival increasing from 10% to now over 50% with combination immunotherapy [Citation3]. These treatments have now moved into the earlier, post-surgical (adjuvant) setting, aiming to reduce recurrence and improve survival similarly ().
Table 1. Summary of published clinical trials for adjuvant systemic therapy in melanoma
Ipilimumab was the first immune checkpoint inhibitor shown to improve survival over placebo in the adjuvant setting (HR 0.75); however, toxicity was a major barrier [Citation4,Citation5]. More recently, the Programmed cell death protein-1 (PD-1) inhibitors nivolumab and pembrolizumab have demonstrated an improvement in recurrence-free survival (RFS) over ipilimumab (HR 0.71) and placebo (HR 0.56), respectively [Citation6,Citation7]. The toxicity profile of these agents is also favorable compared to ipilimumab.
For patients with BRAF V600 mutation-positive melanoma, the COMBI-AD study showed a RFS benefit with adjuvant dabrafenib and trametinib over placebo (HR 0.51) [Citation8,Citation9]. The initial study report also suggested an overall survival (OS) benefit; however, this did not meet the pre-specified P value for significance [Citation10–12].
With the use of these treatments in the ‘real world’ setting comes new challenges for prescribers and our patients. We provide our opinion on how these can be approached.
2. How do we relate the data to the new AJCC 8th edition staging system?
Careful sub-staging and assessment of recurrence risk are important when recommending adjuvant therapy. The clinical trials described above used the American Joint Committee on Cancer (AJCC) 7th edition to group patients, but have since re-analyzed data using the AJCC 8th edition [Citation1]. Trials that recruited AJCC 7th edition IIIA patients required a > 1 mm deposit in the sentinel node.
Patients with stage IIIA disease according to the current AJCC 8th edition have a 5-year melanoma-specific survival (MSS) of 93% compared to 78% with the AJCC 7th edition [Citation1]. Real-world recurrence rates are certainly higher than this, with a recent analysis of tumor registry data of European melanoma patients showing a 5-year MSS of 73–80% for patients with stage IIIA disease [Citation13]. The favorable survival seen with surgery alone and the durable activity of immunotherapy in the metastatic setting may mean the risk/benefit profile of adjuvant therapy in IIIA melanoma favors observation for most. Re-analyses of the Keynote-054 (15-month follow up) and COMBI-AD (44-month follow up) studies using the AJCC 8th edition further suggest limited benefit for adjuvant therapy for patients with stage IIIA disease (although follow-up is short at this time) [Citation11,Citation12,Citation14,Citation15]. In some countries, adjuvant therapy is not reimbursed for IIIA disease, however given the real-world recurrence rates seen it should be discussed with patients and may be recommended in certain scenarios, particularly where patients are at risk of inappropriate downstaging due to not undergoing a completion node dissection (e.g. a 1–2 mm non-ulcerated primary with 1–3 sentinel nodes, T2aN2a).
3. Which adjuvant treatment should we choose if the disease is BRAF mutation positive?
For patients with BRAF mutation-positive melanoma, adjuvant therapy options include immunotherapy with nivolumab or pembrolizumab or targeted therapy with dabrafenib plus trametinib (see ). In the metastatic setting, targeted therapy provides better responses in the short term; however, immunotherapy leads to better overall survival outcomes longer term. Longer follow-up of the adjuvant trials is eagerly awaited, but it is predicted by many that similar patterns will emerge.
There are no direct comparator studies of these two approaches in the adjuvant setting. The European Society of Medical Oncology (ESMO) consensus conference recommendations compare the results from the Keynote-054 and COMBI-AD trials, as these have similar study populations and were conducted at similar times [Citation16]. Treatment with 12 months of targeted therapy has a proven RFS benefit and a suggestion of an OS benefit in the adjuvant setting, although this didn’t meet pre-specified P value for significance [Citation10]. The PD-1 inhibitor studies have shorter follow-up and have not yet reported OS data, but it is anticipated that a benefit will be seen given the long-term RFS benefit and survival benefit seen with adjuvant ipilimumab. Sub-group analyses of the immunotherapy studies have shown a benefit in both BRAF mutation-positive and wild type patients [Citation17,Citation18].
Treatment selection therefore should be guided by sub-stage, patient co-morbidities (e.g. autoimmune conditions), patient preference regarding potential toxicities, and access to available treatments.
4. What about ‘second adjuvant’ therapy?
Many patients treated with adjuvant therapy recur with resectable disease during or after a course of adjuvant therapy. At present, there are no clinical trial data to guide management in this scenario, yet it is an increasingly common situation in the clinic, and particularly important for patients with BRAF mutant melanoma who may be able to receive a second alternate adjuvant therapy.
Emphasizing the need for ‘second adjuvant’ treatment in this setting, a retrospective study found that 43% of patients who recur during or after adjuvant PD-1 inhibitor have locoregional disease only, but 37% of these patients then develop distant disease soon after [Citation19]. Given that the risk of further (distant) recurrence is high and that there is no evidence to suggest that patients become cross-resistant to both targeted and immunotherapies, BRAF mutant patients who recur despite one therapy should be considered for the alternative ‘second adjuvant’ therapy (e.g. initial immunotherapy, subsequent targeted therapy) [Citation16,Citation20].
5. How should unresectable recurrence on adjuvant therapy be managed?
Unresectable recurrence on adjuvant therapy is a significant and increasingly common problem. A recent study of patients with early recurrence, after a median 4.6 months (76% recurred during adjuvant therapy), reported that 57% had distant disease (with or without concurrent local recurrence) [Citation19]. In this scenario, for patients who recur during or after PD-1 inhibitor treatment, combination immunotherapy with ipilimumab plus nivolumab or targeted therapy with BRAF/MEK inhibitors had the highest response rates. Targeted therapy can provide symptom palliation due to rapid responses but is unlikely to provide a long-term survival benefit, and there is also considerable toxicity seen with targeted therapy given after immunotherapy, and often the need for dose modifications [Citation21]. Combination immunotherapy may have a more durable response, but data are unavailable at present [Citation22].
6. Adjuvant therapy toxicity
The acceptance for serious toxicity should be less for patients treated in the adjuvant setting compared to the metastatic setting as a proportion of patients will already be cured by surgery.
Toxicities from immunotherapy can be serious and for some patients, irreversible. There are no reliable biomarkers to predict patients at higher risk of toxicity. It is important when counseling patients about adjuvant immunotherapy that they are aware of the risk of reversible and also permanent toxicity, such as the need for lifelong hormone replacement (thyroid 10%, steroid 1%, insulin 0.5–1%), or rare permanent toxicities such as neuropathies. All toxicities may have long-term sequelae [Citation23]. In the adjuvant setting, comprehensive patient education around reporting symptoms of toxicity with diligent clinician monitoring is imperative. Many clinicians will consider stopping treatment early for non-endocrine toxicity, and it should be stressed to patients that discontinuation may not negatively impact outcomes, rather those who develop toxicity may be less at risk of recurrence [Citation24].
For targeted therapy, permanent toxicities are rare; however, 26% of patients on the adjuvant clinical trial had adverse events leading to treatment discontinuation [Citation10]. These were largely fever/pyrexia, and in clinical practice, early interruption of therapy, treatment breaks, intermittent dosing schedules, and courses of corticosteroids may be required to prevent recurrent pyrexia and prevent early discontinuation. The lower risk of long-term toxicity makes it a favorable choice for some patients in the adjuvant setting, particularly those of lower sub-stage.
There are also more patients of reproductive age being treated with adjuvant therapy, but as yet, there is limited evidence around their effects on fertility. Anecdotally, it is not thought to impact future fertility but a cautious approach is needed, and fertility preservation should be discussed. Also, the unknown effects that may occur if a pregnancy ensues whilst on treatment should be discussed, and hence this would be cautioned against.
7. Dosing schedules for adjuvant immunotherapy
The adjuvant immunotherapy studies used 2-weekly dosing of nivolumab and 3-weekly dosing of pembrolizumab. There is now evidence for similar exposure–response relationships and safety with 4-weekly nivolumab and 6-weekly pembrolizumab dosing in the metastatic setting [Citation25–28]. This is thought to be a reasonable approach to improve convenience and reduce hospital chair time, however with less frequent scheduling comes a greater need for patients to be conscious of reporting toxicity.
It is also uncertain what the most desired length of treatment is. Given its mechanism of action, shorter courses of immunotherapy may be appropriate, and this question warrants further study. It has been seen that patients stopping early due to toxicity do well, and hence stopping due to appropriate toxicity should not be of concern [Citation24]. Of interest, six weeks of therapy in the neoadjuvant setting, and no adjuvant therapy, appear sufficient for most patients, suggesting much shorter courses of adjuvant immunotherapy may be appropriate, and should be tested [Citation29].
8. Should patients with stage IIB and IIC melanoma receive adjuvant therapy?
Patients with stage IIB and IIC disease have a poorer 5-year and 10-year melanoma-specific survival than those with stage IIIA disease [Citation1]. The Keynote-716 study (NCT03553836) is currently evaluating the use of adjuvant pembrolizumab in this patient group, as is the CheckMate-76K trial of nivolumab (NCT04099251).
9. Will the current practice not to perform an immediate completion lymph node dissection affect outcomes?
Completion lymph node dissection (CLND) following the detection of occult (sentinel node positive) disease is no longer the standard of care, after two prospective studies failed to demonstrate an overall survival benefit [Citation30,Citation31]. The initial trials of adjuvant systemic therapies for melanoma required all patients to undergo a completion lymphadenectomy. This approach may potentially downstage patients, and there is the possibility that more locoregional (nodal) recurrences may be seen; however, adjuvant therapies should similarly reduce locoregional and distant recurrence. Recent real-world data for patients with a positive SLNB who underwent adjuvant systemic therapy and no CLND showed similar RFS outcomes to those seen in the adjuvant therapy trials [Citation32]. Some centers recommend regular ultrasound monitoring of nodal basins in those who do not undergo CLND, however, whether this adds value over regular PET/CT scans remains unknown at this time.
It should be noted that all patients who have macroscopic (palpable) lymph node disease should still undergo a completion lymphadenectomy; however, several centers have neoadjuvant clinical trials underway for this scenario. A comparison between outcomes using neoadjuvant with or without adjuvant systemic therapy is also needed.
10. What about non-cutaneous melanoma?
The Checkmate-238 study was the only adjuvant trial that included patients with non-cutaneous melanoma. Patients with mucosal melanoma were allowed, but not uveal melanoma patients. Mucosal melanoma patients made up only 7% of the total study cohort. Data in the metastatic setting suggest combination immunotherapy has much higher efficacy for mucosal melanoma [Citation33]. Adjuvant nivolumab is not an unreasonable consideration in this setting; however, one must be mindful of the impact of using adjuvant nivolumab may have on later options should patients recur during or soon after completing treatment.
11. How should we manage adjuvant therapy for other special patient groups?
Specific patient groups are often excluded from melanoma clinical trials due to concerns regarding the risks of the concomitant illness with the addition of the trial treatment. Patients with autoimmune diseases, viral hepatitis or HIV infection, chronic lymphocytic leukemia, a history of pneumonitis or any serious or unstable medical condition were excluded from the adjuvant melanoma trials.
However, these patients are increasingly being referred to oncology outpatient clinics, and many are likely to benefit from adjuvant therapy. There is increasing retrospective evidence that immunotherapy and targeted therapy are safe and efficacious in these patient populations [Citation34]. Therefore, oncologists will need to weigh up the individual patient’s risk of melanoma recurrence versus the risk of worsening the other health conditions. Collaboration with other oncologists and medical specialists will be crucial to ensuring that the best possible patient outcomes are delivered safely.
12. Future directions
Neoadjuvant systemic therapy in melanoma is not yet an established treatment pathway; however, a number of studies using various agents have been reported [Citation35–39]. Neoadjuvant therapy may have numerous advantages, including reducing tumor burden to facilitate resection and providing information regarding pathological response (which has been used as a surrogate endpoint of improved survival in the treatment of other cancers). This needs to be weighed against the risks of treatment toxicity and surgery delay. Patients who might be suitable for this approach are those with clinically apparent resectable stage IIIB to IIID disease and oligometastatic stage IV disease, and treatment duration should be 6 to 8 weeks [Citation40]. Close clinical and radiological monitoring for potential progression during neoadjuvant therapy is recommended.
The results of the Checkmate 915 study are awaited to see whether the addition of ipilimumab to nivolumab in the adjuvant setting improves RFS. The recently published phase II IMMUNED study of patients with resected stage IV melanoma showed a significant improvement in 2-year RFS with adjuvant ipilimumab plus nivolumab compared to placebo (HR 0.23), particularly in BRAF mutant patients; however, treatment-related adverse events leading to discontinuation occurred in 62% [Citation41]. Trials are also underway adding further agents to an adjuvant nivolumab backbone.
13. Conclusion
Treatment of patients with resected melanoma using immunotherapy or targeted therapy in the ‘real world’ setting comes with various challenges but significant rewards. Further studies are being undertaken using the updated staging system, stage II disease, and combination immunotherapy, and we await these results. As more experience is gained, it will be important to develop a standardized approach to managing these patients.
Declaration of interest
A Menzies has served on the advisory board for BMS, MSD, Novartis, Roche, Pierre Fabre and is supported by a Cancer Institute NSW Fellowship and Melanoma Institute Australia. R Roberts-Thomson has received honorarium from Novartis, BMS, MSD, Roche, has served on the advisory board for Novartis and has conference attendance with MSD. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers
A peer reviewer on this paper has received honoraria for lectures from and served on the advisory boards for Novartis, BMS, MSD, Pierre Fabre, Amgen, Sanofi, and Blueprint Medicines. Peer reviewers have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492.
- Hsueh EC, Essner R, Foshag LJ, et al. Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine. J Clin Oncol. 2002;20(23):4549–4554.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530.
- Eggermont AMM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer. 2019;119:1–10.
- Eggermont AMM, Blank CU, Mandala M, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol. 2020;JCO2002110. DOI:10.1200/JCO.20.02110
- Ascierto PA, Del Vecchio M, Mandala M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020. DOI:10.1016/S1470-2045(20)30494-0
- Hauschild A, Dummer R, Schadendorf D, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441–3449.
- Dummer R, Hauschild A, Santinami M, et al. Five-year analysis of adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2020;383(12):1139–1148.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823.
- Hauschild A, Dummer R, Santinami M, et al. Long-term benefit of adjuvant dabrafenib + trametinib (D+T) in patients (pts) with resected stage III BRAF V600–mutant melanoma: five-year analysis of COMBI-AD. J Clin Oncol. 2020;38(15_suppl): 10001-10001. DOI:10.1200/JCO.2020.38.15_suppl.10001
- Hauschild A, Dummer R, Schadendorf D, et al. longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J Clin Oncol. 2018;36(35):3441-3449.
- Garbe C, Keim U, Suciu S, et al. Prognosis of patients with stage III melanoma according to American joint committee on cancer version 8: a reassessment on the basis of 3 independent stage III melanoma cohorts. J Clin Oncol. 2020;38(22):2543–2551.
- Eggermont AMM, Blank CU, Mandala M, et al. Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs placebo in resected high-risk stage III melanoma. Eur J Cancer. 2019;116:148–157.
- Eggermont AM, Blank CU, Mandala M, et al. Pembrolizumab versus placebo after complete resection of high-risk stage III melanoma: new recurrence-free survival results from the EORTC 1325-MG/Keynote 054 double-blinded phase III trial at three-year median follow-up. J Clin Oncol. 2020;38(15_suppl): 10000-10000. DOI:10.1200/JCO.2020.38.15_suppl.10000
- Michielin O, van Akkooi A, Lorigan P, et al. ESMO consensus conference recommendations on the management of locoregional melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol. 2020. DOI:10.1016/j.annonc.2020.07.005
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835.
- Owen CN, Shoushtari AN, Chauhan D, et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy. Ann Oncol. 2020;31(8):1075–1082.
- Bhave P, Pallan L, Atkinson V, et al. Melanoma recurrence after adjuvant targeted therapy: a multicenter analysis. J Clin Oncol. 2020;38(15_suppl): 10016-10016. DOI:10.1200/JCO.2020.38.15_suppl.10016
- Saab KR, Mooradian MJ, Wang DY, et al. Tolerance and efficacy of BRAF plus MEK inhibition in patients with melanoma who previously have received programmed cell death protein 1-based therapy. Cancer. 2019;125(6):884–891.
- Da Silva IP, Ahmed T, Lo S, et al. Ipilimumab (IPI) alone or in combination with anti-PD-1 (IPI+PD1) in patients (pts) with metastatic melanoma (MM) resistant to PD1 monotherapy. J Clin Oncol. 2020;38(15_suppl):10005-10005.
- Tsang VHM, McGrath RT, Clifton-Bligh RJ, et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab. 2019;104(11):5499–5506.
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6(4):519.
- Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28(8):2002–2008.
- Long GV, Tykodi SS, Schneider JG, et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann Oncol. 2018;29(11):2208–2213.
- Lala M, Li TR, de Alwis DP, et al. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer. 2020;131:68–75.
- Lala M, Akala O, Chartash E, et al. Abstract CT042: Pembrolizumab 400 mg Q6W dosing: first clinical outcomes data from Keynote-555 cohort B in metastatic melanoma patients. Cancer Res August 15. 2020;80(16 Supplement) CT042.
- Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20(7):948–960.
- Leiter U, Stadler R, Mauch C, et al. Final analysis of DeCOG-SLT trial: no survival benefit for complete lymph node dissection in patients with melanoma with positive sentinel node. J Clin Oncol. 2019;37(32):3000–3008.
- Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222.
- Farrow NE, Raman V, Williams TP, et al. Adjuvant therapy is effective for melanoma patients with a positive sentinel lymph node biopsy who forego completion lymphadenectomy. Ann Surg Oncol. 2020. Online ahead of print.
- D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(2):226–235.
- Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–376.
- Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19(2):181–193.
- Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654.
- Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–461.
- Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655–1661.
- Long GV, Saw RPM, Lo S, et al. Neoadjuvant dabrafenib combined with trametinib for resectable, stage IIIB-C, BRAF(V600) mutation-positive melanoma (NeoCombi): a single-arm, open-label, single-centre, phase 2 trial. Lancet Oncol. 2019;20(7):961–971.
- Amaria RN, Menzies AM, Burton EM, et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019;20(7):e378–e89.
- Zimmer L, Livingstone E, Hassel JC, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;395(10236):1558–1568.