ABSTRACT
Background
To evaluate the cost-effectiveness of tofacitinib in comparison to vedolizumab for the treatment of moderate-to-severe ulcerative colitis (UC) after failure or intolerance to conventional therapy (bio-naive) or first-line biologic treatment (bio-experienced), from the Spanish National Health System (NHS) perspective.
Methods
A lifetime Markov model with eight-week cycles was developed including five health states: remission, response, active UC, remission after surgery, and death. Response and remission probabilities (for induction and maintenance periods) were obtained from a multinomial network meta-analysis. Drug acquisition – biosimilar prices included – (ex-factory price with mandatory deductions), administration, surgery, patient management, and adverse event management costs (€, year 2019) were considered. A 3% discount rate (cost/outcomes) was applied. Probabilistic and deterministic sensitivity analyses (PSA) were conducted.
Results
Tofacitinib was dominant versus vedolizumab (both in bio-naive and bio-experienced patients) entailing total cost savings of €23,816 (bio-naïve) and €11,438 (bio-experienced). Differences in quality-adjusted life-year (QALY) were smaller than 0.1 for both populations. PSA results showed that tofacitinib has a high probability of being cost-effective (bio-naïve: 82.5%; bio-experienced: 90.6%) versus vedolizumab.
Conclusions
From the Spanish NHS perspective, tofacitinib could be a dominant treatment (less costly and more effective) in comparison to vedolizumab, with relevant cost savings and similar QALY gains.
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease with impairment limited to the colon and rectum. The usual symptoms are diarrhea, rectal bleeding, urgency, tenesmus, and crampy abdominal pain [Citation1]. In most patients, UC follows a course with alternating periods of remission and relapses [Citation2]. The global incidence and prevalence of UC have been increasing over time. It is more common in northern and western European countries as well as in industrialized countries such as the United States, Canada, and Australia [Citation2,Citation3]. In Spain, its prevalence is around 88.7 cases per 100,000 population, and its annual incidence is 5.7–8 cases per 100,000 population [Citation4].
UC is associated with a high economic burden, especially in severe cases where hospitalizations and surgeries account for the majority of direct costs [Citation5]. Surgery is not the cure, with a significant proportion of patients needing additional surgeries or hospitalizations due to post-surgical complications such as chronic pouchitis [Citation6,Citation7]. In the last years and due to the introduction and widespread use of expensive biologic therapies, there has been a shift away from hospitalizations and toward pharmaceuticals as the predominant driver of direct health-care costs in IBD patients [Citation8,Citation9]. The impact of UC on patient quality of life and work productivity generates significant indirect costs, which may even exceed direct health-care costs [Citation5].
The main objective of management in patients with UC is to induce remission and maintain it over a long-term period, with no need for corticosteroids, in order to prevent disability and surgical procedures, limit the incidence of colorectal cancer, and improve quality of life [Citation2]. Various pharmacological treatments are available for moderate-to-severe UC: conventional treatments (corticosteroids, thiopurines and calcineurin inhibitors), conventional biologic treatments (tumor necrosis factor [TNF] inhibitors), new biologic treatments (anti-integrins [vedolizumab], interleukin 12/23 inhibitors [ustekinumab]) and Janus kinase inhibitors (tofacitinib) [Citation10]. However, up to 15% of patients will ultimately require surgical treatment (colectomy) due to treatment failure or the development of colorectal dysplasia or carcinoma [Citation3].
Given the availability of various treatment options with different mechanisms of action, it is essential to choose the best treatment option for a patient based on his or her individual needs and characteristics. To do this, contraindications for use, patients’ treatment intolerances, the rate of primary non-response, and the potential for secondary loss of response over time must be considered. Secondary loss of response to biologics can be associated with immunogenicity, which is especially relevant for TNF antagonists [Citation11,Citation12].
Consistent with several comparative network meta-analyses (NMAs) [Citation13,Citation14], tofacitinib, vedolizumab, and all other biologic treatments evaluated are considered safe and effective therapies recommended for the treatment of moderate-to-severe active UC, both in induction and in maintenance and both in a bio-naive population and following biologic failure. Specifically, a recent study by Singh et al. [Citation14] classified infliximab as the best drug for inducing remission and mucosal healing in bio-naive patients. It also found tofacitinib and ustekinumab to be the best treatments for inducing remission and mucosal healing in patients previously exposed to TNF inhibitors and possibly even more effective than vedolizumab and adalimumab in these patients [Citation14].
Given the broad range of treatments for UC, comparative economic evaluation and positioning of innovative drugs such as tofacitinib and vedolizumab are currently of special interest to payers and to provide more evidence to support decision-making. It is important to clarify that ustekinumab, another drug with an innovative mechanism of action recently approved by the European Medicines Agency (EMA) for the indication of moderate-to-severe ulcerative colitis, was excluded from the study because, at the time of the analysis, this drug lacked a price and funding for this indication in Spain.
The objective of this study was to evaluate, from the perspective of a European Public Health System (Spain), the efficacy of the use of tofacitinib versus vedolizumab for the treatment of patients with moderate-to-severe active UC following failure, loss of response, or intolerance to conventional treatment (bio-naive) or to an anti-TNF biologic treatment (bio-experienced).
2. Patients and methods
2.1. Population
\This cost-effectiveness analysis considered patients with moderate-to-severe active UC who did not exhibit a suitable response, experienced loss of response, or had an intolerance to conventional treatment, conventional biologic treatment, or new biologic treatments. Two subpopulations were identified based on prior treatment received: patients naive to biologics (bio-naïve) and patients having already been treated with anti-TNF biologics (bio-experienced). Their characteristics were based on the patients enrolled in the OCTAVE Induction 1 and 2 studies [Citation15] (mean age 41.2 years; 59.2% male). A patient weight of 71.93 kg was derived from the weighted mean weight of individuals over 40 years of age in Spain [Citation16].
2.2. Economic model
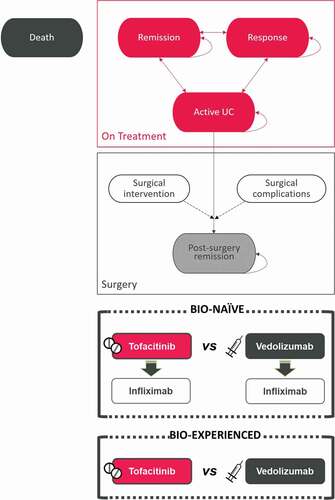
A de novo Markov model was developed in Microsoft Excel to show the course of UC over time according to the treatment received (). It was designed in line with previously published analyses on this disease [Citation17,Citation18] and following the current recommendations [Citation19,Citation20]. An expert panel composed of gastroenterologists and hospital pharmacists validated the parameters of the model and contributed information regarding routine clinical practice.
Treatment consisted of two phases: induction and maintenance. In the induction phase, the treatment objective is to induce response or remission of active UC. In the maintenance phase, the goal is to maintain that response or remission. Four health states in addition to death were considered: remission (Mayo score = 0–2 and all subscores ≤1), response (reduction in the Mayo Clinic score of at least 3 points and a decrease of at least 30% from the baseline score, with a decrease of at least 1 point on the rectal bleeding subscore or an absolute rectal bleeding score of 0 or 1), moderate-to-severe active UC (Mayo score ≥6), and remission following surgery.
An initial cohort of 1,000 patients with moderate-to-severe active UC transited between health states in 8-week cycles. Once they received induction treatment, they could exhibit clinical remission or clinical response or maintain active UC. The induction phase lasted 8 weeks, the same as that of the OCTAVE studies [Citation15] and similar to that seen in other UC clinical trials (6–10 weeks) [Citation14,Citation21]. The treatment alternatives considered in the analysis were tofacitinib and vedolizumab. When patients did not achieve response during induction or experienced loss of response during maintenance, they discontinued the treatment. Bio-naive patients could receive a second line of biologic treatment following discontinuation of the initial treatment. The subsequent treatment following response failure was infliximab (). Patients with active UC in the maintenance phase remained in that health state until they died or underwent surgery. For the purposes of the analysis, although there are other indications of surgery, only patients with active UC could be candidates for surgical treatment, with the possibility of developing complications and achieving remission following surgery ().
Table 1. Probabilities of transition to response and remission (induction and maintenance phases)
This analysis was performed from the perspective of the Spanish National Health System (NHS). The time horizon considered was the patient’s lifetime, with a maximum of 60 years. A 3% annual discount was applied both for costs generated and for health outcomes. Treatment efficacy was evaluated using the incremental cost–effectiveness ratio (ICER) and the incremental cost–utility ratio (ICUR) and expressed in terms of cost per life year (LY) gained and cost per quality-adjusted life year (QALY), respectively. These ratios were estimated using the differences in terms of costs and health outcomes between the alternatives studied. A willingness-to-pay threshold was set at €25,000/QALY to evaluate whether one alternative is cost-effective compared to another. This threshold is not an official one in Spain though the latest publications have suggested ranges of €20,000-€25,000/QALY [Citation22] and, most recently, €25,000-€60,000/QALY [Citation23].
2.3. Efficacy
Without direct comparisons between treatments, the relative efficacy of tofacitinib versus vedolizumab was obtained from a fixed-effects multinomial NMA, using placebo as a comparator for reference [Citation24]. Probabilities of transition between the health states of response and clinical remission were calculated for the induction phase (response and remission at 8 ± 2 weeks) and the maintenance phase (response and remission at 52 ± 4 weeks) [Citation24] (). The latter phase had two independent analyses depending on the design of the studies included: treat-through, where the induction-phase treatment was maintained, and re-randomized, in which some patients were randomized following the induction phase (). Given that, at the time of the study, there were no data for the treat-through analysis with vedolizumab, and the relative risk between vedolizumab and placebo available in the re-randomized analysis was applied for each population (bio-naive, bio-experienced).
For a better adjustment to first-year and long-term efficacy outcomes, probabilities for all treatments were adjusted to two periods: weeks 0–24 and weeks 24–52. This adjustment was based on a post-hoc analysis of the OCTAVE Sustain study, in which differences between the two periods in terms of remission and response were observed. In addition, the second period seemed to show a constant trend over time and that effect was better suited to long-term extrapolation than that obtained over the entire period (weeks 0–52) ().
2.4. Adverse events, risk of surgery, and mortality
Rates of treatment-related serious adverse events were included based on induction and maintenance studies of the medicines considered [Citation15,Citation21,Citation24–27], with no distinction between bio-naive and bio-experienced patients (). An annual rate of surgery in patients with active UC of 1.44% was used in line with an extensive epidemiology study conducted in Spain [Citation28]. Patient mortality was determined based on mortality rates in the general Spanish population by age and gender [Citation16]; in addition, a perioperative mortality rate of 1.18% was used in patients having undergone colectomy [Citation29].
Table 2. Unit costs (administration, patient management, surgery, and adverse event management)
2.5. Quality of life
Utility values drawn from the literature were incorporated to reflect quality of life for patients with UC according to health state [Citation30,Citation31]: remission (0.87), response (0.76), active UC (0.41), and post-surgery remission (0.68).
2.6. Costs
In accordance with the analysis’s perspective (that of the NHS), direct health-care costs were included (): pharmacological costs, administration costs, disease management costs by health state, surgery costs, and serious adverse event management costs. Unit costs were obtained from Spanish national healthcare cost databases [Citation32,Citation33]. Pharmacological costs were calculated based on the dosage regimens of the treatment alternatives described in the summaries of product characteristics [Citation34] and prices were expressed in terms of ex-factory price (EFP) [Citation32], with application of the mandatory deductions according to Royal Decree Law 8/2010 [Citation35], corresponding to a 7.5% discount on the price of tofacitinib and vedolizumab (infliximab does not have a mandatory deduction as it is included in the reference price system). The price of the biosimilar medicine was used, if available. In drugs administered intravenously, the price per milligram was considered, with no vial wastage allowed (). Intravenous administration costs were calculated based on the cost of nursing staff and the infusion time for the drugs (30–90 minutes for vedolizumab and 150 minutes for infliximab, according to the expert panel). Oral administration was assigned no cost. Patient management cost by health state was determined based on health-care resources consumed, which for their part were determined by the expert panel according to clinical practice. Costs of surgery and its complications, including patient management following colectomy, were included in aggregate according to data from a real clinical practice study in Spain [Citation36]. Adverse event costs were obtained from the cost per process in the record of discharges from the database of the Spanish Ministry of Health according to the ICD 9-CM code best matching each event [Citation33,Citation37].
2.7. Sensitivity analyses
Deterministic sensitivity analyses (DSAs) and probabilistic sensitivity analyses (PSAs) were performed to evaluate the uncertainty of the values for the parameters and the robustness of the model. Some scenario analyses were performed modifying values of the time horizon (5, 10, and 20 years), discount rate (0% and 5%), annual surgery risk (alternative value: 4.2% [Citation38]), and perioperative mortality (range 0–2.9% [Citation29]). Univariate and multivariate DSAs modified the following parameters by ±20%: unit price of tofacitinib, unit price of vedolizumab, costs of administration, management by health state, surgery, and management of adverse events as well as utility values for health states. In the PSAs, the parameters (utility values, transition probabilities, risk of surgery, perioperative mortality, pharmacological costs, administration costs, patient management costs, and adverse event costs) were simultaneously varied by performing 1,000 Monte Carlo simulations on the 1,000-patient cohort evaluated. The parametric functions used were beta for utilities, surgery risk and mortality risk due to colectomy, gamma for costs, and Dirichlet for distribution probabilities.
3. Results
The total patient management costs for patients with moderate-to-severe active UC are shown in . Treatment with tofacitinib in patients with moderate-to-severe active UC yielded a reduction in total costs compared to vedolizumab. In bio-naive patients, in the treatment sequence that started with tofacitinib, this reduction was €23,815.58 versus vedolizumab, whereas in the bio-experienced patient population, the reduction in total costs with tofacitinib was €11,437.56. In both subpopulations, the differences in costs were mainly due to the fact that the pharmacological and administration costs of vedolizumab are substantially higher than for tofacitinib, whereas for all other dimensions studied, the results for the two drugs were similar though they were slightly lower with tofacitinib in all cases.
Table 3. Results of cost-effectiveness analysis (base case and scenario analyses)
Regarding health outcomes, similar gains in terms of LYs gained were observed due to the disease’s limited impact on patient survival, with differences between the treatment options of less than 0.0005 LYs gained. In terms of QALYs, the differences between tofacitinib and vedolizumab were somewhat higher in the bio-experienced population (0.042 QALYs) (). In both the bio-naive and bio-experienced patient populations, treatment with tofacitinib was a dominant treatment alternative (lower costs and higher effectiveness) compared to vedolizumab ().
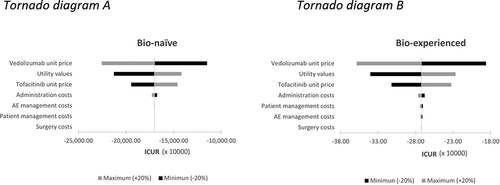
The results of the DSAs and scenario analyses did not show significant variations relative to the base case, especially in the bio-experienced population, in which tofacitinib always remained a dominant alternative (, ). In bio-naive patients, tofacitinib remained a dominant alternative, except when considering a shorter time horizon, a higher discount rate, or a higher surgery risk; these cases showed slight differences in QALYs while maintaining the cost savings associated with tofacitinib (). Other variables with greater impact on the results were the price of vedolizumab and tofacitinib and utility values. However, in these cases, tofacitinib was still a dominant alternative ().
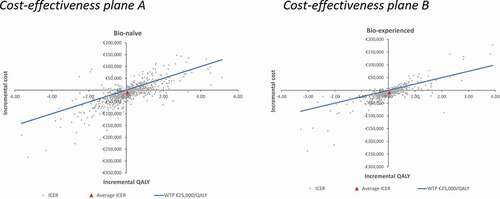
The results of the PSA showed that the probability of tofacitinib being a cost-effective alternative to vedolizumab for a willingness-to-pay threshold of €25,000/QALY was 82.5% in a bio-naive population and 90.6% in a bio-experienced population (). The average costs and QALYs obtained in the PSA were -€10,997.79 and 0.062 QALYs for the bio-naive population and -€8,687.92 and 0.038 QALYs for the bio-experienced population, being tofacitinib a dominant alternative in both scenarios ().
4. Discussion
This analysis showed that tofacitinib is a dominant – i.e. less costly and more effective – treatment alternative compared to vedolizumab in treating patients with moderate-to-severe active UC, regardless of whether it is used following failure with conventional treatment or following an anti-TNF biologic treatment. Tofacitinib yields a reduction in total costs per patient compared to vedolizumab in both patient populations (bio-naive and bio-experienced) largely deriving from the pharmacological costs of the treatments. Among the most relevant findings were those related to administration costs: orally administered treatments account for substantial savings in treatment costs compared to intravenously administered alternatives, which require greater healthcare resource consumption. The results revealed little difference in all other costs analyzed, in which the cost of tofacitinib is shown to be slightly lower. Specifically, disease management with vedolizumab results in a higher cost due to a higher number of patients who remain in a state of active UC and, therefore, with a higher probability of undergoing surgery compared to treatment with tofacitinib. Concerning the difference in costs due to adverse events, the highest cost with vedolizumab derived from a higher incidence of serious infections, upper respiratory tract infections, and acute infusion reactions, according to the studies included. On the other hand, differences in QALYs are slight, with tofacitinib presenting quality-adjusted survival gains in both populations studied.
A recent cost-effectiveness analysis was published comparing tofacitinib and biologic treatments, such as infliximab, adalimumab, golimumab, and vedolizumab, in anti-TNF naive patients with moderate-to-severe UC in Spain. In this 10-year analysis, vedolizumab and tofacitinib were the treatments with higher effectiveness, with an ICUR of €45,253/QALY of vedolizumab compared to tofacitinib. Tofacitinib had differences in costs between -€13,596 and €68,043 and differences in effectiveness between −0.30 and 0,98 QALYs, when compared with the biologic treatments [Citation39]. This author recently published a budget impact analysis conducted in Spain according to which the use of tofacitinib results in savings of up to €3,600,177 after 5 years [Citation40].
Internationally, cost–effectiveness analyses including tofacitinib have indeed been published [Citation41,Citation42]. The results of these publications are consistent with the findings of this analysis. A study by Lohan et al. in the United Kingdom also analyzed two populations according to prior exposure to a TNF inhibitor. This study found cost reductions associated with tofacitinib versus infliximab and vedolizumab in both populations; tofacitinib was dominant in these two comparisons and cost-effective (<₤18,000/QALY) versus adalimumab and golimumab [Citation41]. Another study evaluated the efficiency of tofacitinib in two regions (the United Kingdom and China) using biologic and conventional treatment sequences. The sequences with tofacitinib and vedolizumab were the most cost-effective treatments in the United Kingdom, and the sequences with tofacitinib were the most cost-effective treatments in China. When sequences with a biologic were compared (similar to the bio-experienced analysis), tofacitinib was dominant compared to infliximab, vedolizumab, and golimumab, yielding ratios of ₤11,819-₤22,515/QALY versus adalimumab [Citation42]. In line with these results, in an economic evaluation by Milev et al. [Citation43], tofacitinib followed by infliximab was associated with a lower cost per patient per month compared to treatment with vedolizumab followed by infliximab, in a bio-experienced population. Another study was recently published in Greece, in which tofacitinib was dominant to vedolizumab in both bio-naive and bio-experience populations with a 97% probability of being cost-effective [Citation44]. Finally, a cost-effectiveness analysis in Poland compared tofacitinib and other biologics with conventional treatment, being tofacitinib the most effective treatment in bio-experienced patients and infliximab in bio-naive patients [Citation45].
This analysis did have some limitations. Given the absence of efficacy in direct comparison between the two alternatives, it was necessary to perform indirect comparisons using an NMA. As studies with a treat-through design with vedolizumab were not available at the time of the analysis, estimates had to be made based on the data available from studies with a re-randomized design. This model did not consider administration of conventional treatment following failure with a second line of biologic treatment or tofacitinib, which is included in some published analyses [Citation17,Citation41,Citation42]. However, other existing models also do not incorporate conventional treatment [Citation39]. The expert panel felt that the administration of conventional treatment following biologic treatments did not reflect routine clinical practice; what is typical is to switch to a new biologic treatment at the clinician’s discretion. In line with this approach, a study of patients with UC being treated with biologics was identified in which approximately half the patients who stopped treatment did not subsequently restart the same treatment or switch to another treatment [Citation46]. The bio-naive patient population could only receive a second line of biologic treatment following failure with the first study treatment. To minimize the effects of the subsequent treatment, the same second-line treatment (infliximab) was used in the two comparators, whose selection was in accordance with the opinion of the expert panel based on routine clinical practice. Hence, no further subsequent lines of treatment were used as they could obscure the effect achieved with the treatments to be evaluated and in view of the limited evidence for determining the efficacy of advanced lines of treatment. Finally, possible dose escalations included in the summary of product characteristics, which could lead to increased pharmacological costs for both treatments, were not considered.
Another matter to take into account is that treatments were considered in monotherapy, whereas it has been reported that a high percentage of patients being treated with vedolizumab take it in combination with azathioprine. This would increase the pharmacological cost of this alternative and could be linked to a higher risk of adverse events such as lymphoma, opportunistic infection, and non-melanoma skin cancer [Citation47]. Furthermore, it should be noted that the OCTAVE trials on tofacitinib featured a stricter definition of remission (with the additional requirement of a rectal bleeding subscore of 0) than that used in the studies on vedolizumab, and also required a centralized and therefore more demanding endoscopy reading [Citation15].
To reflect surgery risk in patients with moderate-to-severe active UC, a fixed annual colectomy rate was used throughout the analysis time horizon although in clinical practice surgery risk may have varied over time in these patients with active UC. However, in this case, the use of more recent data published in Spain was preferred. Finally, as a limitation of this study, it should be noted that while the study was under way, ustekinumab, another biologic administered intravenously for the treatment of moderate-to-severe active UC, was approved by the EMA. This drug was not included in the analysis as it lacked a price in Spain and reimbursement at the time of the study.
Overall, it is important to stress that the ultimate choice of a treatment for UC should be the result of a complete assessment that includes patient characteristics, drug efficacy and associated risks, as well as management-derived costs [Citation48]. Economic evaluations are of great utility in assessing a medicine’s therapeutic positioning.
5. Conclusions
From the perspective of the NHS, treatment with tofacitinib could be a cost-effective and dominant (less costly and more effective) alternative than vedolizumab for the management of patients with moderate-to-severe active UC following failure, intolerance, or loss of response to conventional or anti-TNF biologic treatment.
Author contributions
CP, AC, CT, FdA and MAC were responsible for the conceptual development of the analysis. FdA developed the economic model. CP, AC, CT, LM, SG, ASG, BM, ALIA, SG, MAC and FdA validated the study’s structure and premise and provided information on clinical practice in Spain. FdA and ALIA performed the analyses. All the authors took part in the interpretation of the results. FdA and ALIA drafted the manuscript. All the authors reviewed and approved the final version of the manuscript. CP is the guarantor for the general content of this manuscript.
Article highlights
Ulcerative colitis is a recurrent chronic disease with a growing incidence, especially in industrialized countries.
Since a significant percentage of patients do not respond, lose the response or are intolerant to the available treatments, there is a need for new treatments, especially for those who explore new mechanisms of action.
A lifetime Markov model was developed to evaluate the efficiency of the use of tofacitinib versus vedolizumab in patients with moderate-to-severe active ulcerative colitis following failure, loss of response or intolerance to conventional treatment (bio-naïve) or to a TNF-inhibitor biologic treatment (bio-experienced), from the perspective of the Spanish National Health System.
Significant savings in costs per patient with tofacitinib versus vedolizumab were found in both patient populations — €23,816 (bio-naïve) and €11,438 (bio-experienced) — with similar gains in quality-adjusted life years (<0.05).
Treatment with tofacitinib compared to vedolizumab is a dominant (less costly and more effective) alternative from the perspective of the Spanish National Health System.
Declaration of interest
CT has received speaker, advisory board or research fees from MSD, Abbvie, Pfizer, Takeda, Janssen, Ferring, Faes Farma, Dr. Falk Pharma, Amgen, and Tillots.
FdA and MAC are employees of Pharmacoeconomics & Outcomes Research Iberia (PORIB), a consulting firm specialising in economic evaluation of healthcare interventions, which received funding support from Pfizer to conduct this manuscript. CP, AC, SG y ALIA are employees of Pfizer (Spain). CT, LM, SG, ASG and BM have received consultancy fees from Pfizer S.L.U. for their work in this project but have no other relevant financial relationships to disclose. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Data deposition
Not applicable.
Supplemental online material
Not applicable.
Congress presentations
The results of this analysis were presented at the 22nd European Congress of The Professional Society for Health Economics and Outcomes Research (ISPOR Europe 2019, held in Copenhagen, Denmark).
Data availability statement
There is no available data set associated with the paper.
Additional information
Funding
References
- Magro F, Gionchetti P, Eliakim R, et al. European Crohn’s and Colitis Organisation [ecco]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670.
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770.
- Feuerstein JD, Moss AC, Farraye FA. Ulcerative colitis. Mayo Clin Proc. 2019;94(7):1357–1373.
- Marín-Jiménez I, Saro C, Díaz V, et al. Epidemiology and hospital resources use in the treatment of ulcerative colitis at gastroenterology units in Spain (EPICURE study). Drugs Context. 2018;7:212505.
- Cohen RD, Yu AP, Wu EQ, et al. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31(7):693–707.
- Taxonera C, Calvet X, Gisbert JP, et al. On behalf of COSCOL study investigators patient undergoing colectomy because of ulcerative colitis had a very high rate of surgical complications in clinical practice conditions. J Crohns Colitis. 2010;4(1):S59.
- Loftus EV, Delgado DJ, Friedman HS, et al. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. Am J Gastroenterol. 2008;103(7):1737–1745.
- JW K, CK L, JK L, et al. Long-term evolution of direct healthcare costs for inflammatory bowel diseases: a population-based study (2006–2015). Scand J Gastroenterol. 2019;54(4):419–426.
- Kuenzig ME, EI EI, Lee L, et al. The Impact of inflammatory bowel disease in Canada 2018: direct costs and health services utilization. J Can Assoc Gastroenterol. 2019;2(Suppl Supplement_1):S17–S33.
- Harbord M, Eliakim R, Bettenworth D, et al., European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784.
- Strand V, Balsa A, Al-Salch J, et al. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs. 2017;31(4):299–316.
- Van den Berghe N, Verstockt B, Tops S, et al. Immunogenicity is not the driving force of treatment failure in vedolizumab-treated inflammatory bowel disease patients. J Gastroenterol Hepatol. 2019;34(7):1175–1181.
- Bonovas S, Lytras T, Nikolopoulos G, et al. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(4):454–465.
- Singh S, Murad MH, Fumery M, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gasteroenterol Hepatol. 2020;S1542-3565(20):30044–30046.
- Sandborn WJ, Su C, Sands BE, et al. OCTAVE induction 1, OCTAVE induction 2, and OCTAVE sustain investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736.
- INEbase [Internet]. Madrid: instituto nacional de estadística [Spanish National Statistics Institute (INE)]; 2001–2016. Available at: http://www.ine.es.
- Tappenden P, Ren S, Archer R, et al. Evaluation of biologic and non-biologic options for the treatment of adults with moderately-to-severely active ulcerative colitis after the failure of conventional therapy. Pharmacoeconomics. 2016;34(10):1023–1038.
- Xie F, Blackhouse G, Assasi N, et al. Cost-utility analysis of infliximab and adalimumab for refractory ulcerative colitis. Cost Eff Resour Alloc. 2009;7(1):20.
- Caro JJ, Briggs AH, Siebert U, et al., Force I-SMGRPT. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force–1. Value Health. 2012;15(6):796–803.
- Siebert U, Alagoz O, Bayoumi AM, et al.; ISPOR-SMDM modeling good research practices task force. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–3. Value Health. 2012;15: 812–820.
- Feagan BG, Rutgeerts P, Sands BE, et al.; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
- Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–761.
- Sacristán JA, Oliva J, Campillo-Artero C, et al. What is an efficient health intervention in Spain in 2020? Gac Sanit. 2020;34(2):189–193.
- Rubin D, Ashaye A, Zhang Y, et al. Efficacy of tofacitinib and biologics as induction and maintenance therapy for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. United European Gastroenterol J. 2018;6(8S):A252.
- Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146(2):392–400.e3.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. Erratum in: N Engl J Med. 2006;18;354(20):2200.
- Jiang XL, Cui HF, Gao J, et al. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol. 2015;49(7):582–588.
- Chaparro M, Barreiro-de Acosta M, Benítez J, et al. EpidemIBD group. P790 Epidemiology, clinical characteristics, evolution and treatments in newly diagnosed inflammatory bowel disease (IBD): results from the nationwide EpidemIBD study of GETECCU. J Crohns Colitis. 2019;13(Supplement_1):S516–7.
- Peyrin-Biroulet L, Germain A, Patel AS, et al. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther. 2016 Oct;44(8):807–816.
- Woehl A, Hawthorne A, McEwan P. The relation between disease activity, quality of life and health utility in patients with ulcerative colitis. Gut. 2008;57(Suppl. 1):A153.
- Arseneau KO, Sultan S, Provenzale DT, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol. 2006;4(9):1135–1142.
- Consejo General de Colegios Oficiales de Farmacéuticos [Spanish General Council of Official Colleges of Pharmacists]. Conocimiento sanitario [healthcare knowledge] database - Bot PLUS 2.0 [internet]. Madrid: Consejo General de Colegios Oficiales de Farmacéuticos; 2019. Available at: https://botplusweb.portalfarma.com/.
- Oblikue Consulting. eSalud [eHealth] healthcare costs database [Internet]. Barcelona: Oblikue Consulting; 2019 [cited 20 Feb 2019]. Available at: http://www.oblikue.com/bddcostes/.
- Agencia Española de Medicamentos y Productos Sanitarios online Centro de Información de Medicamentos [Spanish Agency of Medicines and Medical Devices online Medicinal Products Information Centre] (AEMPS - CIMA). Madrid: agencia española de medicamentos y productos sanitarios; 2019. Available at: https://cima.aemps.es/cima/publico/home.html.
- Ministerio de Sanidad, Servicios Sociales e Igualdad [Ministry of Health, Social Services and Equality]. Relación informativa de medicamentos afectados por las deducciones establecidas en el real decreto ley 8/2010 de 20 de mayo por el que se adoptan medidas extraordinarias para la reducción del déficit público [Informational list of medicines affected by the deductions established in Spanish Royal Decree/ Law 8/2010, of 20 May, adopting special measures to reduce the public deficit]. [Internet]. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2010. Available at: https://www.mscbs.gob.es/profesionales/farmacia/pdf/DeduccionesAbril19.pdf
- Taxonera C, Gisbert JP, Calvet X, et al. On behalf of COSCOL study investigators. Cost of colectomy in ulcerative colitis patients. Gut. 2009;58(Suppl II):A177.
- Ministerio de Sanidad, Servicios Sociales e Igualdad [Ministry of Health, Social Services and Equality]. Instituto de información sanitaria [Spanish healthcare information institute]. (2018). Record of discharges. CIE9 MC – CMBD 2015 [cited Jan2019]. Available at: http://pestadistico.inteligenciadegestion.mscbs.es/
- Cañas-Ventura A, Márquez L, Ricart E, et al. Spanish GETECCU group (ENEIDA project) risk of colectomy in patients with ulcerative colitis under thiopurine treatment. J Crohns Colitis. 2014;8(10):1287–1293.
- Trigo-Vicente C, Gimeno-Ballester V, López-Del Val A. Cost-effectiveness analysis of infliximab, adalimumab, golimumab, vedolizumab and tofacitinib for moderate to severe ulcerative colitis in Spain. Eur J Hosp Pharm. 2020;27(6):355–360.
- Trigo-Vicente C, Ballester VG. Impacto presupuestario de la introducción de tofacitinib en el tratamiento de colitis ulcerosa moderada a grave [Budget impact of the introduction of tofacitinib in the treatment of moderate to severe ulcerative colitis]. In: Poster session presented at: 64th sociedad española de farmacia hospitalaria [Spanish hospital pharmacy association (SEFH)]. Spain: Seville; 2019 Oct. p. 17–19.
- Lohan C, Diamantopoulos A, LeReun C, et al. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol. 2019;6(1):e000302.
- Wu B, Wang Z, Zhang Q. Cost-effectiveness of different strategies for the treatment of moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2018;24(11):2291–2302.
- Milev S, DiBonaventura MD, Quon P, et al. An economic evaluation of tofacitinib for the treatment of moderately-to-severely active ulcerative colitis: modeling the cost of treatment strategies in the United States. J Med Econ. 2019;22(9):859–868.
- Vellopoulou K, Stefanou G, Tzanetakos C, et al. Cost-effectiveness of tofacitinib for the treatment of moderate to severe active ulcerative colitis in Greece. Eur J Gastroenterol Hepatol. 2020. https://doi.org/10.1097/MEG.0000000000001916.
- Petryszyn P, Ekk-Cierniakowski P, Zurakowski G. Infliximab, adalimumab, golimumab, vedolizumab and tofacitinib in moderate to severe ulcerative colitis: comparative cost-effectiveness study in Poland. Therap Adv Gastroenterol. 2020;13:1756284820941179.
- Null KD, Xu Y, Pasquale MK, et al. Ulcerative colitis treatment patterns and cost of care. Value Health. 2017;20(6):752–761.
- Cross KR. Which patients with inflammatory bowel disease should receive combination therapy? Expert Rev Gastroenterol Hepatol. 2015;9(6):715–717.
- Danese S, Fiorino G, Peyrin-Biroulet L. Positioning therapies in ulcerative colitis. Clin Gasteroenterol Hepatol 2020;18(6):1280.e1.