ABSTRACT
Introduction: The kinds of costs included in cost-effectiveness analyses (CEAs) for vaccines, such as direct medical costs and indirect costs, may affect their outcomes. While some guidelines recommend inclusion of costs associated with productivity losses/gains, very little guidance is provided about the productivity elements to include and their calculation approach.
Areas covered: We conducted a systematic review of CEAs for vaccines and vaccine programs published between 1 January 2010 and 19 November 2019 that included productivity costs using Medline, Embase, and the Cochrane Library. The kind of productivity elements included their calculation approach, and the impact of their inclusion on cost-effectiveness are summarized. Among 88 studies identified, productivity elements included were reported for 71 studies (81%) with absenteeism being the most commonly included element. Only 24 studies (27%) reported the approach used to calculate productivity costs (human capital vs. friction approach). Most studies (81%) reported a more favorable cost-effectiveness with the inclusion of productivity losses/gains.
Expert opinion: Inclusion of productivity losses/gains for CEAs for vaccines has resulted in more favorable cost-effectiveness based on the studies reviewed. However, clearer guidance on the productivity elements to include by disease area and more transparency on the calculation method used may be needed.
1. Introduction
Cost-effectiveness analyses (CEA) are used to compare the cost of two or more interventions relative to the gains in health that they provide. The analysis involves dividing the difference in the cost of the interventions in monetary units by the difference in their health gains. Health gains may be expressed in natural units such as number of lives saved. Many pharmacoeconomic guidelines recommend the use of cost-utility analysis (CUA) which is a type of CEA whereby health gains are expressed in units of quality adjusted life years (QALY) [Citation1].
Cost-effectiveness is also one factor that is sometimes considered when deciding whether or not to recommend vaccines for coverage and/or inclusion in immunization programs [Citation2,Citation3]. Several European countries consider the results of CEA/CUA studies when deciding whether or not to recommend immunization using a vaccine [Citation2]. The Advisory Committee on Immunization Practices (ACIP) in the United States also released updated guidance for health economic studies in October 2019 reaffirming the importance of the results of economic evaluations for ACIP decisions [Citation3].
The inclusion of costs for CEA/CUA studies varies, however, depending on the perspective of the study, the method used to estimate productivity losses/gains, and the kinds of productivity costs included. To begin, the perspective of the study may influence the costs included in the analysis. For CEA/CUA studies conducted based on the healthcare or public payer perspective, costs are typically limited to direct healthcare costs such as the costs of treatment intervention, hospitalization, outpatient visits, etc [Citation4]. In addition to direct healthcare costs, CEA/CUA studies conducted based on the societal perspective may also include other costs borne by the individual patient or their families. For example, under the societal perspective direct non-healthcare costs such as transportation or indirect costs such as costs associated with losses or gains in productivity when undergoing treatment may also be included [Citation5]. The broader scope of costs included can lead to different results in terms of cost-effectiveness. While several studies suggest that fewer than 10% of economic studies include productivity costs, their inclusion has been shown to lead to more favorable outcomes in terms of cost-effectiveness [Citation4,Citation6].
Although the use of CEA/CUA for vaccines is recommended in Europe, the US, and other countries, guidance on the study perspective and the costs that should be included vary by country. The ACIP guidelines recommend that cost-effectiveness evaluations of vaccines be conducted from the healthcare sector perspective, societal perspective, or ideally both perspectives. The consensus framework issued by the European Vaccine Economics Community suggests that a societal perspective be used as the base case with the productivity loss of patients and caregivers taken into consideration [Citation7].
CEA/CUA studies for vaccines may also differ in terms of the types of productivity losses/gains included. Absenteeism and presenteeism for the patient and/or their family members are commonly mentioned components for productivity costs [Citation8,Citation9]. Absenteeism refers to productivity losses or gains associated with the time off required due to an illness or treatment or the ability to forego taking time off due to the benefits of treatment. Presenteeism refers to losses or gains due to loss of productivity while at work due to an illness or treatment without taking off of work. Some CEA/CUA for drug treatments have also considered unemployment and premature mortality both of which involve a permanent loss of employment [Citation10–12]. Unemployment includes a loss of productivity from having to stop working altogether due to a condition. Premature mortality refers to productivity losses/gains that may accumulate due to death from an illness [Citation13]. Unemployment and premature mortality are similar to absenteeism in that they refer to a loss of work associated with an illness, but some studies clearly differentiate those elements as a specific type of productivity element.
There are two commonly mentioned approaches to estimating productivity losses/gains – namely the human capital approach and the friction cost approach and CEA/CUA studies that include productivity losses/gains may use either approach [Citation1,Citation13]. For the human capital approach, costs associated with productivity losses/gains are estimated by multiplying lost work time by discounted gross wages. For the friction cost approach, costs associated with productivity losses are estimated by multiplying the lost work hours during the period that the patient must miss work and when a new employee can be hired and trained to replace them by the discounted gross wages for the patient. Despite apparent challenges in using the friction cost approach, it has been suggested that the human capital approach may overestimate the value of lost production given that it assumes full employment and that loss of employment is not covered by replacing the person with someone else [Citation8]. While both the European and ACIP guidelines mention the societal perspective and the European guidelines mention inclusion of absenteeism, neither specifically mention the approach to be used when measuring productivity losses/gains [Citation2–4].
Despite variations in the inclusion of productivity losses/gains, previous studies have found that their inclusion in economic evaluations may substantially affect cost-effectiveness and may cause different formulary decisions to be made than if they were not incorporated [Citation6,Citation14,Citation15]. Analyses incorporating productivity losses/gains have been shown to increase or decrease the incremental cost-effectiveness ratio (ICER), a measure of the difference in cost between two interventions, divided by the difference in their effect, depending on the direction of their impact on costs. Although multiple reports support the inclusion of productivity losses/gains in CEAs [Citation6,Citation15–17], a systematic review of all health economic evaluation studies published through May 2014 that employed a patient’s perspective found that only half of the included studies examined indirect costs [Citation18]. Moreover, a study conducted in 2000 that examined CUAs found that among 228 studies conducted from 1975 to 1997 only 19 (8.3%) included productivity costs. A systematic literature review examining the inclusion of productivity losses/gains in drugs, excluding vaccines was identified, but no systematic review is currently available that examines the approaches used to include productivity losses/gains and their impact on the outcomes for all CEA and/or CUA studies for vaccines [Citation19].
This systematic literature review was conducted with the objective of understanding the use of indirect costs and in particular productivity losses/gains in CEAs related to vaccines and to examine the impact of their inclusion on the outcome of evaluations. We anticipate that inclusion of productivity losses and gains will have an impact on the cost-effectiveness of vaccines. However, we also anticipate that the productivity elements included and the impact of their inclusion on cost-effectiveness may differ by the disease and target population since vaccines targeting diseases for children typically involve support from one or more parent and vaccines targeting diseases for adults may involve more work-related productivity gains for the patient or a family member.
2. Methods
2.1. Systematic review methodology
A systematic literature review was performed to examine the use of indirect costs in CEA/CUA for vaccines. For this review, indirect costs are defined as costs associated with productivity gains or losses and costs associated with morbidity or mortality that lead to a loss of work time – which is similar to definitions raised in various CEA guidelines [Citation13,Citation20–22]. This review was performed in accordance with recommended international guidelines for the conducting of systematic reviews including the Centre for Reviews and Dissemination (CRD) guidance [Citation23], the Cochrane Handbook [Citation24], and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Citation25,Citation26].
2.2. Sources
A systematic search of three electronic databases was conducted: 1) MEDLINE (including Epub Ahead of Print, In-Process and Other Non-Indexed Citations, MEDLINE Daily, MEDLINE <1946 to 2019>, MEDLINE In-Process Citations and Daily Update), 2) Embase, and 3) Cochrane Library. The initial search period included all publications available up to 19 November 2019 with the earliest search year being 1946 for MEDLINE and 1974 for Embase. The Cochrane Library began in 1996 and does not have a specific start year for searches, but no restrictions on the start date were applied for the initial search. In addition, as recommended by the CRD and Cochrane guidelines [Citation23,Citation24], references cited in each of the included studies and relevant (but not included) systematic reviews were manually searched to identify any additional relevant studies.
2.3. Search criteria
Keywords for the search of the aforementioned databases were identified from the literature for two concepts: 1) type of health economic evaluation (i.e. CEAs and CUAs) and 2) productivity loss components and methods used for calculation [Citation13,Citation18,Citation27]. To identify economic evaluations, keywords such as ‘cost-effectiveness’ and ‘cost-utility’ were included. To further identify those that include indirect cost components, keywords such as ‘productivity’, ‘absenteeism’, ‘time off’, ‘days off’, ‘sick leave’, ‘absence’ ‘unemployment’, etc. were included.
2.4. Study selection and full-text review process
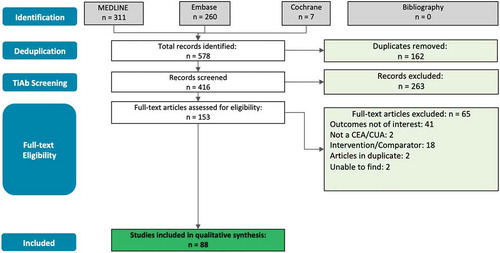
Studies included in the analysis had to be original CEAs or CUAs that fulfil all inclusion criteria and none of the exclusion criteria. An overview of the study inclusion and exclusion criteria is included in Appendix 1 of the supplemental materials. Cost-benefit analyses (CBA), a specific type of CEA whereby outcomes are converted into monetary values, were excluded from this study because they are not commonly used in economic evaluations today. As part of the inclusion criteria, the intervention or comparator examined in a study had to be a vaccine or a vaccination program. Second, the selected study had to provide evidence for one or more of the indirect cost components mentioned above. After exclusion of duplicates, a review of the title and abstract for each publication identified was conducted to confirm whether or not they meet all of the inclusion criteria and none of the exclusion criteria. Full-text articles were then obtained for records that met the inclusion criteria. Each record was reevaluated through a full-text review by two independent analysts. Any disagreements about inclusion or exclusion were resolved through discussion until a consensus was reached, failing which a third reviewer was consulted for a final and irrevocable decision. A summary of the reasons for exclusion of articles based on the full-text article review is provided in the PRISMA flow diagram shown in .
2.5. Data extraction and analysis
Data from the included studies were extracted by a reviewer into data extraction tables created for this analysis. To identify and rectify any errors in data extraction, a second reviewer checked and validated the outcome data by conducting an independent internal data check once all required data had been collected/extracted. Key data extracted from the publications for analysis included publication year, country of origin of the study, target disease/condition, type of evaluation (e.g. CEA or CUA), type of analysis model, intervention/comparator, analysis perspective, analysis time horizon, productivity elements included, approach used to calculate productivity losses/gains, and the impact of productivity losses/gains on the outcome of the study. In addition to a summarization of the studies included, an analysis of the indirect cost components included, the method used to calculate them, and their impact on the outcome of the evaluations was conducted.
Data extraction forms were managed in Microsoft Excel version 14.0 (2010). Synthesis of the extracted evidence was qualitative in nature with numeric data converted to categorical data as necessary. Country of origin of the study was also further subdivided into geographic region and income group based on the World Bank Atlas method [Citation28]. Time horizon was grouped into 5 categories: ‘Less than one year’, ‘1–9 years’, ‘10–30 years’, ‘Lifetime’, and data ‘Not available’. Types of productivity elements included were determined based on a description of the costs included. Productivity elements included absenteeism, presenteeism, unemployment/early retirement, and premature mortality as defined above. Studies that stated that productivity loss was included in the model without explicitly specifying the exact elements included were listed as ‘Other’.
The impact of the inclusion of productivity costs on the ICER was grouped into 4 categories depending on their impact: ‘More favorable’, ‘No substantial impact’, ‘Less favorable’ or ‘Not reported’. Studies were labeled as having a ‘More favorable’ impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a ‘Less favorable’ impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having ‘No substantial impact’ if there was no change in the ICER with the inclusion of productivity costs based on specific figures provided or if their inclusion was reported by the author(s) as having no substantial impact when no specific estimates were provided. When the impact of inclusion of productivity losses/gains was not mentioned, then ‘Not reported’ was noted. A statistical analysis of the relationship between different study characteristics (e.g. disease area, productivity elements included, etc.) and the impact of inclusion of productivity losses/gains on cost-effectiveness was considered. However, that analysis was not conducted due to the small number of studies included and a lack of statistical power.
2.6. Quality assessment
The reporting quality of studies included in this review was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines first published in 2013 by the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) [Citation29]. The CHEERS checklist has since been utilized extensively and helps ensure the consistency of reporting [Citation30–32]. The number (%) of included studies appropriately/partially addressing each item of the CHEERS checklist was calculated according to the criteria they met. Total scores were calculated for each study (maximum score = 24). Each study was then categorized based on the percentage score into one of four categories: excellent (score ≥85%), very good (70 ≤ score < 85%), good (55 ≤ score < 70%), and poor (score < 55%).
3. Results
The search strategy identified 578 publications (). Removal of duplicates resulted in 416 publications to be screened. Review of titles and abstracts (‘TiAb’ screening) by two independent reviewers resulted in exclusion of 263 publications, with 153 publications remaining for the full-text review to assess for inclusion based on the inclusion and exclusion criteria. Sixty-five publications were excluded due to failure to meet one or more of the inclusion criteria or having met one or more of the exclusion criteria. Ultimately, 88 publications were included in the final analysis. No additional publications were identified through the search of references cited in the included studies and relevant (but not included) systematic reviews.
3.1. Characteristics of studies included
The 88 studies meeting the inclusion criteria were analyzed with regard to distribution by income group of the country of origin in which they had been conducted, global regional grouping, target disease/condition, sponsor type, time period of analysis, type(s) of analysis, and perspective(s) adopted for the economic evaluation (). Sixty-four studies (73%) were conducted in high-income countries and 58 (66%) of studies were conducted based in North American and European/Central Asian countries. Fifty-one studies (58%) focused on influenza, rotavirus, or pneumococcal disease including 18 (20%), 17 (19%), and 16 (18%) studies, respectively. Fifty studies (57%) were sponsored by a pharmaceutical company. Thirty-two studies (36%) were published prior to 2010 and 32 studies (36%) were published between January 2010 and December 2014. However, only 24 studies (27%) were published between January 2015 and December 2019 suggesting that the number of CEA/CUA for vaccines that include productivity losses/gains may not be increasing. Fifty-five studies (63%) included both a CEA and a CUA, meaning that the ICER was calculated with QALYs as an outcome measure as well as some other outcome measure (). Nineteen studies (22%) included only a CEA.
Table 1. Distribution of included studies based on global regional grouping, income category of country of study, disease area, study sponsorship, time period of publication, type of pharmacoeconomic analysis, study type, study design, time horizon, and analysis perspective
Eighty studies (91%) were model based, with Markov modeling and decision trees being the most widely employed modeling techniques (). Twenty-seven studies (31%) employed Markov modeling, 22 studies (25%) employed decision trees, and 2 studies (2%) employed both Markov modeling and decision trees. Other commonly used models included disease transition models and static disease/cost models which were used for 15 studies (17%) and 11 studies (13%), respectively. Eight studies (9%) had been conducted in conjunction with clinical studies, a small proportion (4 [5%]) of which were randomized controlled trials (RCTs). The use of Markov modeling was more common for studies that evaluated vaccines for herpes zoster/varicella with 6 out of 9 studies (67%) for herpes zoster vaccines employing Markov modeling compared to 29 out of 88 studies (33%) overall. Time horizons modeled varied substantially by disease area, with studies evaluating vaccines for influenza and rotavirus more frequently evaluating interventions over smaller time periods (less than a year or 1–9 years), while studies evaluating vaccines for herpes zoster/varicella, hepatitis, pneumococcal disease, and other disease used longer time horizons (10–30 years or lifetime). Thirty-eight studies (43%) were conducted based on the societal perspective and 44 studies (50%) were conducted based on the societal perspective and some other perspectives (e.g. healthcare and/or payer perspective). Only three studies (3%) did not include the societal perspective and three studies (3%) did not explicitly state the perspective of the analysis.
3.2. Analysis of inclusion of productivity losses/gains
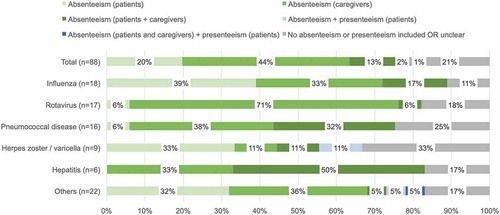
The productivity elements included in the analysis were reported for 71 studies (81%). Absenteeism was the most commonly used element to model productivity losses/gains for vaccines (). Forty-nine studies (56%) used only absenteeism as the productivity element. Most other studies (21 [24%]) modeled absenteeism together with presenteeism and/or costs associated with unemployment and/or premature mortality. Presenteeism alone was not considered in any of the 88 studies examined. Studies that evaluated vaccines for rotavirus more commonly included only productivity losses/gains associated with absenteeism with 12 out of 17 studies (71%) including absenteeism only. Among those, 11 out of 12 studies included absenteeism related to caregivers – likely due to the fact that rotavirus vaccines are geared toward pediatric patients. Studies that evaluated vaccines for influenza and hepatitis more commonly included costs associated with absenteeism for patients only or absenteeism for both patients and caregivers including 10 studies (56%) for influenza vaccines and 3 studies (50%) for hepatitis (). Studies that evaluated vaccines for herpes zoster/varicella and influenza more commonly included absenteeism for patients only – likely due to the fact that herpes zoster vaccines and to a degree influenza as well are geared toward adult patients. Costs associated with premature mortality were modeled for 20 studies (23%) that were included in the review (). CEAs/CUAs for vaccines for influenza (6 [33%]) and pneumococcal disease (5 [26%]) more frequently modeled costs associated with premature mortality ().
Table 2. Productivity loss analysis
Differences in the productivity elements included for studies by geographic region, country income group, and period of evaluation were difficult to ascertain due to limited sample sizes for some groups. Studies conducted for North America more commonly included another element such as unemployment/early retirement and/or premature mortality in addition to absenteeism (). However, this may be driven by the kinds of target diseases covered since 10 out of 18 studies (56%) for North America were for influenza which more commonly includes costs associated with premature mortality, for example. In contrast, only 5 out of 40 studies (13%) included for Europe/Central Asia, where inclusion of absenteeism only was more common, were for influenza. Studies whereby the time horizon was less than one year more commonly included only absenteeism and no other productivity elements.
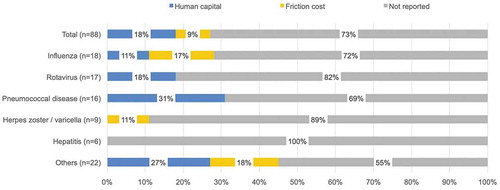
The method used to value productivity losses/gains was explicitly reported in only 24 (27%) of all studies included. The human capital approach and the friction cost approach are two commonly used approaches to value productivity losses. Studies for which the approach used to estimate productivity losses/gains was not explicitly mentioned were also reviewed in more detail to see if the description of productivity loss/gain estimations provided could help confirm the approach used. However, most of the studies that did not specify the approach used only mentioned that costs from lost productivity were included and the approach used to estimate them was uncertain based on the description provided. Therefore, the analysis of the approach used in those cases was based on the explicit mention of the approach used by the author. Sixteen studies (18%) used the human capital approach while 8 studies (9%) used the friction cost approach ().
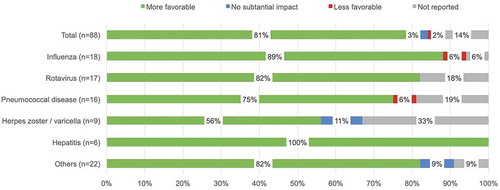
Seventy-six studies (86%) reported the impact of including productivity losses/gains on the ICER, with a majority (93% [71 of 76]) of studies reporting that the inclusion of productivity losses/gains contributed to a more favorable ICER; meaning that their inclusion was said to lead to a reduction in ICER. A very small proportion of studies (4% [3 of 76]) reported no clear change in the ICER with the inclusion of productivity losses/gains (). Among the studies that reported the impact of including productivity losses/gains on the ICER, a very small proportion of studies (3% [2 of 76]) reported a less favorable ICER with the inclusion of productivity losses/gains. Inclusion of productivity losses due to premature mortality seemed to contribute to more favorable cost outcomes and ICERs or lost productivity of a caregiver as a result of acquiring the vaccine.
Figure 4. Productivity loss analysis – impact on ICER. a) Studies were labeled as having a ‘More favorable’ impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a ‘Less favorable’ impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having ‘No substantial impact’ if there was no change in the ICER with the inclusion of productivity costs or if their inclusion was reported by the author(s) as having no substantial impact and no specific figures were provided
3.3. Assessment of the quality of included studies
Of the 88 studies included in this systematic review, 82 were included in the quality assessment exercise based on the CHEERS checklist. Almost half of those studies were considered to be of ‘excellent’ quality and almost one-third were considered to have be of ‘very good’ quality. Only 6% of the studies included in this exercise were deemed to be of ‘poor’ quality.
4. Discussion
This review examined the use of indirect costs, defined as productivity losses/gains associated with morbidity and mortality that leads to loss of work time, in CEAs and CUAs for vaccines and their impact on the findings of those studies [Citation33]*. Most studies were conducted for high-income countries and countries in North America and Europe/Central Asia regions. This is consistent with the previous literature reviews that examined CEA and budget impact studies for vaccines overall [Citation34–36]. Most of the studies identified evaluated the cost-effectiveness of one or more vaccine for influenza, rotavirus, or pneumococcal disease. This, too, is consistent with previous literature reviews of CEAs and budget impact studies for vaccines overall [Citation36–38]. So the findings concerning the origin of the studies and the kinds of condition studies may be characteristic of vaccine cost studies as a whole. Fewer studies were identified that were conducted in the past 5 years than for previous periods suggesting that either fewer CEAs/CUAs related to vaccines have been conducted overall in recent years or that fewer studies have included productivity losses/gains in recent years.
This review showed that most CEA studies for vaccines that include productivity losses/gains provide details on the specific productivity elements that are included in the analysis (e.g. absenteeism, presenteeism, unemployment, premature mortality, etc.). Moreover, most studies included absenteeism as a cost element. However, the specific cost elements included and whether they are productivity losses/gains for patients and/or caregivers differed by the disease area and patient group targeted. Evaluations for vaccines that target conditions that are common among children such as evaluations for rotavirus vaccines predominantly included absenteeism for caregivers. On the other hand, evaluations of vaccines for conditions that are common among adults or both adults and children such as evaluations of influenza vaccines, hepatitis, and pneumococcal disease, varied more widely in their inclusion of absenteeism for patients and/or caregivers. The condition and the age of patients that suffer from the condition that the vaccine targets, therefore, seem to be important factors in the selection of productivity elements for inclusion.
Presenteeism was modeled in only a few studies. One possible reason for its omission from analyses could be lack of data and difficulty in evaluating the loss of efficiency at work which has been shown in a previous study [Citation9]. The findings from the previous reviews showed that losses from reduced productivity while at work are rarely included in CEA/CUA, although presenteeism has been associated with significant costs. Alternatively, it may be that presenteeism has not been included because it assumes that patients attend work even when suffering from their condition which is often an infectious disease in the case of vaccines. Inclusion of premature mortality was more commonly observed for studies that evaluated influenza and rotavirus.
Although the kinds of productivity elements included were typically reported, nearly three out of four studies reviewed did not report the approach used to calculate costs associated with productivity losses/gains. Among the studies that reported the approach used, two-thirds of the studies used the human capital approach and one-third of the studies used the friction cost approach. For the human capital approach, costs associated with productivity losses are calculated by multiplying lost work time by discounted gross wages. For the friction cost approach, costs associated with productivity losses are calculated by multiplying the work hours during the period from when the patient must take off work to when a new employee can be hired and trained to replace them by the discounted gross wages for the patient [Citation24]. All but one study that used the friction cost approach was conducted in The Netherlands where health economic guidelines recommend the use of that approach [Citation36]. More disclosure of information on the approach used to measure productivity losses/gains might contribute to a better understanding of their application to economic evaluations and more transparency in the findings.
We found that inclusion of productivity losses/gains tended to have a favorable impact on the findings of economic evaluations for vaccines with lower ICERs reported based on their inclusion. Some studies also reported that inclusion of productivity losses/gains did not have a significant impact on the ICER which was primarily due to the fact that they formed a very small fraction of total costs in those cases or because the productivity losses/gains were similar in both arms. In most cases whereby the impact of including productivity losses/gains was small, the target population included young children or elderly persons and the productivity losses/gains were small even with the inclusion of caregiver absenteeism.
Two studies reported a less favorable ICER with the inclusion of productivity losses/gains. Among those, one study examined the cost-effectiveness of a pneumococcal disease intervention and one study examined the cost-effectiveness of an influenza intervention. Those studies showed that inclusion of productivity losses associated with a caregiver acquiring the vaccine and other program-related costs can lead to high costs for the intervention group and a higher ICER in some cases. The influenza intervention study examined the cost-effectiveness of influenza vaccinations for healthy children and relatively high productivity losses were reported for the caregiver (i.e. a parent) when supporting the patient with undergoing the vaccine compared to only a modest gain in terms of lost productivity due to lower incidence of the disease. This resulted in a higher ICER with the inclusion of productivity losses/gains. No distinct pattern was observed in the impact on the ICER depending on the productivity elements included; however, studies including productivity losses due to premature mortality seemed to result in more favorable cost outcomes.
It is clear that cost-effectiveness is an important factor considered by decision-makers in countries like the US and several European countries when evaluating whether or not to fund new vaccines and guidelines in those regions. The global COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has sparked further discussion around the cost-effectiveness of vaccines as countries consider the optimal level of coverage for a forthcoming SARS-CoV-2 vaccine. In a recent paper, Neumann et al. suggest that in the context COVID-19 and its impact on society, analyses based on the societal perspective which includes a consideration of productivity losses/gains would be most appropriate [Citation39]. The United States Centers for Disease Control and Prevention’s (CDC) COVID-19 Vaccination Program has announced that vaccines will be administered without cost to patients in the United States through providers – at least in terms of the initial dose [Citation40].
4.1. Limitations
To our knowledge, this review is the most exhaustive research on the use of productivity losses/gains in vaccine-related CEAs and CUAs. This review has some limitations, however. First, as with any review, the quality of reporting in the studies identified had a bearing on the findings of this review. Some studies, for example, did not present separately the ICERs with and without inclusion of productivity losses/gains making it difficult to make a direct inference about the impact of including productivity losses/gains on the ICER. Additionally, we could not elaborate on the approach used to estimate productivity losses/gains (e.g. human capital method, friction cost method, etc.) due to lack of inclusion of that information for many of the publications included.
This review was conducted using three online databases: Medline, Embase, and Cochrane Library. Other databases and gray literature sources including any unpublished reports – including those issued through government agencies – were not included in the review which may have led to non-identification of some relevant studies. However, given that this review had set out to identify CEAs and CUAs for vaccines, the databases searched are widely acknowledged to be very comprehensive with regard to the medical and health economics literature. Moreover, it does not seem likely that inclusion of more databases would have resulted in a substantially higher number of studies that could have altered the conclusion drawn.
Lastly, only CEA and CUA studies that included productivity losses/gains in their analysis were included in this review. A broader analysis that also includes studies that did not include productivity losses/gains may shed more light on the merit and demerit of their inclusion within specific disease areas.
5. Conclusions
This review has provided a comprehensive overview of how costs related to productivity have been used in CEAs and CUAs for vaccines. It was confirmed that inclusion of productivity losses/gains in CEAs and CUAs for vaccines typically results in more favorable cost-effectiveness. However, there is a lack of reporting on how productivity losses/gains were calculated in CEA/CUA studies for vaccines making it difficult to summarize the specific approaches used – e.g. human capital approach or friction method. Moreover, it is unclear how approaches to calculating productivity losses/gains and their impact on cost-effectiveness may differ within specific disease areas since only studies that included productivity losses/gains were included in this review. Further research examining these aspects may lead a clearer understanding of the relative importance of productivity losses/gains within specific disease areas.
6. Expert opinion
Cost-effectiveness analyses based on the societal perspective, which includes considering productivity losses/gains, would be appropriate for some vaccines. However, our review revealed that the specific cost elements included and whether their inclusion results in productivity losses/gains for patients and/or caregivers differed by the disease area and patient group targeted. In the future, clearer guidance on the productivity elements to include by disease area and more transparency on the calculation method used may be needed to better consider the full value of implementing vaccination programs.
Declaration of interest
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Akira Yuasa and Naohiro Yonemoto are full-time employees of Pfizer Japan Inc. Michael LoPresti is a full-time employee of INTAGE Healthcare Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (63.3 KB)Acknowledgments
This study includes research content that Akira Yuasa has conducted during his doctoral program at the International University of Health and Welfare.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Neumann PJ, Russell LB, Siegel JE, et al. Using cost-effectiveness in health and medicine: experiences since the original panel. In: Neumann PJ, Sanders GD, Russell LB, et al., editors. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 2017. p. 1–38.
- Nohynek H, Wichmann O, D’Ancona F, et al. National advisory groups and their role in immunization policy-making processes in European countries. Clin Microbiol Infect. 2013;19:1096–1105.
- Leidner, AJ, Chesson HW, Meltzer MI, Messonier ML, Lee GM, et al. National Center for Immunization and Respiratory Diseases (NCIRD). Guidance for health economics studies present to the advisory committee on immunization practices (ACIP). 2019 Oct 11; Update https://www.cdc.gov/vaccines/acip/committee/downloads/Economics-Guidance-for-ACIP-2019.pdf. cited 2020 Jun 3
- Stone PW, Chapman RH, Sandberg EA, et al. Measuring costs in cost-utility analyses: variations in the literature. Int J Technol Assess Health Care. 2000;16(1):111–124.
- Pike J, Grosse SD. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with human capital estimates: a review. Appl Health Econ Health Policy. 2018;16(6):765–778.
- Krol M, Papenburg J, Tan SS, et al. A noticeable difference? Productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur J Health Econ. 2016;17(4):391–402.
- Ultsch B, Damm O, Beutels P, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a European vaccine economics community. PharmacoEconomics. 2016;34(3):227–244.
- Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. Pharmacoeconomics. 2014;32(4):335–344.
- Kigozi J, Jowett S, Lewis M, et al. Estimating productivity costs using the friction cost approach in practice: a systematic review. Eur J Health Econ. 2016;17(1):31–44.
- Sussman M, Benner J, Neumann P, et al. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia. 2018;38(10):1644–1657.
- Kilian R, Frasch K, Steinert T, et al. Cost-effectiveness of psychotropic polypharmacy in routine schizophrenia care. Results of the ELAN prospective observational trial. Neurol Psychiatry Brain Res. 2018;30:47–55.
- Delea TE, Amdahl J, Chit A, et al. Cost-effectiveness of lapatinib plus letrozole in HER2-positive, hormone receptor-positive metastatic breast cancer in Canada. Current Oncol. 2013;20(5):e371–87.
- Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S17–26.
- Zhang W, Sun H, Woodcock S, et al. Valuing productivity loss due to absenteeism: firm-level evidence from a Canadian linked employer-employee survey. Health Econ Rev. 2017;7(1):3.
- Krol M, Papenburg J, Koopmanschap M, et al. Do productivity costs matter?: the impact of including productivity costs on the incremental costs of interventions targeted at depressive disorders. Pharmacoeconomics. 2011;29(7):601–619.
- Johannesson M, Jonsson B, Jönsson L, et al. Why should economic evaluations of medical innovations have a societal perspective? Office of Health Economics. Briefing. 2009;51:1-32.
- Jönsson B. Ten arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10(4):357–359.
- Tai BB, Bae YH, Le QA. A systematic review of health economic evaluation studies using the patient’s perspective. Value Health. 2016;19(6):903–908.
- Yuasa A, Yonemoto M, LoPresti M, et al. Use of productivity loss/gain in cost-effectiveness analyses for drugs: a systematic review. Pharmacoeconomics. 2020;39:81–97. Available ahead of print.
- Haute Autorité de santé (HAS). Choices in methods for economic evaluation: a methodological guide. Department of Economics and Public Health Assessment, HAS, Saint-Denis La Plaine, France. 2012. cited 2020 Jun 3. https://www.has-sante.fr/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf
- Center for Outcomes Research and Economic Evaluation for Health. Guideline for preparing cost-effectiveness evaluation to the central social insurance medical council (version 2.0). National Institute of Public Health (C2H), Japan, Tokyo. cited 2020 Jun 3. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf
- The Robert Koch Institute. Modelling methods for predicting epidemiological and health economic effects of vaccinations – guidance for analyses to be presented to the German standing committee on vaccination (STIKO). Version 1.0; Mar 2016.
- Centre for Reviews and Dissemination (CRD). University of York. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York, UK: Centre for Reviews and Dissemination (CRD), University of York; 2009.
- Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0. Cochrane, London, UK; Jul 2019. cited 2020 Jun 3. https://training.cochrane.org/handbook
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
- Sakamaki H, Ishida H, Fukuda T, et al. Issues on estimating costs in economic evaluation [in Japanese]. Jpn J Pharmacoepidemiol. 2012;17(1):14–20.
- The World Bank Group, World Bank country and lending groups. cited 2020 Mar 18. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. BMJ. 2013;346:f1049.
- Hettiarachchi RM, Kularatna S, Downes MJ, et al. The cost-effectiveness of oral health interventions: a systematic review of cost-utility analyses. Community Dent Oral Epidemiol. 2018;46(2):118–124.
- Palfreyman SJ, Stone PW. A systematic review of economic evaluations assessing interventions aimed at preventing or treating pressure ulcers. Int J Nurs Stud. 2015;52(3):769–788.
- Rogers HJ, Rodd HD, Vermaire JH, et al. A systematic review of the quality and scope of economic evaluations in child oral health research. BMC Oral Health. 2019;19(1):132.
- Hope SF, Webster J, Trieu K, et al. A systematic review of economic evaluations of population-based sodium reduction interventions. PLoS One. 2017;12(3):e0173600.
- D’Angiolella LS, Lafranconi A, Cortesi PA, et al. Costs and effectiveness of influenza vaccination: a systematic review. Ann dell’Istituto Super Sanità. 2018;54(1):49–57.
- Seto K, Marra F, Raymakers A, et al. The cost effectiveness of human papillomavirus vaccines: a systematic review. Drugs. 2012;72(5):715–743.
- Loze PM, Nasciben LB, Sartori AMC, et al. Vaccines are different: a systematic review of budget impact analyses of vaccines. Vaccine. 2017;35(21):2781–2793.
- Leidner AJ, Murthy N, Chesson HW, et al. Cost-effectiveness of adult vaccinations: a systematic review 2019. . Vaccine. 2019;37(2):226–234.
- Ozawa S, Mirelman A, Stack ML, et al. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: a systematic review. Vaccine. 2012;31(1):96–108.
- Neumann P, Cohen JT, Kim DD, et al. Consideration of value-based pricing for treatments and vaccines is important, even in the COVID-19 pandemic. Health Affairs. 2021;40(1):53–61. (fast track ahead of print article).
- Schwartz K, Freed M, Cubanski J, et al. Vaccine coverage, pricing, and reimbursement in the U.S. Kaiser family foundation. Issue Brief. 2020. https://www.kff.org/coronavirus-covid-19/issue-brief/vaccine-coverage-pricing-and-reimbursement-in-the-u-s/ cited 2020 Jun 3