1. Introduction
Cancer is one of the world’s leading causes of death. The World Health Organization projects the number of global cancer-related deaths to reach 12 million by 2030. Therefore, there is a constant need to improve cancer diagnosis and treatment tools at an early stage. Current therapies employed for cancer treatment include surgery, chemotherapy and radiation therapy. Effective as they may be, these therapies exhibit certain drawbacks and side effects such as the damage of healthy tissues, fatigue, anemia, associated infections, hair loss, extensive duration and expense of treatment. To counter these limitations, it is essential to develop novel anticancer weapons with early tumor detection, effective diagnosis and personalized treatment for different cancer types. In recent years, nanomaterials have become a center of interest for cancer therapeutics. Due to their small size and tunable physicochemical properties, nanoparticles can establish extensive interactions with biomolecules, both on the surface and within cancer cells. This high interaction capacity holds the promise of revolutionizing cancer care. Gold nanoparticles (GNPs) stand out as an up-and-coming agent in cancer diagnostics and treatment due to their high biocompatibility, minimal toxicity, excellent penetration into cancer tissues, and most importantly, their non-immunogenic nature in the human body [Citation1]. Besides, GNPs have unique physical and chemical properties that confer special benefits, such as nanoscale, surface, quantum, electrical and optical effects.
Moreover, GNPs can be easily synthesized, with controlled size and morphology and many available surface modifications. Surface modifications of GNPs have been exploited to attach targeting ligands, imaging labels, therapeutic drugs and various other functionalities [Citation2]. So far, GNPs have been explored for cancer-related applications by different novel approaches, comprising targeted drug delivery, gene delivery, bioimaging, enhanced radiation therapy, diagnostics and light induction therapy. Various shapes of GNPs have been developed to meet the specific cancer needs, such as nanoclusters, nanocages, nanobranches, nanopyramids, nanoflowers, nanorods, nanoshells, nanoplates, nanostars, nanocubes, etc [Citation3]. The following sections will highlight several key areas where GNPs made the most crucial contributions to cancer diagnostics and treatment.
2. Delivery of drugs and gene therapy
GNPs have shown the remarkable potential to improve the pharmacokinetics of many chemotherapeutic drugs by reducing the nonspecific side effects and delivering higher doses of drugs to target sites. This is based on the high drug loading capacity and low cytotoxicity of GNPs. The payload size of GNPs can range from a small drug molecule to a large biomolecule, such as a protein, DNA or RNA. The therapeutics’ loading depends on their specific properties, such as size, charge, and surface chemistry [Citation1]. Once delivered inside the body, these properties also affect their absorption and subsequent intracellular fate. There are several examples in the literature highlighting the enhanced therapeutic effects of chemotherapeutics drugs combined with GNPs. For instance, conjugation of doxorubicin with GNPs and anti-PD-L1 antibody was shown to improve the effectiveness of targeted chemo and photothermal therapy in colorectal cancer [Citation4]. GNPs have also been reported as carriers in gene therapy: conveying nucleic acid into tumor cells. GNPs are very effective transfection agents since they can protect the nucleic acids from nuclease degradation by loading many nucleic acids on the NPs surface and adjusting the surface charge [Citation5]. Currently, GNPs are at the stage of phase I and II clinical trials as drug and gene carriers for cancer treatment . A recent example is the NU-0129 drug designed against gliosarcoma and based on Spherical Nucleic Acid (SNA) arranged on the surface of GNPs. The study suggests that NU-0129 can cross the blood-brain barrier and inhibit the Bcl2L12 gene, promoting tumor growth (NCT03020017). Hu et al. report another example for diagnosing gastric lesions from exhaled breath and saliva using functionalized GNPs biosensors (NCT01420588).
The shape and size have an important impact on the anticancer and cytotoxicity of GNPs. Karol et al. demonstrated the cytotoxicity of GNPs was shape dependent by investigating three different shapes of GNPs as rods (≈39 nm length, 18 nm width), stars (≈ 215 nm) and spheres (≈ 6.3 nm) in human fetal osteoblast, osteosarcoma and pancreatic duct cells. Authors showed that stars shape has the highest anticancer potential were also the most cytotoxic type but has slowest cellular uptake due to their big size. In contrast, the sphere’s form appears to be the safest one had fastest cellular uptake with weak anticancer potential [Citation6]. Lee et al. showed another example of shape-dependent cytotoxicity and cellular uptake of green GNPs of different shapes like stars, sphere and rods in human hepatocyte carcinoma cells. The study concluded that the cytotoxicity was the highest in nanorods, followed by nanostars and finally nanospheres. The cellular uptake of GNPs followed the order nanospheres > nanorods > nanostars [Citation7]. Thus, the studies concluded that the large and complex shape of GNPs has more anticancer potential but lesser cellular uptake and vice-versa.
3. Tumor imaging and radiation-based therapy
The most effective way to improve the prognosis for tumors is by their early detection. Due to biocompatibility, high stability and high atomic number GNPs serve as potentially excellent contrasting agents. GNPs show surface plasmon resonance (SPR) effects and have a high capacity for X-ray absorption that allows them to interact with electromagnetic radiation of different wavelengths and create a contrast image. This can be used to help identify a tumor at a very early stage [Citation8]. The unique SPR property of GNPs allows them to be used in near-infrared (NIR)-resonant imaging modalities such as magnetic resonance imaging (MRI), photoacoustic imaging (PAI), positron emission tomography (PET), fluorescence imaging and X-ray scattering imaging (X-ray CT) [Citation8]. The shape of GNPs plays a critical role in cancer radiotherapy. Ninging et al. compared the different shapes of nanoparticles (GNPs), nanospkies (GNSs) and nanorods (GNRs) and in terms of cellular uptake in KB cancer cells. They found that the GNPs showed higher anticancer efficiency than GNSs and GNRs upon X-ray irradiation due to higher cellular uptake [Citation9]. GNPs have also applied in sensing and diagnostics applications such as immunosensing, colourimetric sensing, plasmonic biosensing etc.
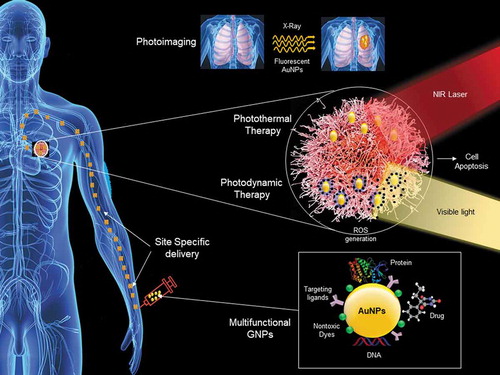
Another essential property of GNPs is their ability to generate heat when exposed to NIR lasers, making them suitable for hyperthermia-based photothermal applications (). In principle, hyperthermia mechanism requires the initiation of heat stress in cells, which further results in various cellular degradation processes such as changes in signal transduction, induction of apoptosis, reduction of perfusion and tumor oxygenation. Taking advantage of these properties of GNPs, many studies have reported topical use of GNPs-based photothermal therapy for effective destruction of cancer cells. This is because GNPs can generate excessive heat inside tumor cells in response to non-harmful radiation from outside the body, which ultimately kills cancers due to locally generated heat [Citation10].
Photodynamic therapy (PDT) is a similar clinically approved therapy, which uses nontoxic dyes with GNPs and harmless visible light in combination with oxygen to produce a high level of reactive oxygen species (ROS) to kill cancer cells via local oxidative stress (). The major drawback of all types of radiation therapy is its inability to distinguish between cancerous and normal tissues. Hence, reducing damage to normal tissue remains the main limiting factor. GNPs, as conveyors of radiation effects in hyperthermia or PDT, offer a tremendous potential to limit such side effects since they provide a possibility of functionalization with specific cellular receptors to improve precise targeting, as well as combining radiation effects with drug delivery since GNPs can be multifunctional [Citation11–13].
4. Combining detection and treatment and other emerging applications
During transport in plasma and solid tissues, anticancer drugs are vulnerable to leakage, restricting their practical applications. GNPs can be used as a stabilizer for other drug carriers, such as liposomes, and at the same time improve their delivery efficiency by promoting the release of drugs at target [Citation14]. One of the recent developments is multifunctional GNPs to combine the diagnostic and therapeutic functions [Citation13]. To obtain these benefits, two or more therapeutic agents, imaging labels, linkers, and drugs can be associated with a single multifunctional GNPs [Citation12]. Such GNPs loaded with drugs, targeting, and imaging agents can form ”theranostic” probes that can detect and cure tumors simultaneously [Citation15]. Another exciting recent development came with discovering that various cancer cells can be induced to form GNPs intracellularly, and these intracellular GNPs can be applied in photothermal applications. For instance, Aron et al. showed in situ biosynthesis of GNPs in MCF7 tumor mouse xenografts and demonstrated that these could be used in photothermal applications [Citation16].
5. Biocompatibility of GNPs
GNPs do not induce adverse and acute toxicity and are therefore considered a biocompatible agent. All cancer-related applications of GNPs mainly depend on their ability to penetrate cancer tissues, depending on the nanoparticles’ size, morphology, and functionality [Citation17]. These factors are directly correlated to cellular internalization, half-life, biodistribution and renal secretion of NPs. In this respect, recently, ultra-small GNPs (diameter around 10 nm) have shown excellent results in lower toxicity, faster body clearance, maximum accumulation, and penetration at the tumor site [Citation18]. Ultra-small GNPs in the range of 2–6 nm can easily pass through the blood-brain barrier and effectively penetrate the tumor region’s nucleus. Moreover, such ultra-small GNPs show excellent body clearance by glomerular filtration, liver and kidney [Citation19]. Bailly et al show an example in which the authors demonstrated the safety, pharmacokinetics and biodistribution of dextran-coated GNPs in the mouse model and concluded that though GNPs mainly accumulated in the liver and spleen, they did not show any hepatic or renal toxicity [Citation20].
6. Limitations of GNPs
Despite having multiple benefits, GNPs have some limitations, which limit their applications in medicines. The first and utmost limitation is the toxicity of bare GNPs without any capping or biocompatible layer, which is a significant concern. The toxicity of bare GNPs depends on numerous parameters, such as composition, shape, size, coating, charge, hydrophobicity, solubility, and reactivity. The different biological environment also influences the toxicity encounters GNPs for instance biofluids, intracellular media, inclusion in biovesicles. The second feature limiting nanoparticles’ use in medicine is the evolution of their biological medium properties. Studies indicate that bare GNPs tend to aggregate within the lysosome, which modifies their optical properties and alters their activation under radiation. Thus, to make them stable, the GNPs need an additional organic or biologic surface coating. Even after achieving the biocompatible GNPs, the most challenging difficulties for gold nanoformulations facing medicine are to cross the biological barriers and specifically recognize their targets. All these constraints limit the approval of GNPs for clinical trials and commercial use [Citation21].
7. Conclusion and perspective
A better understanding of GNPs and their interaction with the human body is expected to lead to more mature technologies. The critical properties of GNPs that are of great value for the diagnosis and treatment of tumors are extremely small size range, effective penetration and deposition power at the tumor site, ability to be functionalized with different proteins and drugs due to their high payload capacity, and excellent biocompatibility. A particular promise comes from designing multifunctional GNPs, with multiple targeting receptors, multimodality imaging, and multiple therapeutic entities. Ultra-small GNPs, which combine excellent biocompatibility with all previously mentioned features, also promise future photodynamic therapy, chemotherapy, and gene therapy applications. A recent example of GNPs cancer applications is the clinical trial of GNPs based photothermal therapy against prostate cancers has been conducted by using Gold-silica NPs, in which researchers claimed that these biocompatible GNPs (AuroLase Therapy) provide localized treatment and save the adjacent organs by avoiding any side effects [Citation22]. However, before GNPs can reach comprehensive cancer diagnostics and therapy, certain hurdles still need to be overcome. These are mainly related to evaluating and optimizing biological toxicity and improving natural stability. The efficient targeting of GNPs based therapies is required to enhance both drug carrier and radiation-based methodologies for maximum tumor accumulation of drugs and less healthy tissue damage. Other important issues determine the optimal doses for different cancer based on various GNPs and monitoring the controlled release of payloads from GNPs upon a change in the physical environment.
8. Expert Opinion
Given the existing limitations of current diagnostic and treatment methods for cancer, GNPs are a promising emergent technology that shows potential for specific action against tumor cells while protecting the healthy tissues. This is due to their non-immunogenic nature, size and shape-dependent tunable properties. The GNPs can emerge as a first choice for cancer diagnostic and treatment by attaining bioavailability and stability in the biological environment. However, in future, applying GNPs for target-based therapies requires in-depth study and understanding of the GNPs formation (inside or outside the tumor), action mechanism and clearance from the body.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Kumar A, Zhang X, Liang XJ. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol Adv. 2013;31(5):593–606.
- Singh P, Pandit S, Mokkapati V, et al. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7).
- Singh P, Kim Y-J, Zhang D, et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016 Jul;34(7):588–599.
- Emami F, Banstola A, Vatanara A, et al. Doxorubicin and Anti-PD-L1 antibody conjugated gold nanoparticles for colorectal cancer photochemotherapy. Mol Pharm. 2019;16(3):1184–1199.
- Amreddy N, Babu A, Muralidharan R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–170.
- Steckiewicz KP, Barcinska E, Malankowska A, et al. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anticancer potential. J Mater Sci Mater Med. 2019;30(2):22.
- Lee YJ, Ahn EY, Park Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res Lett. 2019;14(1):129.
- McQuaid HN, Muir MF, Taggart LE, et al. Imaging and radiation effects of gold nanoparticles in tumour cells. Sci Rep. 2016;6(1):19442.
- Ma N, Wu F-G, Zhang X, et al. Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: comparison of gold nanoparticles, nanospikes, and nanorods. ACS Appl Mater Interfaces. 2017;9(15):13037–13048.
- León Félix L, Sanz B, Sebastián V, et al. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci Rep. 2019;9(1):4185.
- Mieszawska AJ, Mulder WJ, Fayad ZA, et al. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol Pharm. 2013;10(3):831–847.
- Yin X, Yang B, Chen B, et al. Multifunctional gold nanocluster decorated metal–organic framework for real-time monitoring of targeted drug delivery and quantitative evaluation of cellular therapeutic response. Anal Chem. 2019;91(16):10596–10603.
- Dykman LA, Khlebtsov NG. Gold nanoparticles in chemo-, immuno-, and combined therapy: review [Invited]. Biomed Opt Express. 2019;10(7):3152–3182.
- Liu J-W, Wang Y-M, Zhang CH, et al. Tumor-targeted graphitic carbon nitride nanoassembly for activatable two-photon fluorescence imaging. Anal Chem. 2018;90(7):4649–4656.
- Chen Q, Wang H, Liu H, et al. Multifunctional dendrimer-entrapped gold nanoparticles modified with RGD peptide for targeted computed tomography/magnetic resonance dual-modal imaging of tumors. Anal Chem. 2015;87(7):3949–3956.
- Schwartz-Duval AS, Konopka CJ, Moitra P, et al. Intratumoral generation of photothermal gold nanoparticles through a vectorized biomineralization of ionic gold. Nat Commun. 2020;11(1):4530.
- Kumar A, Ma H, Zhang X, et al. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials. 2012;33(4):1180–1189.
- Loynachan CN, Soleimany AP, Dudani JS, et al. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat Nanotechnol. 2019;14(9):883–890.
- Sokolova V, Mekky G, Van Der Meer SB, et al. Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model. Sci Rep. 2020;10(1):18033.
- Bailly A-L, Correard F, Popov A, et al. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci Rep. 2019;9(1):12890.
- Feliu N, Docter D, Heine M, et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem Soc Rev. 2016;45(9):2440–2457.
- Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Nat Acad Sci. 2019;116(37):18590–18596.