ABSTRACT
Background: This study aims to quantify medication costs in juvenile idiopathic arthritis (JIA), based on subtype.
Research design and methods: This study is a single-center, retrospective analysis of prospective data from electronic medical records of JIA patients, aged 0–18 years between 1 April 2011 and 31 March 2019. Patient characteristics (age, gender, subtype) and medication use were extracted. Medication use and costs were reported as: 1) mean total annual costs; 2) between-patient heterogeneity in these costs; 3) duration of medication use; and, 4) costs over the treatment course.
Results: The analysis included 691 patients. Mean total medication costs were €2,103/patient/year, including €1,930/patient/year (91.8%) spent on biologicals. Costs varied considerably between subtypes, with polyarticular rheumatoid-factor positive and systemic JIA patients having the highest mean costs (€5,020/patient/year and €4,790/patient/year, respectively). Mean annual medication costs over the patient’s treatment course ranged from <€1,000/year (71.1% of patients) to >€11,000/year (2.5% of patients). Etanercept and adalimumab were the most commonly used biologicals. Cost fluctuations over the treatment course were primarily attributable to biological use.
Conclusions: Polyarticular rheumatoid-factor positive and systemic JIA patients had the highest mean total annual medication costs, primarily attributable to biologicals. Costs varied considerably between subtypes, individuals, and over the treatment course.
1. Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatologic disorder in childhoodFootnote1, affecting approximately 1 in 1,000 children [Citation1,Citation2]. JIA is characterized by pain, stiffness, joint damage, growth abnormalities and (long-term) functional impairment, and consequently, a lower quality of life [Citation3–6]. To minimize the (long-term) impact of JIA, early recognition and adequate treatment is crucial [Citation7].
Treatment of JIA consists of pharmaceutical treatment, combined with physical therapy, occupational therapy, and psychosocial support [Citation8–10]. Previous studies have shown that medication use contributes to approximately half of the total cost of managing JIA [Citation11–13]. The development of biological disease-modifying anti-rheumatic drugs (DMARDs), in particular, has increased treatment opportunities in JIA. These medicines are however 20 up to almost 500 times as expensive compared to conventional medicines like methotrexate (MTX) [Citation14]. Despite the higher costs attributed to these medications, a more effective disease control may decrease other treatment-related costs (e.g. fewer consultations with pediatric rheumatologists or physiotherapists, or fewer hospitalizations). In addition, it may decrease the (long-term) burden of JIA to society in terms of missed school or work days by patients, parents and/or caregivers [Citation15,Citation16].
Based on clinical and laboratory features, the International League of Associations for Rheumatology (ILAR) classification distinguished seven subtypes of JIA [Citation17]. These subtypes differ in terms of treatment prescribed, as well as in disease severity, response to treatment and prognosis [Citation17–19]. In addition, significant variation has been reported among patients of the same subtype [Citation20,Citation21]. Beside this patient-level heterogeneity, previous research found that early, aggressive treatment with biologicals was more effective in lowering disease activity compared to conservative delayed treatment [Citation22], and that treat-to-target approaches proved to be superior to routine care in reaching remission [Citation23,Citation24]. All in all, these findings highlight the need for personalized treatment strategies in JIA [Citation25].
Such personalized treatment strategies will inevitably lead to differences in medication use between patients, as well as in differences in terms of health outcomes and costs. Quantifying patient-level variations in current medication use and the accompanying costs therefore is a crucial first step in determining the potential impact of these personalized treatment strategies. However, the majority of studies that investigated medication costs of JIA either focussed on one specific JIA subtype, did not distinguish between JIA subtypes, or did not consider costs on the individual patient level [Citation26]. Therefore, the current study aims to quantify the total costs of medication use, as well as the costs of JIA-related medication use in particular (i.e. immune modulating medication), for all JIA patients and depending on subtype, by means of 1) quantifying the mean total annual costs of medication use, 2) provide insights in between-patient heterogeneity in costs of JIA-related medication use, 3) investigate the duration of use of JIA-related medication, and 4) investigate costs of JIA-related medication over the patient’s treatment course.
2. Methods
2.1. Data sources
This study was a retrospective analysis of prospective data extracted from electronic medical records from the Wilhelmina Children’s Hospital (Utrecht, the Netherlands), using a previously developed research data platform [Citation27]. Patients with a diagnosis of JIA and treated in the Wilhelmina Children’s Hospital between 1 April 2011 and 31 March 2019 were included. Data on medication use within this time period were extracted from this platform using a unique, de-identified patient number. As treatment guidelines in JIA have changed quickly with the increasing availability of biologicals and because the availability of data in electronic form after 1 April 2011, this date was chosen as starting date of the analysis. As this study focuses on children, from patients who turned 18 before 31 March 2019 only data up until the patient’s 18th birthday was included.
2.2. Data selection and extraction
Exclusion criteria involved: patients turning 18 years of age before 1 April 2011; patients diagnosed with idiopathic uveitis; patients not primarily treated in the Wilhelmina Children’s Hospital (e.g. second opinion only); patients with major comorbidities besides their JIA (such as inflammatory bowel disease); patients who received treatment as part of a pharmaceutical trial which they would not have received outside the trial setting; patients with a follow-up of less than 1 year. Patients who were lost to follow-up, for example because they continued treatment in another hospital, were included until that point in time. None of the patients included in the database died during follow-up. Resource use and costs were included up to 10 years after JIA diagnosis. As some types of biologicals were prescribed to small numbers of patients, the biologicals that were prescribed to fewer than five patients were reported as such (instead of mentioning the exact number), to prevent results from being traced back to individual patients.
2.3. Medication use and costs
Data on medication use and accompanying costs were quantified from a payer’s perspective. All medication use (including type, dosage, and frequency and mode of administration) was derived from the data extracted from the hospital pharmacy. The associated drug prices were obtained from Dutch pharmaceutical list prices [Citation14] in 2019 Euros, regardless of the year in which they were prescribed. Daily cost of medication use per patient were calculated by multiplying the frequency and dosage used in each individual patient with its unit price. All daily cost accounted for fluctuations in medication dosage and/or frequency over the patient’s treatment course (for example for biologicals). When medicines were taken or administered at regular intervals (for example bi-weekly injections with biologicals), the costs per administration were equally distributed over this interval and expressed as costs per day.
All medication prescribed during the inclusion period which was assumed to be either directly or indirectly attributable to JIA was included in the initial analysis to estimate the overall impact of JIA on medication use. In case of uncertainty, this was decided in consultation with a pediatric rheumatologist. For the in depth analyses, we excluded medication classified as ‘other medication’ that is exclusively used to treat symptoms associated with JIA since a substantial part of this medication is not primarily aimed at achieving clinically inactive disease, but instead at alleviating pain (e.g. ibuprofen), treating side effects of JIA-related medication (e.g. MTX-related nausea), or to treat (potential) complications of JIA (e.g. eye drops for uveitis). So this study focuses on medication which is aimed at achieving clinically inactive disease (i.e. immune modulating medication), including biological DMARDs (i.e. biologicals), non-biological DMARDs, intra-articular injections, and steroids. These medications will be referred to as JIA-related medication in the remainder of this manuscript. Although some of this JIA-related medication may also be prescribed to treat uveitis (as complication of the patient’s JIA), or to treat both uveitis and joint inflammation, these medicines were included in the analysis regardless of their indication for use. A detailed overview of all assumptions made with regard to resource use and cost estimates, as well as the unit prices used for JIA-related medication is provided in the Electronic Supplementary Material.
2.4. Analysis
Patients were classified into JIA subtype according to the ILAR criteria [Citation17]. As persistent oligoarticular JIA was the most common subtype, and because the risk of developing JIA-related uveitis depends on a patient’s antinuclear antibody (ANA) status [Citation28], this subtype was further subdivided into ANA– oligoarthritis and ANA+ oligoarthritis. The duration of medication use and accompanying costs were calculated for all JIA patients in general and by JIA subtype.
The date of JIA diagnosis was set as starting point of the analysis, resulting in a different duration of follow-up between patients, as well as a different part of the patient’s treatment course that is captured. In other words, for a patient diagnosed in March 2017, the data collected between 1 April 2011 and 31 March 2019 will only capture the first 2 years after JIA diagnosis, whereas for other patients, only a period of inactive disease may have been captured. Therefore, the duration of medication use and accompanying costs were expressed as days/patient/year and costs/patient/year, respectively, instead of as the total duration and total costs per patient. For the analysis of the mean duration of biological use per year, only patients who received a biological during their follow-up period were included. For each of these patients, the first month at which a biological was prescribed was used as a starting point of the analysis. In other words, in this analysis, the time period from JIA onset to the first time a biological was prescribed was excluded.
Since part of the variation observed between patients was attributable to the part of the treatment course that was captured in the database, reporting 95% confidence intervals would not have been of added value. Therefore, patient-level variations in costs were visualized using histograms. All analyses were performed using R (version 3.5.3), and the packages dplyr, ggforce, ggplot2, lubridate and plotrix [Citation29–34].
3. Results
There were 969 patients in the database of which 691 patients fulfilled the inclusion criteria, as described in Figure S1 of the Electronic Supplementary Material. Of these 691 patients, 211 (30.5%) received a biological at some point in time during the period of follow-up captured in the current analysis. The median age at JIA diagnosis was 8.0 years for all patients and 9.0 years for biological users, respectively. The median duration of follow-up of was 4.9 and 4.7 years ().
Table 1. Characteristics of patients included in the analysis, reported separately for all patients and for patients who received a biological during the period of follow-up in this study
3.1. Mean total annual costs of medication use in JIA
The total mean costs of medication use among all JIA patients was €2,103/patient/year. Of these costs, €1,930/patient/year was attributable to biologicals (i.e. 91.8%), €97/patient/year to non-biological DMARDs, intra-articular injections and steroids (i.e. 4.6%), and €76/patient/year to other medication (i.e. 3.6%). As mentioned previously, the costs for other medications were excluded from the analysis.
In , mean total medication costs per patient per year are shown for all JIA patients in general and depending on subtype. The mean total annual costs per patient were the highest in patients with polyarticular RF+ JIA (i.e. €5,020/patient/year), followed by patients with systemic JIA (i.e. €4,790/patient/year). When multiplying these costs with the number of patients in each JIA subgroup, the subgroup to which (overall) most medication costs were spent involved patients with polyarticular RF– JIA (20.8% of all JIA patients, 25.5% of total expenditures).
Table 2. Heat map showing an overview of mean total annual costs of medication per patient for all JIA patients and specified per subtype
When considering costs of JIA-related medication (but excluding biologicals), €66/patient/year was spent on MTX, €26/patient/year on DMARDs other than MTX (including hydroxychloroquine, leflunomide, sulfasalazine, mycophenolic acid, and tofacitinib), €3/patient/year on steroids, and €2/patient/year on intra-articular injections. The three JIA subgroups with the highest mean annual costs when excluding biologicals were extended oligoarticular JIA, polyarticular RF+ JIA, and polyarticular RF– JIA.
Of all medication costs in JIA, 91.8% was attributable to biologicals, which were used by 211 patients (i.e. 30.5%) of all patients included in the database. Costs of biologicals were analyzed in more detail in . The highest mean annual costs of biological use were observed in patients with polyarticular RF+ JIA (i.e. €4,788/patient/year) and systemic JIA (i.e. €4,725/patient/year). Of all costs for biologicals, most costs were spent on adalimumab (40.2%) and etanercept (27.4%), which were used by 108 and 101 patients, respectively. Canakinumab came in third place, with 13.4% of total expenditures on biologicals, but these costs were skewed as these were attributable to a small number of systemic JIA patients (i.e. <5).
Table 3. Heat map showing an overview of mean annual total costs of biologicals per patient, among users only, for all JIA patients and specified per subtype
3.2. Between-patient heterogeneity in medication costs
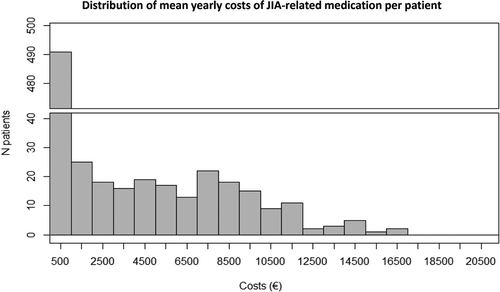
As do not represent differences in costs on an individual patient-level, this patient-level heterogeneity in mean annual total costs of JIA-related medication was visualized in a histogram, showing a strongly right skewed distribution (). More specifically, in 491 out of 691 patients (i.e. 71.1%) the mean annual total costs of JIA-related medication ranged between €0 and €1,000 over their episode of follow-up, with 187 patients (i.e. 27.1%) having no costs of medication within their episode of follow-up. In addition, 17 patients had mean annual costs of €11,000 or higher. Four of these patients were however out of range in this figure, which included patients with systemic JIA or polyarticular RF+ JIA, in which these high costs were attributable to treatment with canakinumab, tocilizumab (intravenous), golimumab, and adalimumab. Note that these analyses present mean annual costs on an individual patient-level, not accounting for the duration of follow-up. As shown in Figures S2 a-i of the Electronic Supplementary Material, similar right-skewed distributions were observed regardless of JIA subtype.
Figure 1. Distribution of mean total annual costs of JIA-related medication per patient (including biologicals, non-biological DMARDs, intra-articular injections, and steroids), regardless of JIA subtype. In this figure, 4 patients were excluded because they were out of range in this figure. These involved patients with systemic JIA or polyarticular RF+ JIA. DMARDs = disease-modifying anti-rheumatic drugs; JIA = juvenile idiopathic arthritis, RF = rheumatoid factor
3.3. Duration of medication use
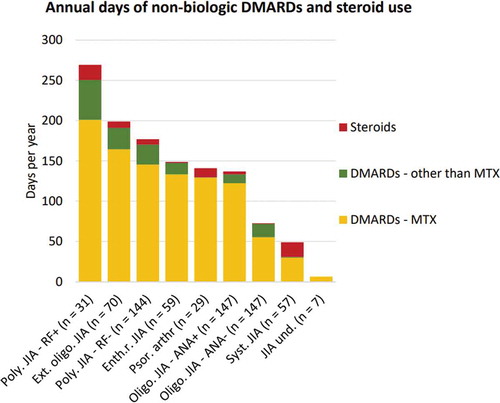
When considering the duration of use of non-biologic DMARDs and steroids over the entire follow-up episode of all 691 patients, patients with polyarticular RF+ JIA, on average, received these drugs for most days per year (). More specifically, this figure indicates that, on average, patients with polyarticular RF+ JIA received MTX for 201 days per year, other DMARDs for 50 days per year, and steroids for 18 days per year. As intra-articular injections involve single-day treatments, these were excluded from this figure.
Figure 2. Mean annual number of days non-biological DMARDs and steroids. This figure represents the mean annual number of days of non-biological DMARD use (divided into MTX and other DMARDs) and systemic steroids among all JIA patients during the entire follow-up period, specified per JIA subtype. ANA = antinuclear antibody; DMARDs = disease-modifying anti-rheumatic drug; Enth.r. = enthesitis-related; Ext. = extended; JIA = juvenile idiopathic arthritis; MTX = methotrexate, Oligo. = oligoarticular; Poly. = polyarticular; Psor. arthr = psoriatic arthritis; RF = rheumatoid factor; Syst. = systemic; und. = undifferentiated
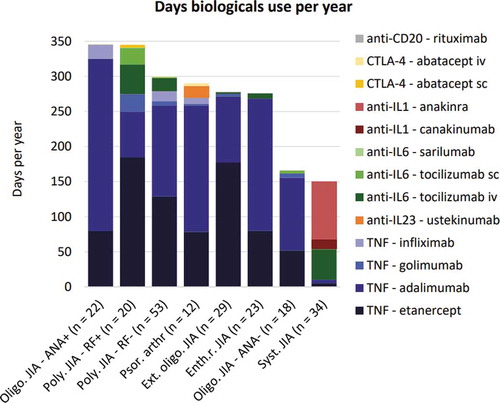
When only considering the subgroup of patients who were prescribed a biological during their follow-up period (n = 211), patients with oligoarticular ANA+ JIA were found to receive, on average, a biological for the highest number of days (i.e. 346 days/year) (). In contrast, systemic JIA patients received on average the fewest days of biologicals (i.e. 150 days/year), apart from the 7 patients with undifferentiated JIA who did not receive biologicals at all. This figure also illustrates that Tumor Necrosis Factor (TNF) alfa inhibitors were the class of biological prescribed for the majority of time (especially etanercept and adalimumab), regardless of JIA subtype, except for patients with systemic JIA. Anakinra and canakinumab were however exclusively prescribed to systemic JIA patients.
Figure 3. Mean days of biological use per year among users. This figure represents the mean days of biological use per year among JIA patients who were prescribed a biological during the follow-up period n = 211), specified per subtype. The first month in which a biological was prescribed was used as starting point of the analysis for each individual patient. ANA = antinuclear antibody; CTLA-4 = cytotoxic T-lymphocyte-associated protein 4; Enth.r. = enthesitis-related; Ext. = extended; IL = interleukin; JIA = juvenile idiopathic arthritis; Oligo. = oligoarticular; Poly. = polyarticular; Psor. arthr = psoriatic arthritis; RF = rheumatoid factor; Syst. = systemic; TNF = tumor necrosis factor; und. = undifferentiated
3.4. Medication costs over the patient’s treatment course
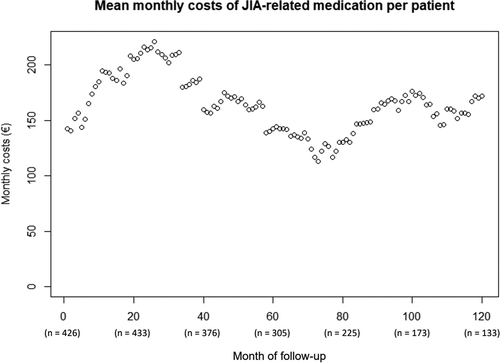
As costs for JIA-related medication use likely fluctuate over the course of JIA treatment, shows the mean monthly costs of medication over the 10-year follow-up period. Each point in the graph represents the mean monthly costs per patient, when taking the mean over the patients for which data are available for each of the 120 months of follow-up (with ‘0ʹ representing the moment of JIA diagnosis). This figure indicates that costs of JIA-related medication use increased after JIA diagnosis and peaked after approximately 25 months of follow-up. A second (although lower) peak was observed around 100 months of follow-up. To get insight about the extent to which these fluctuations in costs over time were attributable to the different types of medication, these mean monthly costs per patient were plotted for each type of medication separately (Figures S3a-q of the Electronic Supplementary Material). This indicates that the peak at approximately 25 months in was mainly attributable to the use of canakinumab, whereas the peak at approximately 100 months was mainly attributable to adalimumab. In addition, Figure S3b of the Electronic Supplementary Material shows that anakinra was often prescribed as first-line treatment (among patients with systemic JIA), and that its use decreased strongly over a time period of approximately 20 months.
Figure 4. Overview of mean monthly costs of JIA-related medication per patient over the course of follow-up. Each point represents the mean costs of JIA-related medication for the set of patients of which data is available during each of the 120 months. ‘0ʹ represents the moment of JIA diagnosis. ‘n’ represents the number of patients included at the different months of follow-up
4. Discussion
Overall, the highest mean annual costs of medication were observed in patients with polyarticular RF+ JIA, although major variation was observed between JIA subtypes and between individual patients. MTX was the most commonly used medicine, but the great majority of costs was attributable to the use of biologicals, in particular etanercept and adalimumab. These were also the biologicals which were prescribed for the highest number of days per year. In systemic JIA patients however, canakinumab and anakinra were the main cost drivers. Although canakinumab was only prescribed to <5 patients (as compared to 31 patients who received anakinra), the daily costs of canakinumab were approximately 12 times higher compared with anakinra (i.e. €428/day vs. €35/day), resulting in higher mean annual costs.
When only considering patients from the moment at which a biological was prescribed onwards, patients with oligoarticular ANA+ JIA and polyarticular RF+ JIA were found to have the highest mean annual duration of biological use. However, as biologicals are currently not registered for oligoarticular ANA+ JIA, the high duration of biological use in this subgroup (n = 22) is likely attributable to treating JIA-related uveitis, as this JIA subgroup has the highest risk of developing uveitis [Citation28]. When considering this subgroup in detail it is observed that the mean duration of follow-up is 4.8 years, of which they receive a biological for on average 2.2 years. In addition, when biologicals are used to treat uveitis, a relatively long duration of remission is required before tapering is considered as compared to tapering decisions in JIA. This also explains why the costs of biologicals (over the entire follow-up period) are relatively low in this subgroup, although the duration of biologicals is the highest among JIA subtypes.
Although it may be expected that the mean total costs of JIA-related medication would decrease over the course of follow-up, attributable to the increasing number of patients who reach clinically inactive disease, this is not confirmed in the current analysis. More specifically, results indicate that these costs vary over the course of follow-up. This variation is mainly attributable to fluctuations in the number of patients using costly biologicals (mainly adalimumab and canakinumab). Anakinra was the only medicine in which a strong decrease in costs was observed over the first 20 months after JIA diagnosis, likely due to the strict tapering regime starting 3 months after start anakinra which is applied in the Netherlands [Citation35].
The current analysis used fixed cost prices (i.e. 2019 Euros), implying that we did not account for price differences over time, for example due to the introduction of biosimilars as an alternative to biologicals, or due to discounts attributable to price negotiations with pharmaceutical companies. Incorporating these price fluctuations was not possible because the moment of JIA diagnosis served as starting point for the analysis. To illustrate this, patients were considered in their first year of follow-up in the year following their diagnosis of JIA, regardless of whether this diagnosis was established in (for example) 2012 or 2017. In addition, as the availability and use of biologicals has increased strongly over the past decades (i.e. from 5% in 2003, to 10% in 2006 and 31% in the current study [Citation11]), it was decided to limit the follow-up to 10 years after JIA diagnosis. This maximizes the likelihood that patients in the same year of follow-up are comparable in terms of the availability of biologicals (and other treatment options) during their disease course.
4.1. Strengths
This study is unique as it reports a patient-level analysis of costs of (JIA-related) medication in a large database, distinguished according to subtype of JIA, and over a patient’s treatment course. Although costs of medication use in JIA patients have been investigated extensively [Citation12,Citation13,Citation16,Citation36–41], most of these studies either focus on one specific type of medication (or one specific biological), they do not distinguish between JIA subtypes, nor do they investigate changes in medication costs over the course of treatment.
In addition, as the current study includes patients based on a diagnosis of JIA, regardless of their current disease state, this study provides an accurate representation of the mean medication costs of the entire population of JIA patients. This likely also explains why a recent study reported higher annual medication costs in JIA patients in five different countries [Citation16]. However, similar to the findings in the current study, another study reported that costs vary strongly between patients with active disease and patients in remission (i.e. €5,681/patient/year vs. €782/patient/year) [Citation42]. Therefore, the cost benefits of a patient being in remission, which is likely attributable to the use of adequate medication, are captured in the current analysis.
An illustration of how inactive disease is captured in the current database is observed in the subgroup of patients with systemic JIA. More specifically, the relatively high annual costs of biologicals in this subgroup are explained by the rare use of canakinumab (costing €12,000 per injection), as well as by the use of anakinra as first-line treatment in systemic JIA instead of MTX. A study by the Utrecht group has shown excellent responses to anakinra as first-line treatment in systemic JIA [Citation43]. This treatment strategy has therefore been incorporated in current Dutch treatment guidelines. Furthermore, this study reported that in the majority of responding patients, treatment with anakinra could be stopped within 1 year, with remission being preserved during follow-up [Citation43]. This remission is observed in the current analysis by the strong decrease in the use (and accompanying costs) of anakinra in the first 20 months following JIA diagnosis. In turn, it also explains why systemic JIA patients have the fewest number of days of biological use per annum of all JIA subtypes.
4.2. Limitations
The impact of patents on prices of biologicals and its potential accompanying impact on medication use could not be captured. For example, after the patent expired and biosimilars were approved, the price of the originator adalimumab has been reduced up to 80% by the end of 2018. This could have resulted in more patients starting on adalimumab then on etanercept for example. As the current analysis only includes data until 1 April 2019, it is unlikely that these developments affected the results reported in this manuscript.
The costs of intra-articular injections as well as the additional costs of biologicals which are administered intravenously may have been underestimated, as the current analysis only incorporates the costs of the medication itself, and do (for example) not incorporate the time spent within a hospital, which often involves a daycare admission and potentially even anesthesia for joint injection.
Third, costs of medication use are based upon the medication prescribed by the treating physician and dispensed by the hospital pharmacy, indicating that aspects like treatment compliance could not be captured. Although compliance is likely critical for treatment success, the current analysis aims to quantify costs from a payer’s perspective, indicating that costs should be quantified in terms of medication dispensed without accounting for the extent to which this medication is actually used.
4.3. Generalizability of the results
The study was conducted as a single-center study, known to be the largest JIA research center in the Netherlands. Results are expected to be highly representative of current practice, because patients participating in pharma-sponsored studies were excluded.
However, as previously illustrated for anakinra, treatment guidelines and (accompanying) reimbursement decisions differ strongly between countries or healthcare systems. The findings of this study should therefore be interpreted in the context of the Dutch healthcare system. As an example, in the Netherlands, while the prescription of biologicals is not restricted by regulations or protocols, the reimbursement is restricted by scientific evidence for its use. In the end, if the treating physician has strong scientific evidence for a treatment or even a guideline that backs it up, one can easily prescribe that biological off label and reimbursement will follow. As a consequence, the generalizability of some of the results of the current study may be limited to countries or healthcare systems with comparable treatment guidelines, especially with regard to biologicals. In addition, aspects like waiting times prior to treatment initiation are, in contrast to some other countries [Citation44], not an issue in the Netherlands.
4.4. Implications for practice and future research
The current study provides insight in differences in the duration of medication use and accompanying costs depending on JIA subtype. It reveals the between-patient heterogeneity in these costs, as well as in fluctuations in costs over time. These insights are likely of added value to clinicians as it facilitates decisions regarding which medicine to prescribe to which patient. In addition, the results of this study are likely of added value to health technology assessment agencies as it provides insight in medication costs (and fluctuations herein) over the course of JIA treatment. In practice however, decisions regarding which biological to start in which patient may not only be affected by its expected effectiveness, but also by financial agreements about prices of biologicals and/or biosimilars. Such agreements can be made between (individual) hospitals and health insurance companies and likely differ between countries and may thus affect the medication costs as reported in this study.
In the current study, the impact of the different treatment strategies in terms of treatment efficacy or health outcomes has not been considered. Therefore, future studies should examine whether quick and adequate treatment will (as expected) lead to reaching clinically inactive disease faster, and quantify the accompanying impact in terms of healthcare-related resource use, costs and health outcomes. This would inform whether biologicals should also be prescribed as first-line therapy to other subgroups of JIA patients and not be restricted to systemic JIA patients. This is currently investigated in a multicenter, international collaborative project named UCAN CAN-DU, which is the Canada-Netherlands Personalized Medicine Network in Childhood Arthritis and Rheumatic diseases.
4.5. Conclusion
Patients with polyarticular RF+ and systemic JIA have on average the highest mean total costs of medication, primarily attributable to biologicals. Substantial between-patient heterogeneity in medication costs was found. MTX was by far the most commonly used medicine, whereas adalimumab and etanercept were the most commonly used biological. Fluctuations in costs of medication use over time were primarily attributable to variations in the number of patients receiving biologicals and their accompanying costs.
Declaration of interest
DM reports non-financial support from consultancy (Illumina) and ISPOR, and personal fees from Analytica, outside the submitted work; RY reports consulting fees from Novartis and Lily outside the submitted work. SV reports grants and personal fees from SOBI and Novartis during the conduct of the study; JS reports grants from SOBI, outside the submitted work; MK, SdR, MS, GC, LG, MT, SB, NW, AvR and MIJ have nothing to disclose. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Author contributions
MK, SdR, GC, DM, LGL, MT, MS, ARK, JS and MIJ were involved in the conception and design of the study. MK, SdR, MS, SV, NW, ARK and JS collected the data, and MK, SdR, MS, ARK, JS, and MIJ analyzed the data. All authors were involved in interpreting the results. MK drafted the manuscript and all other authors were major contributors in critically reviewing the manuscript. All authors read and approved the final manuscript.
Availability of data and material
The data that support the findings of this study are available upon reasonable request and by contacting the corresponding author but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of dr. J.F. Swart.
Ethics approval
The use of data from the research data platform, as described in this manuscript, was classified by the institutional review board as exempt from the Medical Research Involving Human Subjects Act (14/684). The study was conducted according to good CPGs and the Declaration of Helsinki. The ethical committee of the faculty of Behavioural, Management and Social Sciences of the University of Twente approved the study (no. 190,216).
Supplemental Material
Download MS Word (1.1 MB)Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
Notes
1. Part of this work, reporting preliminary study outcomes, was presented as a poster presentation at the conference of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in Copenhagen, 2–6 November 2019.
References
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377(9783):2138–2149.
- Shiff NJ, Oen K, Kroeker K, et al. Trends in population-based incidence and prevalence of juvenile idiopathic arthritis in Manitoba, Canada. Arthritis Care Res (Hoboken). 2019;71(3):413–418.
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369(9563):767–778.
- Barth S, Haas JP, Schlichtiger J, et al. Long-term health-related quality of life in german patients with juvenile idiopathic arthritis in comparison to German general population. PLoS One. 2016;11(4):e0153267.
- Tollisen A, Selvaag AM, Aulie HA, et al. Physical functioning, pain and health-related quality of life in adults with juvenile idiopathic arthritis: a longitudinal 30-year follow-up study. Arthritis Care Res (Hoboken). 2017.
- Muller-Godeffroy E, Lehmann H, Kuster RM, et al. [Quality of life and psychosocial adaptation in children and adolescents with juvenile idiopathic arthritis and reactive arthritis]. Z Rheumatol. 2005;64(3):177–187.
- Albers HM, Wessels JA, van der Straaten RJ, et al. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Rheumatism. 2009;61(1):46–51.
- Wallace CA. Current management of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol. 2006;20(2):279–300.
- Hashkes PJ, Laxer RM. Medical treatment of juvenile idiopathic arthritis. JAMA. 2005;294(13):1671–1684.
- Ilowite NT. Current treatment of juvenile rheumatoid arthritis. Pediatrics. 2002;109(1):109–115.
- Minden K, Niewerth M, Listing J, et al. The economic burden of juvenile idiopathic arthritis-results from the German paediatric rheumatologic database. Clin Exp Rheumatol. 2009;27(5):863–869.
- Haapasaari J, Kautiainen HJ, Isomaki HA, et al. Etanercept does not essentially increase the total costs of the treatment of refractory juvenile idiopathic arthritis. J Rheumatol. 2004;31(11):2286–2289.
- Bernatsky S, Duffy C, Malleson P, et al. Economic impact of juvenile idiopathic arthritis. Arthritis Rheumatism. 2007;57(1):44–48.
- Zorginstituut Nederland. Farmacotherapeutisch Kompas. 2019.
- Bouaddi I, Rostom S, El Badri D, et al. Impact of juvenile idiopathic arthritis on schooling. BMC Pediatr. 2013;13:2.
- Kuhlmann A, Schmidt T, Treskova M, et al. Social/economic costs and health-related quality of life in patients with juvenile idiopathic arthritis in Europe. Eur J Health Econ. 2016;17(Suppl 1):79–87.
- Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–392.
- Lee JJY, Schneider R. Systemic juvenile idiopathic arthritis. Pediatr Clin North Am. 2018;65(4):691–709.
- Davies R, Gaynor D, Hyrich KL, et al. Efficacy of biologic therapy across individual juvenile idiopathic arthritis subtypes: a systematic review. Semin Arthritis Rheum. 2017;46(5):584–593.
- Vastert SJ, Nigrovic PA. Editorial: toward personalized treatment for systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70(8):1172–1174.
- Funk RS, Becker ML. Disease modifying anti-rheumatic drugs in juvenile idiopathic arthritis: striving for individualized therapy. Expert Rev Precis Med Drug Dev. 2016;1(1):53–68.
- Huang B, Qiu T, Chen C, et al. Timing matters: real-world effectiveness of early combination of biologic and conventional synthetic disease-modifying antirheumatic drugs for treating newly diagnosed polyarticular course juvenile idiopathic arthritis. RMD Open. 2020;6(1):e001091.
- Klein A, Minden K, Hospach A, et al. Treat-to-target study for improved outcome in polyarticular juvenile idiopathic arthritis. Ann Rheum Dis. 2020;79(7):969–974.
- Klein-Wieringa IR, Brinkman DMC, Ten Cate R, et al. Update on the treatment of nonsystemic juvenile idiopathic arthritis including treatment-to-target: is (drug-free) inactive disease already possible? Curr Opin Rheumatol. 2020;32(5):403–413.
- Ravelli A, Consolaro A, Horneff G, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77(6):819–828.
- Kip MMA, Currie G, Marshall DA, et al. Seeking the state of the art in standardized measurement of health care resource use and costs in juvenile idiopathic arthritis: a scoping review. Pediatr Rheumatol Online J. 2019;17(1):20.
- Swart JF, van Dijkhuizen EHP, Wulffraat NM, et al. Clinical juvenile arthritis disease activity score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77(3):336–342.
- Conti G, Chirico V, Porcaro F, et al. Frequency and identification of risk factors of uveitis in juvenile idiopathic arthritis: a long-term follow-up study in a cohort of Italian children. J Clin Rheumatol. 2019. DOI:https://doi.org/10.1097/RHU.0000000000001104.
- Pedersen TL. ggforce: accelerating ‘ggplot2ʹ. R package version 0.3.1. 2019.
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
- Wickham H, François R, Henry L, et al. A grammar of data manipulation. R package version 0.8.3. 2019.
- Wickham H. ggplot2: elegant graphics for data analysis. New-York: Springer-Verlag; 2016.
- Grolemund G, Wickham H. Dates and times made easy with lubridate. J Stat Softw. 2011;40(3):1–25.
- Lemon J. Plotrix: a package in the red light district of R. R-News. 2006;6(4):8–12.
- Ter Haar NM, van Dijkhuizen EHP, Swart JF, et al. Treatment to target using recombinant interleukin-1 receptor antagonist as first-line monotherapy in new-onset systemic juvenile idiopathic arthritis: results from a five-year follow-up study. Arthritis Rheumatol. 2019;71(7):1163–1173.
- Allaire SH, DeNardo BS, Szer IS, et al. The economic impacts of juvenile rheumatoid arthritis. J Rheumatol. 1992;19(6):952–955.
- Angelis A, Kanavos P, Lopez-Bastida J, et al. Socioeconomic costs and health-related quality of life in juvenile idiopathic arthritis: a cost-of-illness study in the United Kingdom. BMC Musculoskelet Disord. 2016;17:321.
- Ens A, Lang B, Ramsey S, et al. The financial burden of juvenile idiopathic arthritis: a Nova Scotia experience. Pediatr Rheumatol Online J. 2013;11(1):24.
- Epps H, Ginnelly L, Utley M, et al. Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis. Health Technol Assess. 2005;9(39):iii–iv, ix–x, 1–59.
- Luca NJ, Burnett HF, Ungar WJ, et al. Cost-effectiveness analysis of first-line treatment with biologic agents in polyarticular juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2016;68(12):1803–1811. .
- Shepherd J, Cooper K, Harris P, et al. The clinical effectiveness and cost-effectiveness of abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis: a systematic review and economic evaluation. Health Technol Assess. 2016;20(34):1–222.
- Minden K, Niewerth M, Listing J, et al. Burden and cost of illness in patients with juvenile idiopathic arthritis. Ann Rheum Dis. 2004;63(7):836–842.
- Vastert SJ, de Jager W, Noordman BJ, et al. Effectiveness of first-line treatment with recombinant interleukin-1 receptor antagonist in steroid-naive patients with new-onset systemic juvenile idiopathic arthritis: results of a prospective cohort study. Arthritis Rheumatol. 2014;66(4):1034–1043. .
- Barber CEH, Barnabe C, Benseler S, et al. Patient factors associated with waiting time to pediatric rheumatologist consultation for patients with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2020;18(1):22. .
