?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
Chronic lymphocytic leukemia (CLL) management has witnessed a transformative shift with the advent of time-limited venetoclax and anti-CD20 monoclonal antibody (mAb) regimens, as exemplified by the groundbreaking MURANO and CLL14 trials.
Area covered
This article delves into the long-term follow-up data of fixed duration (FD) venetoclax combined with anti-CD20 mAb across various lines of CLL therapy. The data discussed here, not yet available in current literature, was unveiled at the 23rd European Hematological Association (EHA) congress held in Frankfurt in June 2023.
Expert opinion
Combinations of venetoclax with anti-CD20 mAbs represent a compelling therapeutic option due to their finite treatment duration and remarkable achievement of undetectable minimal residual disease (uMRD). This not only ensures more enduring responses but also presents a manageable toxicity profile that suits a broad spectrum of CLL patients, including those who are elderly or less medically fit.
Importantly, the integration of venetoclax/anti-CD20 mAb FD regimens may diminish the likelihood of CLL patients developing target mutations. This, in turn, enhances the potential for eliciting secondary clinical responses upon retreatment with venetoclax. Additionally, from an economic perspective, the cost-effectiveness of targeted therapy may further advocate for the selection of FD therapy as a frontrunner in CLL treatment.
1. Introduction
In recent years, there has been a significant shift in the treatment paradigm for chronic lymphocytic leukemia (CLL), moving away from chemo-immunotherapy (CIT) toward targeted therapies [Citation1–7]. The earliest clinical trial results demonstrated that Ibrutinib, a first-in-class Bruton’s tyrosine kinase inhibitor (BTKi), outperforms both chemotherapy and CIT in terms of progression-free survival (PFS) [Citation1–7]. Subsequently, next-generation BTKis, such as acalabrutinib and zanubrutinib, have shown efficacy at least equivalent to that of ibrutinib with the added advantage of improved toxicity profiles [Citation8–11]. BTKis are administered until disease progression or the occurrence of toxicity. However, despite their outstanding efficacy, some patients experience cardiovascular toxicities and acquire BTK mutations associated with resistance to BTKi therapy [Citation12,Citation13].
In the initial clinical trials demonstrating the efficacy of venetoclax, a BCL2 (B-cell lymphoma 2) inhibitor, the drug was administered continuously until disease progression [Citation14]. Subsequent studies investigated the efficacy of venetoclax in combination with an anti-CD20 monoclonal antibody (mAb) as a time-limited therapy [Citation15,Citation16]. The MURANO and CLL14 trials, two innovative phase 3 studies in which the combinations of venetoclax and an anti-CD20 mAb (rituximab or obinutuzumab) were studied, have now reported long-term outcome data that further support the broad usage of these combinations in clinical practice () [Citation17,Citation18].
This concise review highlights the long-term results of the MURANO and CLL14 studies, recently presented at the 23rd European Hematology Association (EHA) meeting [Citation17,Citation18]. Notably, these updated results provide evidence to support retreatment with venetoclax-based therapies for selected CLL patients
2. Long-term benefits of fixed-duration venetoclax-rituximab (VR) in R/R CLL and evidence for retreatment efficacy
The original design of the MURANO study involved 389 patients with relapsed/refractory (R/R) CLL who were randomly assigned to one of two treatment groups [Citation15]:
The first patient group received 400 mg of venetoclax daily for 2 years in combination with rituximab (VR), with the latter administered monthly for the initial 6 months.
The second group received the combination of bendamustine and rituximab (BR) for 6 months.
Remarkably, in both treatment groups, fewer than 3% of patients had prior exposure to a B-cell receptor inhibitor [Citation15].
The final analysis of the MURANO trial, conducted at a median follow-up of 7 years, primarily focuses on the updated PFS and overall survival (OS) outcome data. Additionally, the evaluation of minimal residual disease (MRD) has been reported for both patient groups: those initially treated in the main study and those who experienced relapse and subsequently underwent retreatment with VR [Citation17]
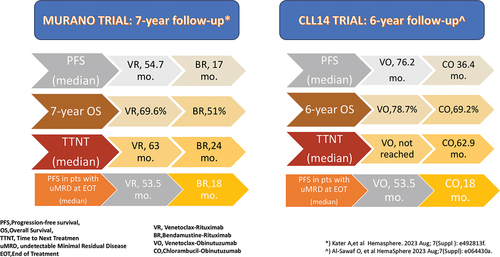
In this long-term analysis, the 194 patients treated with VR experienced a median PFS of 54.7 months, compared to 17.0 months for the 195 BR-treated patients. The hazard ratio (HR) for PFS was 0.23 (95% confidence interval [CI], 0.18–0.29), indicating a significant benefit for VR. The 7-year OS was 69.6% for patients treated with VR and 51.0% for those treated with BR (HR 0.53; 95% CI, 0.37–0.74). Notably, there was a substantial benefit in terms of time to next treatment (TTNT) favoring VR over BR-treated patients. VR-treated patients had a median TTNT of 63.0 months, while BR-treated patients had a median TTNT of 24.0 months, with an HR of 0.30 (95% CI, 0.23–0.39).
The long-term follow-up data also emphasizes the importance of undetectable MRD (uMRD) as a surrogate endpoint for PFS. Among the patients who received VR treatment and achieved uMRD at the end of treatment (EOT) (83 out of 118; 70.3%), the PFS was 52.5 months, in contrast to 18.0 months (P < .0001) for patients who were tested positive for MRD at the EOT (35 out of 118; 29.7% [Citation17].
Among the patients who achieved uMRD at EOT with VR (n = 83), 14 (16.9%) remained free from either progressive disease (PD) or conversion to detectable MRD at the seven-year follow-up. For the 63 patients (75.9%) who underwent MRD conversion, the median time to detectable MRD was 19.4 months. Among this patient cohort, 39 individuals (62%) subsequently experienced either PD or death. The median time from MRD conversion to PD was 28.3 months. This translates into a highly clinically significant four-year treatment-free interval (TFI) before patients require new treatment, demonstrating not only the efficacy of VR but also confirming the highly predictive value of uMRD with the VR combination.
Of note, in this extended follow-up no new safety signals were identified. Rates of Richter’s transformation remained balanced between treatment arms (7 or 3.6% cases in the VR arm and 6 or 3.2% in the BR arm) [Citation17].
A sub-study was opened in 2018 within the original MURANO trial protocol to allow patients who developed progressive disease following treatment with either regimen to receive VR.
Upon disease progression 34 patients were enrolled in this MURANO sub-study. Out of these 34 patients, 25 (73.5%) were re-treated with VR. Notably, the majority (92.0%) of these patients exhibited at least one high-risk feature, such as IGHV-unmutated disease, genomic complexity, or deletion of chromosome 17p and/or TP53 mutations. Despite these high-risk factors, 56.0% (14 out of 25) of patients achieved uMRD at the EOT [Citation17].
Overall, in this final analysis of MURANO, sustained survival benefits are shown with VR over BR. This survival advantage is maintained for up to 5 years after the completion of FD VR treatment with all the patients off-therapy. uMRD at EOT with VR is associated with an improvement in both PFS and OS while re-treatment with VR seems to be a feasible option open to a significant number of patients with recurrent CLL [Citation17].
3. Breaking down the CLL14 trial: sustained efficacy of the venetoclax-obinutuzumab (VO) combination
The CLL14 trial included 432 TN CLL patients, with a median age of 72 years, who were deemed unfit for intensive CIT due to a Cumulative Illness Rating Scale (CIRS) score exceeding six points or presence of impaired renal function, indicated by a creatine clearance below 70 mL/min. The trial studied two groups of 216 patients TN CLL who received either venetoclax in combination with obinutuzumab (VO) (oral venetoclax initiated on day 22 of a 28-day cycle, with a 5-week dose ramp-up [20 mg, 50 mg, 100 mg, and 200 mg, then 400 mg daily for 1 week], followed by 400 mg daily until the completion of cycle 12) combined with intravenous obinutuzumab for six cycles or chlorambucil in combination with obinutuzumab (CO) for 6 cycles followed by oral chlorambucil as monotherapy for an additional 6 cycles [Citation16].
At a median follow-up of 76.4 months, the VO group exhibited a median PFS of 76.2 months, compared to 36.4 months of the CO cohort. In a six-year landmark analysis, 53.1% of patients treated in the VO arm remained progression-free as compared with 21.7% in the control arm with a hazard ratio (HR) of 0.40 (95% CI, [0.31–0.52)(P < 0.0001) [Citation18].
The median TTNT, defined as death from any cause or initiation of another line of treatment, was not reached for the VO group, whereas the PFS was 52.9 months for the CO group (HR 0.44, 95% CI 0.33–0.58; P < 0.0001). After six years follow-up 65.2% of patients in the VO cohort had not initiated another treatment in comparison to 37.1% in the control arm. These differences were observed across all risk groups, including those with TP53 mutations or gene deletions.
Although the initial CLL14 study results did not show a difference in OS, the now mature data indicates a trend toward a survival benefit for VO with a six-year OS of 78.7% as compared to 69.2% in the CO group (HR 0.69, 95% CI, 0.48–1.01; P = 0.052).
At the EOT 74.% in the VO arm and 32.8% in the CO arm had uMRD (<10 −4 by NGS in peripheral blood) [Citation19]. Five years after the EOT, 1.9% and 7.9% of patients had sustained uMRD in the CO and VO arms, respectively. In the VO arm, the EOT MRD status significantly correlated with both PFS and OS, with a shorter PFS observed in patients with MRD ≥ 10−4 (i.e. >1 CLL cell per 10,000 leukocytes) [Citation18].
No new safety signals were observed in this six-year analysis of the CLL14 clinical trial. The most frequently occurring Grade 3 (≥2%) adverse events (AEs) in patients receiving the VO was neutropenia, often successfully managed with dose interruptions, intermittent use of colony-stimulating factor and dose reductions, if required. Notably, the reported rates of grade ≥ 3 febrile neutropenia was low following VO with an incidence of 5.2% in VO treated trial participants.
Second primary malignancies were reported in 30 patients in the VO and 18 in the CO arm; cumulative incidences 6 years after randomization were 14.2% and 8.5%, respectively (p = 0.071). Two Richter transformations were reported in the VO arm and four in the CO arm. No new safety signals were observed [Citation18].
The 6-year follow-up results of CLL14 has demonstrated the significant long-term PFS benefit of FD VO across all subgroups of patients with previously untreated CLL in the presence of medical comorbidities. These survival benefits of VO were consistently observed in high-risk patient groups, such as those with TP53 deletions/mutations or IGHV unmutated disease. In addition, more than 60% of VO-treated patients did not require second-line treatment [Citation18]. This study also highlights the prognostic value of EOT MRD status with respect to PFS and OS, emphasizing the need for MRD-guided approaches [Citation19–22]. It is worth noting that the ongoing study continues to monitor the secondary malignancy rate, with anticipated further follow-up results to be presented later this year.
4. Expert opinion
The long-term results of the MURANO and CLL14 trials, recently presented at the 23rd EHA meeting, have definitively established the role of FD venetoclax/anti-CD20 mAb combinations as a breakthrough in the treatment of both TN and R/R CLL (). Venetoclax-anti-CD20 mAb combination is an appealing treatment option due to time-limited administration, associated high rate of uMRD which is predictive of durable responses, and the acceptable toxicity profile, especially in older or medically less fit patients. Notably, the use of venetoclax-anti CD20 mAb FD regimens may reduce the likelihood of patients developing new CLL targeting mutations, thus increasing the potential for eliciting secondary clinical responses upon retreatment with venetoclax [Citation23]. The acquisition of the recurrent Gly101Val mutation in the BCL2 gene, which confers resistance to venetoclax, is a well-described mechanism of secondary resistance seen only with continuous venetoclax therapy and which has never been reported following 12 to 24 months of FD therapy [Citation24].
Results of MURANO trial show that venetoclax retreatment is promising, however, additional data are required, and the optimal anti-CD20 mAb to combine with venetoclax may not be rituximab. Moreover, prospective studies should be conducted to better define the TFI needed to consider a patient eligible for retreatment. The ReVenG trial (NCT04895436) is a phase 2 study designed to assess whether patients with CLL who have completed first-line VO treatment can derive clinical benefit from VO retreatment. The primary objective of this trial is to evaluate the ORR of VO retreatment in patients who have progressed more than 24 months after their initial VO treatment.
Some practical considerations accompany venetoclax therapy. These include the need for clinical and laboratory monitoring during the initial venetoclax dose ramp-up phase with the attendant possibility of hospital admissions and the need to implement prophylactic strategies to mitigate the risk of tumor lysis syndrome (TLS), particularly in patients with a higher tumor burden [Citation25]. It is worth noting that the phase three trials of VO and VR have demonstrated a low incidence of TLS events [Citation15–18].
Some logistical challenges around the delivery of venetoclax may arise for patients who face difficulties with transportation, live in location which is remote from the hematology unit or who lack caregiver support with such factors potentially impeding the access to venetoclax-based therapies [Citation23]. However, after the ramp-up phase and the intravenous administration of anti CD20 mAb, the frequency of clinical monitoring decreases for the remainder of venetoclax therapy [Citation15–18].
Neutropenia, which is the most common adverse event observed with venetoclax, can be effectively managed through various strategies, including discontinuation, intermittent use of granulocyte colony-stimulating growth factors, and adjusting the venetoclax dose as required. Based on data from the CLL14 and CLL13 trial, the incidence of grade ≥ 3 febrile neutropenia in the VO arm is relatively low (5.2% and 3.1%, respectively) [Citation18,Citation26].
An important aspect to consider is how potential immune recovery with FD therapy aligns with the risk of infection. A recent meta-analysis assessed the prevalence of infections in CLL patients treated with targeted agents. The pooled cumulative incidence of severe infections across patients treated with BTKi was 19.8%, compared to 17.4% in patients treated with venetoclax-based therapies [Citation27]. Notably, both randomized controlled trials (RCTs) evaluating venetoclax-based treatments, namely the MURANO and CLL14 trials, included regimens that incorporated anti-CD20 mAbs [Citation15,Citation16].
Following FD treatment, patients demonstrated immune recovery. In a post hoc analysis of the MURANO trial, post-treatment recovery of IgG, IgA, and IgM levels, as well as normalization of CD3+ T cells, was observed, irrespective of EOT MRD status. The overall infection rate was low, with non-statistically significant differences in grade ≥ 3 infections occurring during treatment between patients who achieved uMRD at EOT and those who did not [Citation28]. In the CAPTIVATE MRD cohort, infection rates generally decreased over time. Notably, a trend emerged indicating lower infection rates in patients who achieved confirmed uMRD [Citation29]. The GLOW study provided compelling evidence of restoration of normal B cells in patients receiving a FD treatment of ibrutinib plus venetoclax [Citation30]. These findings offer promising indications of the restoration of a normal blood immune composition when utilizing FD venetoclax-based regimens.
One of the advantages of using venetoclax-anti CD20 mAb combinations is the absence of cardiovascular (CV) toxicities [Citation15–18]. This characteristic makes such an approach particularly suitable for individuals over 70 years of age, as they often have a high prevalence of CV comorbidities, reported in up to 60% of persons in this age range [Citation31]. However, the decision between FD venetoclax-anti-CD20 mAb combinations and a BTKi agent given continuously becomes more complex in patients without CV comorbidities [Citation23].
While limited compelling data supports the efficacy of BTKis over venetoclax-based regimens, it is crucial to individualize treatment selection. illustrates the advantages and disadvantages of continuous vs. FD therapy based on specific disease-related factors (e.g. TP53 and IGHV mutational status, the presence of a complex karyotype) and treatment-related features (e.g. cardiovascular risk, infection risk, the likelihood of developing target mutations, costs, challenges of re-treatment with FD therapy, or achieving uMRD). However, this is a generalized representation, clinical decisions should be made in consultation with a healthcare professional, taking into account the the burden of patients’ comorbidities, logistical aspects, and individual patients’ needs [Citation23].
Figure 2. Visual representation of potential advantages and disadvantages of continuous versus fixed-duration therapy based on specific disease-related (IGVH mutational status, TP53 mutational status, presence of complex karyotype [KC]) or treatment-related (infection risk, high cardiovascular [CV] risk, potential for retreatment, potential for achieving undetectable minimal residual disease [uMRD], costs, risk of developing targeting mutations) features. The sign (+) indicates a potential advantage while the sign (-) a potential disadvantage.
![Figure 2. Visual representation of potential advantages and disadvantages of continuous versus fixed-duration therapy based on specific disease-related (IGVH mutational status, TP53 mutational status, presence of complex karyotype [KC]) or treatment-related (infection risk, high cardiovascular [CV] risk, potential for retreatment, potential for achieving undetectable minimal residual disease [uMRD], costs, risk of developing targeting mutations) features. The sign (+) indicates a potential advantage while the sign (-) a potential disadvantage.](/cms/asset/f5d8c8e8-d5bb-40a9-ada4-71d626363e6f/iery_a_2288899_f0002_oc.jpg)
In the context of FD CLL therapy, a significant development has emerged with the recent approval by the European Commission of the all-oral combination of ibrutinib and venetoclax for the frontline treatment of patients with CLL [Citation32]. This approval is based on the results of the phase 3 GLOW trial, which enrolled unfit CLL patients, and the CAPTIVATE clinical trial, a phase 2 study that evaluated both MRD-guided treatment discontinuation and FD therapy in untreated patients with CLL/SLL [Citation21,Citation30]. The results of the ongoing CLL17 (NCT04608318) phase 3 trial, a prospective, multicentre, open-label randomized study comparing the efficacy and safety of continuous ibrutinib monotherapy versus fixed-duration VO or fixed-duration ibrutinib plus venetoclax in previously untreated CLL is therefore eagerly awaited. When these results become available, they will provide valuable assistance to clinicians in selecting the most suitable therapeutic approach. This guidance extends beyond the choice between continuous BTK inhibitors and a venetoclax-based FD approach; it also encompasses the decision between an all-oral FD regimen and an FD regimen that includes an anti-CD20 mAb.
It is crucial to recognize that the conventional method for assessing adverse events, primarily reliant on the Common Terminology Criteria for Adverse Events (CTCAE), often hinges solely on basic incidence rates. However, this approach fails to consider a critical aspect: the duration of exposure to targeted agent treatments. To enable meaningful comparisons of adverse event profiles across clinical trials for CLL involving targeted agents, it is crucial to establish a comprehensive measure of adverse event burden. Such a measure should encompass both the severity and the number of reported adverse events during a trial, while also accounting for the duration of administered medication [Citation33,Citation34]
Finally, the economic burden of continuous BTKi therapy may favor the choice of FD therapy. The increased benefits and costs associated with oral targeted therapies are projected to improve CLL survivorship but can impose a substantial financial burden on both patients and payers, particularly for those treated with a continuously administered BTKi [Citation35]. A recent study conducted in Canada on the costs associated with CLL management for both continuous- and FD treatment which included drug acquisition, follow-up/monitoring, adverse event, and palliative care suggests that FD therapy is expected to result in major reductions in cost burden over the 5-year projection, compared to continuous therapy. Changes in pricing, improved management of AEs, and tailored treatment plans based on genetic and patient profiling can help to reduce the financial burden of managing CLL [Citation36].
Article highlights
Fixed-duration venetoclax combined with an anti-CD20 monoclonal antibody (mAb) therapy has demonstrated efficacy and safety, as indicated by the long-term follow-up results from the MURANO and CLL14 trials.
Updated findings from the MURANO trial provide additional support for the effectiveness of retreatment with venetoclax-based therapies in selected CLL patients.
The combination of venetoclax and an anti-CD20 mAb presents an attractive treatment option for both treatment-naïve (TN) and relapsed/refractory (R/R) CLL patients. This is due to its time-limited administration, high rates of achieving undetectable minimal residual disease (uMRD), which is predictive of long-lasting responses, a manageable toxicity profile, and a controlled economic burden.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437.
- Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib- rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–443. doi: 10.1056/NEJMoa1817073
- Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528.
- Moreno C, Greil R, Demirkan F, et al. Ibrutinib plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicenter, randomized, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5
- Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94(12):1353–1363.
- Barr PM, Owen C, Robak T, et al. Up to 8-year follow-up from RESONATE-2: firstline ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6:5641–5654. doi: 10.1182/bloodadvances.2021006434
- Molica S, Matutes E, Tam C, et al. Ibrutinib in the treatment of chronic lymphocytic leukemia: 5 years on. Hematol Oncol. 2019;38:129–136. doi: 10.1002/hon.2695
- Sharman JP, Egyed M, Jurczak W, et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022;36(4):1171–1175.
- Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031–1043.
- Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in Previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–3452.
- Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319–332.
- Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134(22):1919–1928.
- Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370(24):2286–2294.
- Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311–322.
- Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N Engl J Med. 2018 Mar 22;378(12):1107–1120. doi: 10.1056/NEJMoa1713976
- Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019 Jun 6;380(23):2225–2236. doi: 10.1056/NEJMoa1815281
- Kater A, Harrup R, Kipps TJ, et al. Final 7-year follow-up and retreatment substudy analysis of MURANO: venetoclax-rituximab (venr)-treated patients with relapsed/refractory chronic lymphocytic leukemia (R/R CLL). Hemasphere. 2023 Aug;7(Suppl):e492813f. doi: 10.1097/01.HS9.0000967716.49281.3f
- Al-Sawaf O, Robrecht S, Zhang C, et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized CLL14 study. Hemasphere. 2023 Aug;7(Sull):e064430a. doi: 10.1097/01.HS9.0000967492.06443.0a
- Al-Sawaf O, Zhang C, Lu T, et al. Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: extended off-treatment follow-up from the randomized CLL14 study. J Clin Oncol. 2021 Dec 20;39(36):4049–4060. doi: 10.1200/JCO.21.01181
- Kater AP, Levin M-D, Dubois J, et al. Minimal residual disease guided stop and start of venetoclax plus ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia (Hovon141/VISION): primary analysis of an open-label, randomised, phase 2 trial. Lancet Oncol. 2022;23:818–828. doi: 10.1016/S1470-2045(22)00220-0
- Wierda WG, Allan JN, Siddiqi T, et al. Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol. 2021 Dec 1;39(34):3853–3865. doi: 10.1200/JCO.21.00807
- Molica S, Allsup DJ. Time-limited, chemotherapy-free treatment comes of age in chronic lymphocytic leukaemia. Lancet Oncol. 2022 Jun;23(6):699–701. doi: 10.1016/S1470-2045(22)00266-2
- Bennett R, Anderson MA, Seymour JF. Unresolved questions in selection of therapies for treatment-naïve chronic lymphocytic leukemia. J Hematol Oncol. 2023 Jul 8;16(1):72. doi: 10.1186/s13045-023-01469-7
- Blombery P, Anderson MA, Gong J, et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019;9(3):342–353.
- Wierda WG, Tambaro FP. How I manage CLL with venetoclax-based treatments. Blood. 2020;135(17):1421–1427. doi: 10.1182/blood.2019002841
- Eichhorst B, Niemann CU, Kater AP, et al. First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023 May 11;388(19):1739–1754. doi: 10.1056/NEJMoa2213093
- Vassilopoulos S, Shehadeh F, Kalligeros M, et al. Targeted therapies in CLL/SLL and the cumulative incidence of infection: a systematic review and meta-analysis. Front Pharmacol. 2022 Sep 14;13:989830. eCollection 2022. doi: 10.3389/fphar.2022.989830
- Kater AP, Eichhorst B, Owen C, et al. Long-term host immune changes following treatment with venetoclax plus rituximab in relapsed/refractory chronic lymphocytic leukemia. Blood. 2022;140(Supplement 1):7010–7012.
- Moreno C, Solman IG, Tam CS, et al. Immune restoration with ibrutinib plus venetoclax in first-line chronic lymphocytic leukemia: the phase 2 CAPTIVATE study. Blood Adv. 2023;7(18):5294–5303.
- Kater AP, Owen C, Moreno C, et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid. 2022;1(7). doi: 10.1056/EVIDoa2200006
- Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019 Apr 27;6(2):19. doi: 10.3390/jcdd6020019
- European Commission approves IMBRUVICA® (ibrutinib) in a fixed-duration combination regimen for adult patients with previously untreated chronic lymphocytic leukaemia (CLL). News Release. Johnson & Johnson. [2022 Aug 4; cited 2022 Aug 25]. Available from: https://bit.ly/3AMP4yh
- Niemann CU. BTK inhibitors: safety + efficacy = outcome. Blood. 2023 Aug 24;142(8):679–680. doi: 10.1182/blood.2023020974
- Molica S. Redefining efficacy and safety endpoints for chronic lymphocytic leukemia in the era of targeted therapy. Expert Rev Hematol. 2023 Oct;13:1–4. Online ahead of print. doi: 10.1080/17474086.2023.2271170
- Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017 Jan 10;35(2):166–174. doi: 10.1200/JCO.2016.68.2856
- Lachaine J, Guinan K, Aw A, et al. Impact of fixed-duration oral targeted therapies on the economic burden of chronic lymphocytic leukemia in Canada. Curr Oncol. 2023;30(5):4483–4498.