ABSTRACT
Introduction
Oral squamous cell carcinoma (OSCC) and oral potentially malignant disorders (OPMD) are a significant health burden globally. Smoking, alcohol, and betel quid are the main risk factors. Lack of screening methods has been highlighted as a significant challenge in management. Salivary biomarkers are proposed as noninvasive diagnostic tools. The aim of this systematic review was to study salivary biomarkers reported in OSCC and OPMD. Specific objectives were to select a salivary biomarker panel suitable for early detection of OSCC and OPMD and to assess relationships between salivary biomarkers and risk factors.
Methods
Electronic literature search was conducted in academic databases (Scopus, Medline, Embase and Web of Science) without any restrictions. Following calibration, two blinded reviewers screened the studies and extracted data. A risk of bias assessment was conducted using Newcastle Ottawa scale. 295 studies were included with descriptive data analysis.
Expert opinion
A salivary biomarker panel including Interleukin (IL) 1β, IL6, and IL8 was selected for OSCC and OPMD. Reported relationships between salivary biomarkers and risk factors are discussed and research gaps are highlighted. Future research should be directed to assess potential salivary biomarkers and their relationships to risk factors in order to understand the biomarker’s role in disease initiation.
1. Introduction
Oral cancer is a significant public health problem worldwide. More than 90% of oral cavity malignancies are oral squamous cell carcinomas (OSCC) [Citation1]. Some patients develop OSCC from clinically distinguishable pre-cancer stage. These conditions are collectively identified as oral potentially malignant disorders (OPMD). OPMD are defined as clinical presentations that carry an increased risk to develop into OSCC [Citation2]. Common OPMD conditions are leukoplakia, erythroleukoplakia, oral lichen planus, and oral submucous fibrosis.
According to the global health statistics, lip and oral cavity cancers reported more than 177,000 deaths and accounted for more than 350,000 new cases in the year 2020 [Citation3]. The worldwide prevalence of OPMD was estimated as 4.47% [Citation4]. More than two-third of OSCC were reported from Asia [Citation5]. In 2012, OSCC was the 12th common cancer type in Asia; in 2018, it had advanced to the 11th position demonstrating an increasing trend with time [Citation6].
Compared to other cancers, OSCC demonstrate low five-year survival rates, the survival rate is about 20% when diagnosed at advance stage and it can improve up to 80% when diagnosed at early stages [Citation7]. The five-year survival rate has not improved with time despite advances in treatment [Citation8,Citation9]. Early detection is important to reduce mortality and morbidity associated with this disease. Lack of effective screening protocols was highlighted as a major barrier for early detection [Citation10]. Identifying which OPMDs will develop into a malignancy remains a challenge, as the malignant transformation of OPMD is not consistent [Citation11]. Hence, the need of biomarkers for screening, diagnosis and prognosis in OSCC and OPMD has been emphasized [Citation12,Citation13].
A biomarker is defined as ‘A characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention’ [Citation14]. Different DNA, RNA, proteins and metabolites were identified as biomarkers in OSCC [Citation13]. Due to the noninvasiveness and the presence of variety of biomolecules, saliva was proposed as a suitable biological sample to study biomarkers [Citation15]. In OSCC, certain biomarkers that appeared non-significant when analyzed in serum reported significant differences when analyzed in saliva [Citation16,Citation17]. There is no consensus on the most suitable salivary biomarkers for clinical use in head and neck cancer, up to date [Citation18]. In-depth assessment of already identified salivary biomarkers is as important as introduction of novel targets as biomarkers [Citation19].
Smoking, high alcohol consumption and betel quid are the main risk factors for OSCC and OPMD [Citation20]. Human papillomavirus (HPV) infection, smokeless tobacco use, genetic predisposition, poor oral hygiene, denture wearing, mouthwash use, dietary factors, low socio economic status, co-morbidities, and genetic disorders are other associated risk factors for OSCC and OPMD [Citation21,Citation22].
This systematic review was conducted with the aim of studying salivary biomarkers reported in OSCC and OPMD with two primary objectives. The first objective was to identify suitable salivary biomarkers for early detection and screening of OSCC and OPMD. The second objective was to identify reported relationships between salivary biomarkers and risk factors in OSCC and OPMD.
2. Methodology
2.1. Data Sources
A systematic electronic literature search was conducted to identify relevant published studies using Medline (Ovid), Web of Science, Embase and Scopus databases. The original literature search was conducted in May 2018, and the search was updated in September 2020.
2.2. Search strategy
The keywords () were combined with AND/OR Boolean to generate the search syntax. The search was conducted without time or language restrictions. Search syntax used for Web of Science database was as follows: ‘oral cancer*’ MeSH terms (mouth cancer, mouth neoplasm, mouth carcinoma, squamous cell carcinoma) OR ‘oral premalignant’ MeSH terms (pre-cancerous) AND ‘saliva* biomarkers*’ MeSH terms (saliva, biomarkers).
2.3. Screening and study selection
Abstracts retrieved from the search were exported to Refwork library. Following title and abstract screening 346 articles were selected for the full-text screening. These were screened by two blinded reviewers with four pairs of reviewers (NP+SR, NP+PC, NP+RA, NP+RMSR) assessing approximately 65 papers per pair. Study selection at all stages was conducted using the following eligibility criteria.
2.3.1. Inclusion criteria
Original research articles containing primary data.
Studies including patients with head and neck cancer including oral cavity, OSCC or OPMD aged 18 and above.
2.3.2. Exclusion criteria
Reviews, systematic reviews, meta-analysis, conference proceedings, case reports and case series.
Full text articles published in languages other than English.
Studies using non-human subjects.
Studies that did not analyze biomarkers in saliva or salivary rinse of participants.
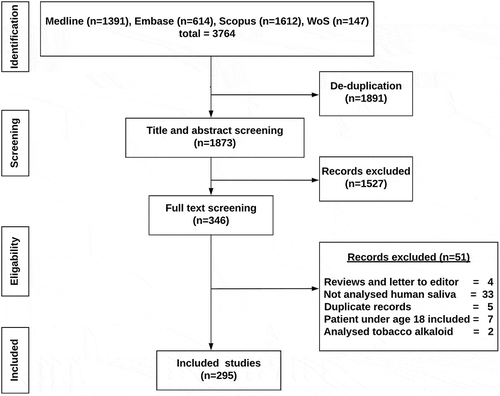
Study selection is summarized using PRISMA (preferred reporting systems for systematic reviews and meta-analysis) flow chart ().
2.4. Reviewer calibration
All reviewers extracted and analyzed data from five randomly selected papers for training and calibration. Once calibration was achieved two reviewers extracted data from each paper independently and blinded to one another’s scores. Four pairs of reviewers (NP+SR, NP+PC, NP+RA, NP+RMSR) conducted the data extraction. Disagreements were resolved through discussion and when necessary with the involvement of a third reviewer (EG).
2.5. Data extraction
The variables extracted from included articles were: first author, published year, country where the study sample was obtained, study design, age, gender, sample size, biomarkers, method used to analyse the biomarkers, relationships between salivary biomarkers and risk factors and main conclusions. The data were recorded and summarized using bespoke Microsoft Excel spreadsheet and descriptive data analyses were performed.
2.6. Quality assessment
Risk of bias assessment of the studies was conducted using Newcastle Ottawa scale. A star (*) was awarded to the feature of the study that minimized risk of bias in each category. Studies with 6–9 stars were graded with high quality. Studies with 5–4 stars were graded with fair quality. Studies with 3 or less stars were graded with poor quality.
3. Results
From the literature search, total of 3764 abstracts were retrieved. Following de-duplication, 1873 abstracts were subjected to title and abstract screening by application of exclusion criteria. Subsequently, 346 articles were selected for the full-text screening stage. From the screened full-text articles, a total of 295 met the selection criteria and were included in the systematic review. Biomarkers reported in the studies were categorized based on molecular type. 52% of the studies reported protein biomarkers followed by DNA (12%), RNA (8%), metabolites (3%), and microbial (2%) biomarkers.
3.1. Identification of a salivary biomarker panel for OSCC and OPMD
From different biomarker categories, proteins were selected for further study. Among proteins, Interleukins (IL) were selected as suitable biomarkers to assess disease progression in OSSC and OPMD. Studies that reported IL biomarkers in saliva in cases with OSCC and OPMD compared to controls were selected for the objective one (n = 28).
Manuscripts assessed for objective one were published from 2004 to 2020 (). Majority of the studies were from USA (n = 6), followed by India (n = 5), Croatia (n = 3), Hungary (n = 2), Iran (n = 2), Pakistan (n = 2), Taiwan (n = 2), and one study each from Iraq, Japan, New Zealand, Poland, Serbia and Spain. Sample size ranged from 18 to 300. Enzyme-Linked immuno-sorbent assay (ELISA) and magnetic-bead-based assay were used for biomarker quantification. All researchers used resting whole saliva samples. In the risk of bias assessment, four studies were graded with high quality with low risk of bias, majority of the studies (n = 19) were graded with fair quality with moderate risk of bias and five studies were graded with poor quality with high risk of bias ().
Table 1. Studies reporting interleukin biomarkers in saliva
Table 2. Risk of bias assessment of studies reporting interleukin biomarkers
Nine IL were reported in the work assessed for objective one. These were IL1α, IL1β, IL2, IL4, IL5, IL6, IL8, IL10, and IL13. Statistical significance of the salivary biomarker concentrations were reported using p values and area under the curve (AUC) of the receiver operating characteristics curves. P values less than 0.05 and the AUC values more than 0.65 were taken as statistically significant difference. From the reported data, IL1β, IL6, and IL8 were selected as most suitable salivary biomarkers for early detection of OSCC and OPMD.
3.2. Relationships between salivary biomarkers and risk factors
Studies that reported relationships between biomarkers in saliva (all biomarker types were included) and risk factors in OSCC and OPMD with statistical data were selected for the objective two (n = 33). Findings of these manuscripts are reported in . Three studies used cohort designs, the rest (n = 30) used case control design. Sample size ranged from 18 to 747. Majority of the data was from USA (n = 8), followed by India (n = 3), Taiwan (n = 3), Brazil (n = 3), Croatia (n = 3), Italy (n = 2), Pakistan (n = 3), and one study each from Australia, Japan, Poland, New Zealand, Sri Lanka, Syria, Hungary, China, and Thailand. Most research analyzed protein biomarkers (n = 22), followed by DNA (n = 8), antioxidant (n = 1), metabolite (n = 1) and both protein and RNA (n = 1). For biomarker analysis, one research has used oral rinse obtained using a mouthwash, four studies used normal saline mouth rinse, three studies used normal saline mouth rinse with exfoliative brush, four studies used stimulated saliva and the rest of the studies (n = 21) used resting whole saliva samples.
Table 3. Studies reporting relationships between salivary biomarkers and risk factors
In the risk of bias assessment using Newcastle Ottawa scale, four studies were graded with high quality with low risk of bias. Majority of the studies (n = 24) were graded with fair quality with moderate risk of bias and five studies were graded with poor quality with high risk of bias ().
Table 4. Risk of bias assessment of studies reporting relationships between salivary biomarkers and risk factors using the Newcastle Ottawa scale
3.2.1. Salivary biomarkers and smoking
Significant differences between epidermal growth factor (EGF), EDNRB gene, loss of hetero-zygosity (LOH), secretory leukocyte protease inhibitor (SLPI), IL10, hypermethylation of the promoter region of MGMT gene and macrophage migration inhibitory factor (MIF) in patients with smoking habit were reported [Citation47,Citation54,Citation48–52].
Protein biomarker SLPI was studied in saliva of patients with OSCC. SLPI level in current smokers was nearly 1.5-fold higher compared to former smokers, and sixfold higher to never smokers [Citation49]. LOH in 25 gene loci was studied in DNA extracted from salivary cell pellet in patients with OSCC. This study found that LOH in former smokers was in between of current smokers and never-smokers [Citation48]. These researchers identified a pattern where the studied biomarkers were lowest in never-smokers, followed by former smokers with the highest level in current smokers.
Protein biomarkers (IL 4, IL10, IL13 and IL 1 receptor antigen) were assessed for their correlation with smoking, yielding statistically non-significant relationships [Citation53]. Associations between mitochondrial DNA biomarkers (cox 1 and 2 genes), and smoking status were variable; one study identified a significant difference with smoking in the healthy control group but not in the patient cohort [Citation54]. The mean change of mitochondrial DNA biomarker in pre-operative and post-operative saliva samples significantly differed with smoking category in patients with head and neck cancer [Citation55]. On the contrary, a study reported a non-significant difference of mitochondrial DNA biomarker with smoking habit [Citation56].
Protein biomarker MIF was analyzed in patients with OSCC before and after surgical treatment. In this research MIF was decreased in current smokers compared to former-smokers, the difference was statistically significant in the post-operative samples [Citation47]. Significantly low EGF levels in smokers in patients with OSCC were identified [Citation57]. IL10 was significantly higher in smokers in patients with OSCC [Citation50]. EDNRB gene studied in salivary rinse was significantly low in smokers compared to never-smokers in patients with head and neck cancer [Citation58].
Studies reported non-significant differences of IL6, IL1, IL1a, IL8, TIMP3, CCNA1, DCC, DAPK, p16, CD44, malondialdehyde, HP, IGHA2, PRDX-2, ZAG, TNF-α, VGEF, TGF, mitochondrial DNA, choline, betaine, pipecolinic acid, L-carnitine, MMP1, ANXA2, KNG, HSPA and EGFR biomarkers in saliva in smokers compared to nonsmokers [Citation47,Citation50,Citation51,Citation54–56,Citation70,Citation59–68,Citation57,Citation58,Citation69].
3.2.2. Salivary biomarkers and alcohol
The potential associations of the biomarkers EGF, TIMP3, MGMT, MINT31, CCNA1, DCC, DAPK and p16, CD44, HP,IGHA2, PRDX-2, ZAG, Cox 1 and cox 2 genes, SLPI, IL10, TNF-α, VGEF, TGF-β, choline, betaine, pipecolinic acid, L-carnitine and EGFR with alcohol consumption habit were assessed, all these revealed statistically non-significant relations [Citation47,Citation49,Citation50,Citation55,Citation78,Citation61–63,Citation66–70].
3.2.3. Salivary biomarkers and betel quid chewing
Researchers reported non-significant associations of betel quid chewing habit with malonaldehyde, lactate dehydrogenase, ANXA protein, KNG protein, and HSPA protein analyzed in saliva [Citation61,Citation71,Citation72].
Hypermethylation of the promoter region of genes were studied in DNA in saliva of patients with OSCC and oropharyngeal cancer [Citation73]. This study reported a significant difference in the methylation profile of the studied gene panel in patients with and without betel quid chewing habit [Citation73]. Protein biomarker S100A7 was studied in saliva in patients with oral sub-mucous fibrosis, there was a significant negative correlation between biomarker concentration and duration of Arecanut (dried nuts of the plant Areca catechu, this is a component of the betel quid and smokeless tobacco products) use [Citation74].
3.2.4. Salivary biomarkers and viral infections
The potential correlations between HPV and Epstein Barr virus (EBV) infections and salivary biomarkers were analyzed. DNA biomarkers (hypermethylation of the promoter region of genes TIMP3, MGMT, MINT31, CCNA1, DCC, DAPK, and p16), reported non-significant differences with HPV infection in patients with head and neck cancer [Citation67]. Lim and colleagues (2016) reported that methylation status of the promoter region of RASSF1α, p16INK4a, TIMP3 and PCQAP genes identified in saliva was able to discriminate both HPV status and the presence of head and neck cancer [Citation75]. Possible associations between protein biomarkers IL10, TGFβ, TNFα, VGEF and HPV infection were assessed in patients with OSCC [Citation50]. This study reported significant difference of the IL10 biomarker in patients with smoking habit and viral infections. Low methylation profile of the promoter region of the tumor suppressor genes was reported in HPV positive OSCC and oropharyngeal cancer patients [Citation73].
3.2.5. Salivary biomarkers and other risk factors
Assessment of the possible relationships between mitochondrial DNA and oral health status, SLPI protein and mouthwash use, body mass index, education level, IL6 biomarker with periodontitis revealed non-significant relationships [Citation49,Citation56,Citation76]. There were significant associations between salivary IL1β and IL8 levels and microbial species in the oral cavity in head and neck cancer patients [Citation77]. A significant relationship of salivary IL6 mRNA and DMFT (decayed, missing, filled number of teeth due to decay) was reported in OSCC [Citation78].
4. Discussion
More than two-third of OSCC is diagnosed at advance stage of disease [Citation79]. Delayed diagnosis was identified as a major reason for increased mortality, morbidity and low five-year survival rates [Citation7]. Screening and early detection is important to upgrade the management of OSCC [Citation10,11]. Early detection of OPMD lesions will allow the chance to apply secondary preventive measures and thereby reduce the incidence of malignant transformation [Citation2]. Due to noninvasiveness of the sample and presence of variety of biomolecules, saliva is proposed as a suitable specimen to identify biomarkers associated with diseases [Citation80]. As it bathes the oral cavity, salivary biomarkers are proposed as important diagnostic and screening adjuncts for oral diseases, particularly OSCC and OPMD. This systematic review was conducted to identify salivary biomarkers reported in OSCC and OPMD.
The first objective of the present study was to select suitable salivary biomarkers for OSCC and OPMD for the purpose of early detection. Proteins are the working state of molecules and therefore prone to demonstrate acute changes with disease. As proteomic biomarkers, cytokines [Citation81], growth factors [Citation82], angio-genic factors [Citation83], antigens [Citation84], cytokeratin [Citation85], cell surface receptors [Citation86], and enzymes [Citation87,Citation88] were reported in saliva. IL have a diverse role in inflammation and immune reactions in carcinogenesis. IL are involved in the pathogenesis of OSCC and malignant transformation of OPMD [Citation89,Citation90]. Hence, IL were selected as suitable biomarkers to assess disease progression in OSCC and OPMD. Through descriptive data analysis of 28 studies, IL1β, IL6 and IL8 were identified as suitable biomarkers for early detection of OSCC and OPMD. These three biomarkers reported significant differences in the disease group compared to controls. Majority of the studies were graded with fair quality with moderate risk of bias.
Absence of a meta-analysis is a limitation of the present study. Rezaei and colleagues (2019) conducted a meta-analysis on IL6 and IL8 biomarkers in OSCC [Citation91]. This study concluded that these biomarkers have significant predictive power for OSCC.
The second objective was to identify relationships between salivary biomarkers and risk factors. In the phases of biomarker development presented by Pepe and colleagues (2001), one requirement is the assessment of the relationships between biomarkers and variables such as age, sex, and risk factors [Citation92]. If such factors were found to have strong associations with the studied biomarker, threshold levels must be defined separately for subpopulations. In a study by Csosz and colleagues (2017), researchers highlighted that salivary biomarker expression in OSCC is population tailored as different protein biomarkers were discovered in different population groups with OSCC [Citation93]. This variation reflects the heterogeneity of the pathogenesis of OSCC. This heterogeneity can be due to the difference in risk factors in populations. Risk factors induce molecular and cellular changes in the body. These changes accumulate to give rise to cancer. With different risk factors, the mechanisms of disease initiation can be different; these can be reflected in salivary analysis.
Relationships between biomarkers and risk factors can help to understand the mechanisms involved in disease initiation through risk factors. Loss of heterozygosity (LOH) is a genetic event identified in cancer cells. Significantly high amount of LOH in 25 gene loci was identified in saliva of patients with OSCC. These biomarkers were high in patients who were smokers, followed by ex-smokers and lowest in nonsmokers [Citation48]. These findings suggest that LOH may be an event induced by smoking in the initiation of OSCC.
SLPI is a protein also known as ‘antileukoproteinase’. Its main function is inhibition of enzymes secreted by leukocytes. A significant increase in the SLPI biomarker was observed in patients who were smokers compared to never smokers. However, there was no statistically significant elevation of the risk of head and neck cancer with elevated SLPI levels [Citation49]. This result suggests that even though salivary SLPI levels may be induced by smoking, this may not be involved in disease initiation in head and neck cancer.
EGF and EGF receptors are involved in signaling pathways commonly altered in cancer cells. Significantly low levels of salivary EGF in patients with OSCC compared to controls were identified. The same biomarker was significantly lower in smokers compared to never smokers [Citation52]. These findings indicate that decrease in EGF may be involved in the pathogenesis of OSCC by smoking.
Silencing of tumor suppressor genes (TSG) via promoter hypermethylation is an event in the malignant transformation of normal cells into cancer cells. Significantly low methylation status of the TSG was reported in HPV positive head and neck cancer patients compared to HPV negative counterparts [Citation75]. The same biomarkers were assessed in saliva of patients with OSCC and oropharyngeal cancer, the findings of this study support the results reported by Lim and colleagues (2016) as low methylation levels were reported in HPV positive cases [Citation73]. In addition, a higher methylation profile of the same gene panel was reported in patients with betel quid chewing [Citation73]. Results reported in these studies suggest that methylation status of the promoter region of TSG may be a biomarker differently expressed in saliva in relation to risk factors.
Protein biomarkers (IL10, TNFα, TGFβ and VEGF) were studied in patients with head and neck cancer, this study reported higher salivary levels of all biomarkers in cases compared to controls [Citation50]. In the same research, significantly higher IL10 levels were reported in smokers and cases infected with HPV and EBV. From these results, it may be deduced that elevation of anti-inflammatory cytokine IL10 is a mechanism involved in disease initiation in both smoking and viral induced head and neck cancer.
From the results of the present study, following research gaps were identified. From the many salivary biomarkers reported in OSCC and OPMD, only a small proportion was assessed for their relationships to risk factors. Most biomarkers were related to smoking and alcohol habits. Other risk factors such as betel quid, oral health indices and viral infections were less frequently assessed. No records were identified assessing the relationships between biomarkers and risk factors such as genetic predisposition, smokeless tobacco preparations such as snuff, indicators of socioeconomic status, denture wearing, dietary factors, genetic diseases and drug use. These were identified as research needs in the assessment of salivary biomarkers in OSCC and OPMD.
5. Expert opinions
The present systematic review included data extracted from 295 research articles reporting salivary biomarkers in OSCC and OPMD. Identifying biomarkers with high performance in terms of sensitivity and specificity to OSCC help to accelerate future research. Through descriptive data analysis, the present study has identified a proteomic salivary biomarker panel (IL1β, IL6 and IL8) useful for early detection and screening of OSCC and OPMD. This biomarker panel is proposed as suitable for clinical validation as screening and early detection tools for OPMD and OSCC. One disadvantage of the selected biomarkers is that they are inflammatory cytokines; this can be overcome by using a combination of three biomarkers, and using threshold values optimized for the specific patient population.
Relationships between biomarkers reported in saliva and risk factors have been discussed to elucidate mechanisms induced by risk factors in disease initiation. Research gaps in the assessment of salivary biomarkers with respect to their relationships to risk factors have been highlighted. Results of this systematic review indicate that future studies should be directed to assess potential salivary biomarkers for their relationships to risk factors in order to understand the biomarker’s role in initiation of disease.
Article highlights
Interleukin 1 beta, 6 and 8 is a biomarker panel suitable for early detection of OSCC and OPMD through salivary analysis
Salivary biomarkers in OSCC and OPMD have relationships with risk factors
Relationships between salivary biomarkers and risk factors are important to assess the biomarker’s role in disease initiation
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
N Piyarathne was financially supported by the University of Aberdeen (Elphinstone scholarship), UK and the University grants commission (UGC/VC/DRIC/PG2017(11)/PDN/03) Sri Lanka. Authors would like to thank the library staff at Medical library and Sir Duncan Rice library of the University of Aberdeen for their support in the literature search and recovery of research articles.
Additional information
Funding
References
- Conway DI, Purkayastha M, Chestnutt IG. The changing epidemiology of oral cancer : definitions, trends, and risk factors. Nat Publ Gr. 2018;225(9):867–873.
- Warnakulasuriya S. Oral potentially malignant disorders: a comprehensive review on clinical aspects and management. Oral Oncol. 2020;102: 104550.
- World Health Organization. GLOBOCAN 2020 [Internet]. Cancer today. 2020. Available from: https://gco.iarc.fr/today/home
- Mello FW, Miguel AFP, Dutra KL, et al. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med. 2018 Aug;47(7):633–640.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov 1;68(6):394–424.
- Sarode G, Maniyar N, Sarode SC, et al. Epidemiologic aspects of oral cancer. Disease-a-Month. 2020;66(12):100988.
- Jin LJ, Lamster IB, Greenspan JS, et al. Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016 Oct;22(7):609–619.
- Ghani WMN, Ramanathan A, Prime SS, et al. Survival of oral cancer patients in different ethnicities. Cancer Invest. 2019;37(7):275–287.
- Capote-Moreno A, Brabyn P, Muñoz-Guerra MF, et al., Oral squamous cell carcinoma: epidemiological study and risk factor assessment based on a 39-year series. Int J Oral Maxillofac Surg. 49(12): 1525–1534. 2020.
- Tirelli G, Gatto A, Bonini P, et al. Prognostic indicators of improved survival and quality of life in surgically treated oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018 Jan;126(1):31–40.
- Speight PM, Epstein J, Kujan O, et al. Screening for oral cancer-a perspective from the Global Oral Cancer Forum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017 Jun;123(6):680–687.
- Nikitakis NG, Pentenero M, Georgaki M, et al. Molecular markers associated with development and progression of potentially premalignant oral epithelial lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):650–669.
- Rai V, Mukherjee R, Ghosh AK, et al. “Omics” in oral cancer: new approaches for biomarker discovery. Available from Arch Oral Biol. 2018;87February2017:15–34.
- Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood). 2018 Feb;243(3):213–221.
- Kaczor-Urbanowicz KE, Martin Carreras-Presas C, Aro K, et al. Saliva diagnostics – current views and directions. Exp Biol Med. 2017;242(5):459–472.
- Chi LM, Hsiao YC, Chien KY, et al. Assessment of candidate biomarkers in paired saliva and plasma samples from oral cancer patients by targeted mass spectrometry. J Proteomics. 2020;211:103571.
- Lee LT, Wong YK, Hsiao HY, et al. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2018;47(6):699–707.
- Amenabar JM, Da Silva BM, Punyadeera C. Salivary protein biomarkers for head and neck cancer. Expert Rev Mol Diagn. 2020;1–9.
- Adeoye J, Brennan PA, Thomson P. “Search less, verify more”-Reviewing salivary biomarkers in oral cancer detection. J Oral Pathol Med. 2020;49(8): 711–719.
- Warnakulasuriya S. Risk assessment in oral cancer. Springer; 2020. p. 119–132.
- Al Moustafa AE. Development of oral cancer. 2017.
- Diajil A, Thomson P Risk factors of oral cancer and potentially malignant disorders (PMDs) – developing a high/low risk profiling system. 2016.
- Arellano-Garcia ME, Hu S, Wang J, et al. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008 Nov;14(8):705–712.
- Babiuch K, Kuśnierz-Cabala B, Kęsek B, et al. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J Clin Med. 2020;9(3):867.
- Bagan L, Sáez GT, Tormos MC, et al. Salivary and serum interleukin-6 levels in proliferative verrucous leukoplakia. Clin Oral Investig. 2016;20(4):737–743.
- Brinkmanna O, Kastratovicb DA, Milovan V, et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2012;47(1):51–55.
- Gleber-Netto FO, Yakob M, Li F, et al. Salivary biomarkers for detection of oral squamous cell carcinoma in a Taiwanese population. Clin Cancer Res. 2016;22(13):3340–3347.
- Hamad AWR, Gaphor SM, Shawagfeh MT, et al. Study of serum and salivary levels of proinflammatory cytokines, potential biomarkers in the diagnosis of oral squamous cell carcinoma. Acad J Cancer Res. 2011;4(2):47–55.
- Hamzavi M, Tadbir AA, Rezvani G, et al. Tissue expression, serum and salivary levels of IL-10 in patients with head and necksquamous cell carcinoma. Asian Pacific J Cancer Prev. 2013;14(3):1681–1685.
- Khyani IAM, Qureshi MA, Mirza T, et al. Detection of interleukins-6 and 8 in saliva as potential biomarkers of oral pre-malignant lesion and oral carcinoma: a breakthrough in salivary diagnostics in Pakistan. Pak J Pharm Sci. 2017 May;30(3):817–823.
- Korostoff A, Reder L, Masood R, et al. The role of salivary cytokine biomarkers in tongue cancer invasion and mortality. Oral Oncol. 2011;47(4):282–287.
- Lisa Cheng Y-S, Jordan L, Gorugantula LM, et al. Salivary interleukin-6 and −8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J Periodontol. 2014;85(7):956–965.
- Panneer Selvam N, Sadaksharam J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac J Clin Oncol. 2015;11(3):236–241.
- Punyani SR, Sathawane RS. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin Oral Investig. 2013;17(2):517–524.
- Rajkumar K, Nandhini G, Ramya R, et al. Validation of the diagnostic utility of salivary interleukin 8 in the differentiation of potentially malignant oral lesions and oral squamous cell carcinoma in a region with high endemicity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(3):309–319.
- Rhodus NL, Cheng B, Myers S, et al. The feasibility of monitoring NF-κB associated cytokines: TNF-α, IL-1α, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog. 2005;44(2):77–82.
- Sahebjamee M, Eslami M, Atarbashimoghadam F, et al. Salivary concentration of TNF? IL1? IL6, and IL8 in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2008;13(5):292–295.
- Sharma M, Bairy I, Pai K, et al. Salivary IL-6 levels in oral leukoplakia with dysplasia and its clinical relevance to tobacco habits and periodontitis. Clin Oral Investig. 2011;15(5):705–714.
- Singh P, Verma JK, Singh JK. Validation of salivary markers, IL-1β, IL-8 and Lgals3bp for detection of oral squamous cell carcinoma in an Indian population. Sci Rep. 2020 Apr;10(1):7365.
- St. John MAR, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol - Head Neck Surg. 2004;130(8):929–935.
- Tan W, Sabet L, Li Y, et al. Optical protein sensor for detecting cancer markers in saliva. Biosens Bioelectron. 2008;24(2):266–271.
- Lee LT, Wong YK, Hsiao HY, et al. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2018;47(6):699–707.
- Hu SY, Lin MH, Yang SC, et al. Areca quid chewing enhances the expression of salivary matrix metalloproteinase-9. J Formos Med Assoc. 2005;2(104):113–119.
- Krishnan R, Thayalan DK, Padmanaban R, et al. Association of serum and salivary tumor necrosis factor-α with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pacific J Cancer Prev. 2014;15(17):7141–7148.
- Liu SY, Lin MH, Yang SC, et al. Increased expression of matrix metalloproteinase-2 in oral cells after short-term stimulation and long-term usage of areca quid. J Formos Med Assoc. 2005;06(104):390–397.
- Wang Q, Gao P, Cheng F, et al. Measurement of salivary metabolite biomarkers for early monitoring of oral cancer with ultra performance liquid chromatography-mass spectrometry. Talanta. 2014;119:299–305.
- De Souza MB, De Carvalho MB, De Carvalho MB, et al. Serum and salivary macrophage migration inhibitory factor in patients with oral squamous cell carcinoma. Oncol Lett. 2014;8(5):2267–2275.
- El-Naggar AK, Mao L, Staerkel G, et al., Genetic heterogeneity in saliva from patients with oral squamous carcinomas. J Mol Diagnostics. 3(4): 164–170. 2001.
- Pierce Campbell CM, Giuliano AR, Torres BN, et al. Salivary secretory leukocyte protease inhibitor (SLPI) and head and neck cancer: the cancer prevention study II nutrition cohort. Oral Oncol. 2016;55:1–5.
- Polz-Dacewicz M, Strycharz-Dudziak M, Dworzański J, et al. Salivary and serum IL-10, TNF-α, TGF-β, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infect Agent Cancer. 2016;11(1):1–8.
- Rettori MM, De carvalho AC, Bomfim longo AL, et al. Prognostic significance of TIMP3 hypermethylation in post-treatment salivary rinse from head and neck squamous cell carcinoma patients. Carcinogenesis. 2013;34(1):20–27.
- Bernardes VF, Gleber-Netto FO, Sousa SF, et al. EGF in saliva and tumor samples of oral squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2011;19(6):528–533.
- Aziz S, Ahmed SS, Ali A, et al. Salivary immunosuppressive cytokines IL-10 and IL-13 are significantly elevated in oral squamous cell carcinoma patients. Cancer Invest. 2015;33(7):318–328.
- Jiang WW, Masayesva B, Zahurak M, et al. Increased mitochondrial DNA content in saliva associated with head and neck cancer. Clin Cancer Res. 2005;11(7):2486–2491.
- Jiang WW, Rosenbaum E, Mambo E, et al. Decreased mitochondrial DNA content in posttreatment salivary rinses from head and neck cancer patients. Clin Cancer Res. 2006;12(5):1564–1569.
- Sayall L, Mashlah A, Kassem I. Evaluation of mitochondrial DNA content in saliva of oral squamous cell carcinoma and leukoplakia as non-invasive biomarker. Int J PharmTech Res. 2015;7(4):573–579.
- Bernardes VF, Gleber-Netto FO, Sousa SF, et al. Clinical significance of EGFR, Her-2 and EGF in oral squamous cell carcinoma: a case control study. J Exp Clin Cancer Res. 2010;29(1):1–7.
- Demokan S, Chang X, Chuang A, et al. KIF1A and EDNRB are differentially methylated in primary HNSCC and salivary rinses. Int J Cancer. 2010;127(10):2351–2359.
- Arduino PG, Menegatti E, Cappello N, et al. Possible role for interleukins as biomarkers for mortality and recurrence in oral cancer. Int J Biol Markers. 2015;30(2):e262–6.
- Brailo V, Vučićević-Boras V, Cekić-Arambašin A, et al. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006;42(4):370–373.
- Yu J-S, Chen Y-T, Chiang W-F, et al. Saliva protein biomarkers To detect oral squamous cell carcinoma in a high-risk population in Taiwan. Proc Natl Acad Sci U S A. 2016;113(41):11549–11554.
- Zanotti L, Paderno A, Piazza C, et al. Epidermal growth factor receptor detection in serum and saliva as a diagnostic and prognostic tool in oral cancer. Laryngoscope. 2017;127(11):E408–14.
- Wang Q, Gao P, Wang X, et al. Investigation and identification of potential biomarkers in human saliva for the early diagnosis of oral squamous cell carcinoma. Clin Chim Acta. 2014;427:79–85.
- Rhodus NL, Ho V, Miller CS, et al. NF-κB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29(1):42–45.
- Brailo V, Vucicevic-Boras V, Lukac J, et al. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med Oral Patol Oral Cir Bucal. 2012;17(1):6–11.
- Juretić M, Cerović R, Belušić-Gobić M. et al. Salivary levels of TNF-α and IL-6 in patients with oral premalignant and malignant lesions. Folia Biol. 2013;59(2):99–102.
- Carvalho AL, Henrique R, Jeronimo C, et al. Detection of promoter hypermethylation in salivary rinses as a biomarker for head and neck squamous cell carcinoma surveillance. Clin Cancer Res. 2011;17(14):4782–4789.
- Franzmann EJ, Reategui EP, Carraway KL, et al. Salivary soluble CD44: a potential molecular marker for head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(3):735–739.
- Heawchaiyaphum C, Pientong C, Phusingha P, et al. Peroxiredoxin-2 and zinc-alpha-2-glycoprotein as potentially combined novel salivary biomarkers for early detection of oral squamous cell carcinoma using proteomic approaches. J Proteomics. 2018;173:52–61.
- Franzmann EJ, Reategui EP, Mateus Pereira LH, et al. Salivary protein and solcd44 levels as a potential screening tool for early detection of head and neck squamous cell carcinoma. Head Neck. 2012;34(5):687–695.
- Ganesan A, Gautham Kumar N. Assessment of lipid peroxides in multiple biofluids of leukoplakia and oral squamous cell carcinoma patients - A clinico- biochemical study. J Clin Diagn Res. 2014;8(8):10–13.
- Sivaramakrishnan M, Sivapathasundharam B, Jananni M. Evaluation of lactate dehydrogenase enzyme activity in saliva and serum of oral submucous fibrosis patients. J Oral Pathol Med. 2015;44(6):449–452.
- Liyanage C, Wathupola A, Muraleetharan S, et al. Promoter Hypermethylation of Tumor-Suppressor Genes p16(INK4a), RASSF1A, TIMP3, and PCQAP/MED15 in Salivary DNA as a Quadruple Biomarker Panel for Early Detection of Oral and Oropharyngeal Cancers. Biomolecules. 2019;9(4):148.
- Raffat MA, Hadi NI, Hosein M, et al. Differential expression of salivary S100A7 in oral submucous fibrosis. Saudi Dent J. 2019;31(1):39–44.
- Lim Y, Wan Y, Vagenas D, et al., Salivary DNA methylation panel to diagnose HPV-positive and HPV-negative head and neck cancers. BMC Cancer. 16(1): 1–12. 2016.
- Sato J, Ohuchi M, Wada M, et al. Differences in sequential posttreatment salivary IL-6 levels between patients with and patients without locoregional recurrences of oral squamous cell carcinoma: part III of a cohort study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(6):751–760.e2.
- Vesty A, Gear K, Biswas K, et al. Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin Exp Dent Res. 2018 Nov 28;4(6):255–262.
- Márton IJ, Horváth J, Lábiscsák P, Márkus B, Dezső B, Szabó A, Tar I, Piffkó J, Jakus P, Barabás J, Barabás P, Olasz L, Kövér Z, Tőzsér J, Sándor J, Csősz É, Scholtz B, Kiss C. Salivary IL-6 mRNA is a Robust Biomarker in Oral Squamous Cell Carcinoma. J Clin Med. 2019 Nov 13;8(11):1958. doi:10.3390/jcm8111958. PMID: 31766212; PMCID: PMC6912409
- Jayasooriya PR, Pitakotuwage TN, Lombardi T. Descriptive study of 896 Oral squamous cell carcinomas from the only University based Oral Pathology Diagnostic Service in Sri Lanka. BMC Oral Health. 2016 Jan 8;16(1). 10.1186/s12903-015-0139-y.
- Pfaffe T, Cooper-White J, Beyerlein P, et al. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57(5):675–687.
- Katakura A, Kamiyama I, Takano N, et al. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull Tokyo Dent Coll. 2007;48(4):199–203.
- Upile T, Jerjes W, Kafas P, et al. Salivary VEGF: a non-invasive angiogenic and lymphangiogenic proxy in head and neck cancer prognostication. Int Arch Med. 2009;2(1):1–4.
- van der Merwe L, Wan Y, Cheong HJ, et al. A pilot study to profile salivary angiogenic factors to detect head and neck cancers. BMC Cancer. 2018 Jul;18(1):734.
- He H, Chen G, Zhou L, et al. A joint detection of CEA and CA-50 levels in saliva and serum of patients with tumors in oral region and salivary gland. J Cancer Res Clin Oncol. 2009;135(10):1315–1321.
- Malhotra R, Urs AB, Chakravarti A, et al. Correlation of Cyfra 21-1 levels in saliva and serum with CK19 mRNA expression in oral squamous cell carcinoma. Tumor Biol. 2016;37(7):9263–9271.
- Ghalwash DM, El GK, Zahran FM, et al. The diagnostic and prognostic value of salivary sCD44 level determination in oral malignant and potentially premalignant lesions. Adv Environ Biol. 2012;6(1):302–310.
- Lokesh K, Kannabiran J, Rao MD. Salivary lactate dehydrogenase (LDH)- A novel technique in oral cancer detection and diagnosis. J Clin Diagn Res. 2016;10(2):ZC34–7.
- Totan A, Miricescu D, Parlatescu I, et al. Possible salivary and serum biomarkers for oral lichen planus. Biotech Histochem. 2015;90(7):552–558.
- Sahibzada HA, Khurshid Z, Khan RS, et al. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics. 2017;7(2):21.
- Dikova VR, Principe S, Bagan JV. Salivary inflammatory proteins in patients with oral potentially malignant disorders. J Clin Exp Dent. 2019 Jul;11(7):e659–64.
- Rezaei F, Mozaffari HR, Tavasoli J, et al. Evaluation of serum and salivary interleukin-6 and interleukin-8 levels in oral squamous cell carcinoma patients: systematic review and meta-analysis. J Interferon Cytokine Res. 2019 Dec;39(12):727–739.
- Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054–1061.
- Csosz É, Lábiscsák P, Kalló G, et al., Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS One. 12(5): 1–22. 2017.